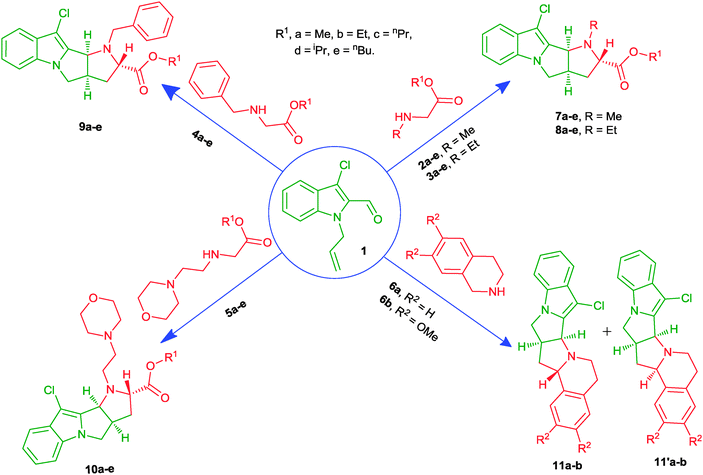
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient synthesis of some new antiproliferative N-fused indoles and isoquinolines via 1,3-dipolar cycloaddition reaction in an ionic liquid†
Tushar R.
Sutariya
a,
Balvantsingh M.
Labana
a,
Narsidas J.
Parmar
*a,
Rajni
Kant
b,
Vivek K.
Gupta
b,
Gabriela B.
Plata
c and
José M.
Padrón
c
aDepartment of Chemistry, Sardar Patel University, Vallabh Vidyanagar-388120. Dist. Anand, Gujarat, India. E-mail: njpchemdeptspu@yahoo.co.in; Fax: +91-2692-236475; Tel: +91-2692-226858
bPost-Graduate Department of Physics, University of Jammu, Jammu, Tawi-180006, India
cBioLab, Instituto Universitario de Bio-Orgánica “Antonio González” (IUBO-AG), Centro de Investigaciones Biomédicas de Canarias (CIBICAN), C/Astrofísico Francisco Sánchez 2, 38206 La Laguna, Spain
First published on 23rd January 2015
Abstract
Syntheses of some new pyrrolo-fused pyrrolo[1,2-a] indole derivatives have been achieved by combining N-allyl-indole-2-carbaldehyde with a variety of N-alkyl-glycine esters as well as tetrahydroisoquinolines in an ionic liquid, triethylammonium acetate (TEAA), a recyclable reaction medium, via intramolecular [3+2] cycloaddition reaction. This new method is highly efficient, and the ionic liquid employed is recyclable. The stereochemistry of all the compounds was confirmed by 2D NMR NOESY and in some cases single crystal X-ray diffraction data. The in vitro screening of all new candidates against various bacterial strains and representative human solid tumor cell lines, A549 (lung), HeLa (cervix), SW1573 (lung), T-47D (breast) and WiDr (colon), revealed that many of them have good antibacterial, antifungal and antitubercular and antiproliferative activities.
Introduction
The heterocycles with a N-fused indole/isoquinoline-heterocyclic framework as one of their integral structural units constitute an important class of bioprofiles, and have attracted the interest of many in the research fields of medicines and pharmaceuticals.1 Containing the pyrrolo-indole nucleus, terpenoidal alkaloids2 and mitomycins exhibited antitumor activity (Fig. 1A).3 With pyrrolo[1,2-a]indole as a specific fusion, cyclopropamitosenes revealed cytotoxicity through bacterial cell division and DNA alkylation.4 Likewise, antimalarial Flinderole B,5 bioactive Isatisine A (Fig. 1B)6 and hallucinogenic Yuremamine (Fig. 1C), which are potential candidates of this class have attracted the interest of many chemists and biochemists.7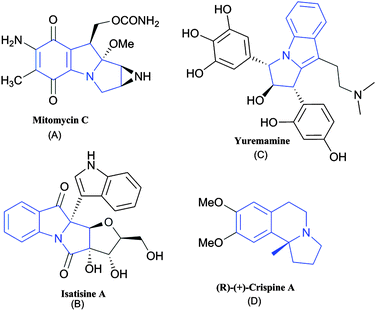 | ||
| Fig. 1 Some biologically active heterocycles containing pyrrolo-indole and pyrrolo-isoquinoline units. | ||
Pyrrolo-isoquinoline alkaloids, on the other hand, showed promising results for depression in animals, with the pyrrolo-isoquinoline nucleus.8 A tricyclic hydropyrrolo [2,1-a] isoquinoline is a key fused-ring system with (2)-trolline structure, which can be derived from Trollius Chinese flowers9 and its Portulaca oleracea L weed-derived antipode (+)-oleracein E.10,11 Studies showed that (2)-trolline has remarkable activity against influenza viruses A and B, and so revealed antiviral properties. (+)-Oleracein E displayed DPPH-radical scavenging activity.10 In addition, the members of the trolline family are active against respiratory Staphylococcus aureus and pneumonia bacteria.9 Like trolline, (+)-crispine A (Fig. 1D) also incorporates an analogous heterocyclic framework and has potential anti-proliferative activity against SKOV3, KB, and HeLa human cancer lines.12
Finally, pyrrolizidine present in many heterocycles represents another useful framework of biological interest. In addition to anti-inflammatory and analgesic properties, it confers heterocycles with effective aromatase and tumour growth inhibitory action.13 Besides, 5-HT2c receptor agonists, known to be effective in hyperglycaemia and other diseases, come from the same family and make it an interesting source of bioactive scaffolds.14
In view of the above, it follows that incorporation of N-fused indole, N-fused isoquinoline and pyrrolizidine into a molecular assembly helps generate a new and useful class of heterocycles, finding a great importance in medicinal chemistry. Development and enlargement of a molecular library of these heterocycles is thus desirable and worth studying too.
Intermolecular alkylation,15 radical cyclization,16trans annulation reaction,17 carbene-based rearrangement,18etc. are general synthetic approaches for N-fused indoles. Cycloaddition reaction involving in situ azomethine ylide generation, nevertheless, seems to be seldom studied for such heterocycles.19 This protocol is highly atom economic, and has been used so far for many complex heterocyclic systems. Many aldehydes reacted with amino acid derivatives20 to afford 6–5 fused-ring systems. Indole-based substrates however are very few in number for designing 5–5 fused-ring systems.21 The ring system exists in biotin, an important naturally occurring heterocycle.21d
As part of our research program, we have reported the synthesis of many pyran-heterocycles.20h including aminobenzopyrans20h,ivia 1,3 dipolar cycloaddition. Here, we describe pyrrolo-indole and pyrrolo-isoquinoline derivatives as new bioprofiles, constructed from N-allyl-3-chloro-indole-2-carbaldehyde 1 with a variety of α-amino acid esters as well as tetrahydroisoquinolines, in ionic liquid TEAA as an effective reaction medium.
Use of ionic liquids (ILs) helped translate many synthetic routes into green methodologies, with adopting requisite practice not only as economic one, but as environmentally friendly too.22 To the best of our knowledge, very few reports appeared on use of ionic liquid in 1,3-dipolar cycloaddition reaction.23 And those reported in ILs still suffer from preparation cost, use and recyclability of ILs, and, of course, promotion of the reaction.23c Conventional methods in general suffers from many disadvantages like use of conventional solvent, longer reaction time and tedious work-up procedure.24
Results and discussion
Chemistry
All requisite secondary amines 2–6, except tetrahydro-isoquinolines 6; N-methyl/ethyl/benzyl/(4-morpholinyl) ethyl glycine esters, are liquids and prepared by methods reported elsewhere.25 Teterahydroisoquinolines 6 were used as received from commercial sources. The substrate, N-allyl-3-chloro-indole-2-carbaldehyde 1, was obtained by reacting 3-chloro-indole-2-carbaldehyde with ally bromide in the presence of anhydrous K2CO3, suspended in DMF (dimethylformamide) solution (Scheme 1), in 95% yield. 3-Chloro-indole-2-carbaldehyde was obtained as the Vilsmeier–Haack product of (phenylglycine)-O-carboxylic acid, with excellent purity.26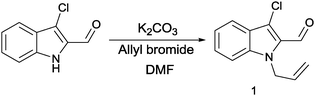 | ||
| Scheme 1 Synthesis of N-allyl-3-chloro-indole-2-carbaldehyde 1, the reagents and conditions (1) K2CO3, allyl bromide, DMF, 10–12 h, room temp. | ||
The reaction between amine 3a and aldehyde 1 was taken as a model to optimize the reaction conditions (Table 1). Initially, we heated the aldehyde and amine in refluxing methanol (entry 1), acetonitrile (entry 2), toluene (entries 3 and 4) and xylene (entries 5 and 6), in the presence and absence of Na2SO4. Although results in toluene and xylene using Na2CO3 were good, prolonged heating was discouraging to accept this method (6 h). Even when tried reported methods, they failed to run present conversion effectively.22c,d Thus, we opted for heating in a solvent-free environment at 100 °C. Here, the reaction time could be improved but not the yields (entry 7). The conventional way was thus abandoned in favour of the TEAA promoted one (entry 8). It showed improved results in yield and reaction time at 80 °C that were improved further at 100 °C (entry 9). Above 100 °C, however no further improvement was seen. This improved method was then generalised to receive other products, too (Scheme 2 and Table 2). The advantage of the present protocol is that it takes relatively less reaction time (3 h) than others.24c It should be noted further that the present combination allows pyrrolizidine to act as a highly significant bioactive unit to incorporate effectively into N-fused indole/isoquinoline heterocycles.
| Entry | Compound | R | R1 | R2 | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 7a | Me | Me | — | 2.75 | 82 |
| 2 | 7b | Me | Et | — | 2.75 | 80 |
| 3 | 7c | Me | nPr | — | 3.0 | 77 |
| 4 | 7d | Me | iPr | — | 3.0 | 84 |
| 5 | 7e | Me | nBu | — | 3.5 | 77 |
| 6 | 8a | Et | Me | — | 2.5 | 85 |
| 7 | 8b | Et | Et | — | 2.75 | 82 |
| 8 | 8c | Et | nPr | — | 3.0 | 84 |
| 9 | 8d | Et | iPr | — | 3.0 | 78 |
| 10 | 8e | Et | nBu | —— | 3.0 | 79 |
| 11 | 9a | Bn | Me | — | 2.5 | 80 |
| 12 | 9b | Bn | Et | — | 3.0 | 82 |
| 13 | 9c | Bn | nPr | — | 3.0 | 78 |
| 14 | 9d | Bn | iPr | — | 3.5 | 76 |
| 15 | 9e | Bn | nBu | — | 3.5 | 74 |
| 16 | 10a | Mp | Me | — | 3.0 | 76 |
| 17 | 10b | Mp | Et | — | 3.0 | 78 |
| 18 | 10c | Mp | nPr | — | 3.0 | 72 |
| 19 | 10d | Mp | iPr | — | 3.5 | 74 |
| 20 | 10e | Mp | nBu | — | 3.0 | 75 |
| 21 | 11a | — | — | H | 2.75 | 45 |
| 22 | 11′a | — | — | H | 2.75 | 37 |
| 23 | 11b | — | — | OMe | 3.0 | 46 |
| 24 | 11′b | — | — | OMe | 3.0 | 38 |
A plausible mechanism of the reaction has been depicted in Scheme 3. Addition of electrons from the tethered-alkene terminal carbon on imine follows the addition of the enolized ester on the other end of the alkene via a semi bicyclic transition state that may force the system to adopt the most favoured cis-fusion product. The exo or endo attack of dipolarophile alkene on azomethine ylide dipole determines the stereoselectivity of the reaction. The spectral data support the cis-fusion between central pyrrolidine rings in all heterocycles, hence favouring the endo transition state. In 11′a–b, however, the cis-fusion may involve a further isomerization of the ylide.27
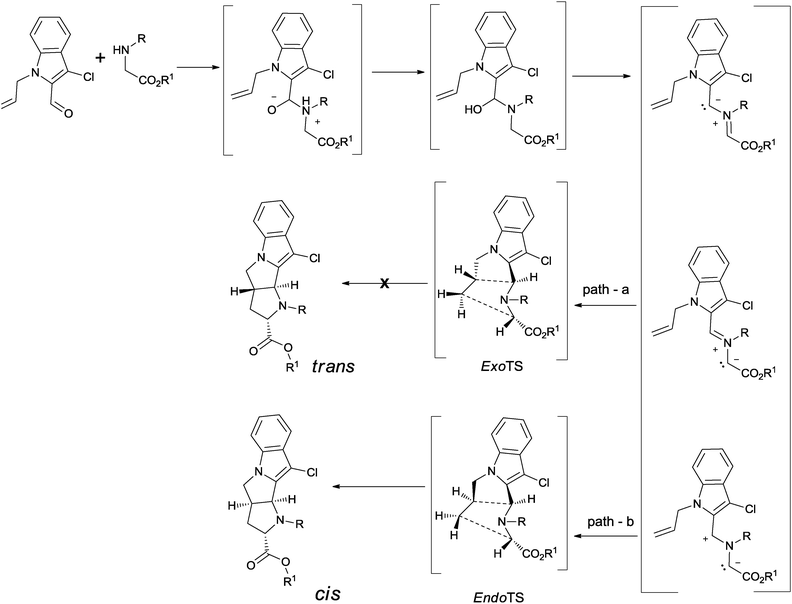 | ||
| Scheme 3 A plausible mechanism of the reaction between aldehyde 1 and secondary amine via 1,3-dipolar cycloaddition reaction. | ||
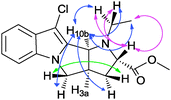
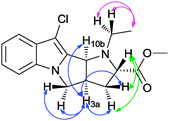
The proposed structures of all newly synthesized heterocycles fully agreed with the observed elemental analyses, mass, IR and NMR spectral data. In the 1H NMR spectra, all compounds except 7 showed a multiplet in the region δ 2.93–4.09 ppm, due to diastereotopic methylene protons of the pyrrolidine ring. In 7, instead the N-methyl proton appeared as a singlet at δ ∼ 2.75 ppm. The carbonyl group in 7–10 showed a characteristic IR band at ∼1730 cm−1, and a 13C NMR signal ∼δ 173 ppm. In all compounds, a doublet in the δ 4.00–4.80 ppm range, with the J value in the 7.6–8.4 Hz range, can be attributed to a bridge-head proton 10b or 12b, suggestive of cis-fusion between central pyrrolidine rings. Another bridge-head proton 3a or 5a appeared as a multiplet at δ ∼ 3.80 ppm is therefore orientated cis to this 10b or 12b proton. Analysing cross-peaks in 2D NMR NOESY (nuclear Overhauser effect spectroscopy) and DQF-COSY (double quantum filtered correlation spectroscopy) of representative 8a (Fig. 2 and 3), a similar relationship between these protons could be confirmed. The bridge-head proton 2 or 4b is however trans with respect to proton 10b or 12b, appearing at δ 4.00–4.40 ppm, except in 11′a–b. In 11′a–b, it is cis oriented.
Finally, with the single-crystal X-ray diffraction data of 9b and 11′b, we could be able to establish important stereo-chemical considerations (Fig. 4).
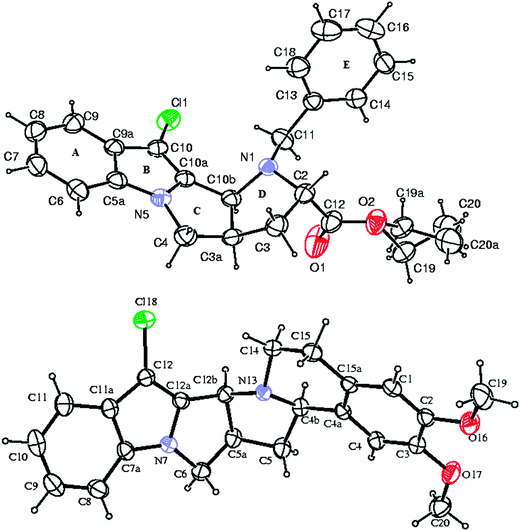 | ||
| Fig. 4 The ORTEP views of compounds 9b and 11′b, with displacement ellipsoids at the 40% probability level. | ||
Biological results
Table 3 summarizes in vitro antimicrobial screening test results of all the compounds. The majority of compounds displayed good resistance against bacteria, at least, in line with one of the standard reference drugs ampicillin. The activity of some of them was found to be equal to that of a more potent drug. Analyzing results in terms of maximum how many bacterium species a compound can kill effectively revealed that the compound can cover a maximum of five species at least with the potency equivalent to ampicillin. Examples include 7c, 9c, 10e and 11b. Among them, 7c revealed excellent activity against Gram-positive Clostridium tetani (reaching to potency of even more potent norfloxacin) and Gram-negative Escherichia coli bacteria (reaching the potency of even more potent chloramphenicol). Similarly, compound 9c resembled more potent standard norfloxacin drug in activity, against Gram-positive Bacillus subtilis bacteria. Compounds 7a, 7d, 8a, 8c, 8d, 9e, 10a, 10b, 10c and 11a, on the other hand, had recorded better resistance against at least four types of bacteria. Among them, 7a, 7d, 8c, and 10a registered excellent activity against Gram-positive Clostridium tetani bacteria, with MIC values in line with ciprofloxacin which is more potent than ampicillin. Compounds 7d and 10a with chloramphenicol-equivalent potency showed good results against Bacillus subtilis and Salmonella typhi bacteria respectively. It is noted that both ciprofloxacin and compound 7d recorded similar MIC values against Bacillus subtilis bacteria. Next, those with at least activity against three types of bacterium species include 7b, 7e, 8b, 8e, 9b, 9d and 10d. Among them 8b, 8c and 9d are very close to standard ciprofloxacin in potency, against Clostridium tetani bacteria. Further, a few of the compounds showed good antifungal activity, particularly against Candida albicans fungus. Examples include 7a, 7e, 8c, 8e, 9c and 10d all having griseofulvin-equivalent power. As anti-fungal agents, compounds 7d, 10a and 10b are relatively more active.| Compound | Antimicrobial activity (MIC μg mL−1) | Anti TBa (%) inhibition | Antioxidant activityb FRAPc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gram-positive bacteria | Gram-negative bacteria | Fungi | ||||||||
| S.p. | C.t. | B.s. | S.t. | V.c. | E.c. | A.f. | C.a. | |||
| S.p.: Streptococcus pneumoniae, C.t.: Clostridium tetani, B.s.: Bacillus subtilis, S.t.: Salmonella typhi, V.c.: Vibrio cholerae, E.c.: Escherichia coli, A.f.: Aspergillus fumigatus, C.a.: Candidaalbicans; [A]: gentamycin, [B]: ampicillin, [C]: chloramphenicol, [D]: ciprofloxacin, [E]: norfloxacin, [F]: nystatin, [G]: griseofulvin, [H]: isoniazide.a Concentration of compounds used against M. tuberculosis H37Rv bacteria = 250 μg mL−1, standard antimicrobials used: isoniazide (0.2 μg mL−1).b Concentration of compounds = 200 μg mL−1 and standard: A.A. (ascorbic acid) = 176 μg mL.c A.A. mm per 100 g sample. | ||||||||||
| 7a | 250 | 100 | 250 | 250 | 125 | 100 | >500 | 500 | 46 | 225.11 |
| 7b | 200 | 125 | 100 | 500 | 100 | 200 | >500 | >500 | 20 | 220.49 |
| 7c | 250 | 62.5 | 200 | 125 | 125 | 62.5 | >500 | >500 | 13 | 215.27 |
| 7d | 100 | 100 | 62.5 | 200 | 200 | 100 | >500 | 250 | 47 | 216.87 |
| 7e | 100 | 250 | 100 | 250 | 250 | 250 | >500 | 500 | 57 | 227.32 |
| 8a | 125 | 200 | 200 | 200 | 250 | 62.5 | >500 | >500 | 84 | 219.28 |
| 8b | 125 | 100 | 250 | 200 | 200 | 200 | >500 | >500 | 59 | 217.28 |
| 8c | 100 | 100 | 250 | 250 | 250 | 125 | >500 | 500 | 58 | 210.25 |
| 8d | 125 | 200 | 250 | 250 | 250 | 125 | >500 | >500 | 33 | 213.26 |
| 8e | 200 | 125 | 200 | 200 | 250 | 100 | >500 | 500 | 47 | 222.90 |
| 9a | 200 | 200 | 125 | 250 | 250 | 200 | >500 | >500 | 80 | 213.46 |
| 9b | 250 | 250 | 200 | 250 | 200 | 100 | >500 | >500 | 33 | 228.72 |
| 9c | 100 | 250 | 100 | 100 | 125 | 200 | >500 | 500 | 87 | 234.95 |
| 9d | 250 | 100 | 200 | 100 | 200 | 250 | >500 | >500 | 91 | 236.35 |
| 9e | 200 | 250 | 100 | 100 | 125 | 250 | >500 | >500 | 12 | 245.99 |
| 10a | 125 | 125 | 100 | 62.5 | 200 | 200 | 500 | 250 | 84 | 232.14 |
| 10b | 200 | 200 | 100 | 125 | 125 | 250 | 500 | 250 | 92 | 285.15 |
| 10c | 500 | 250 | 250 | 125 | 250 | 100 | >500 | >500 | 25 | 236.15 |
| 10d | 200 | 250 | 100 | 125 | 250 | 250 | >1000 | 500 | 74 | 239.37 |
| 10e | 500 | 200 | 250 | 125 | 125 | 100 | 500 | >500 | 65 | 241.37 |
| 11a | 100 | 200 | 250 | 100 | 200 | 250 | 500 | 500 | 65 | 224.51 |
| 11′a | 500 | 250 | 100 | 200 | 250 | 200 | 250 | 500 | 78 | 252.22 |
| 11b | 200 | 125 | 125 | 125 | 100 | 100 | >500 | >500 | 58 | 254.23 |
| 11′b | 250 | 200 | 200 | 200 | 250 | 250 | 500 | >500 | 88 | 217.28 |
| [A] | 0.5 | 5 | 1 | 5 | 5 | 0.05 | — | — | — | — |
| [B] | 100 | 250 | 250 | 100 | 100 | 100 | — | — | — | — |
| [C] | 50 | 50 | 50 | 50 | 50 | 50 | — | — | — | — |
| [D] | 50 | 100 | 50 | 25 | 25 | 25 | — | — | — | — |
| [E] | 10 | 50 | 100 | 10 | 10 | 10 | — | — | — | — |
| [F] | — | — | — | — | — | — | 100 | 100 | — | — |
| [G] | — | — | — | — | — | — | 100 | 500 | — | — |
| [H] | — | — | — | — | — | — | — | — | 99 | — |
Anti-tubercular activity study shows that compounds 9d and 10b have highest M. tuberculosis H37Rv bacterial resistance, with growth inhibition in the 90–100% range. Compounds 8a, 9a, 9c, 10a and 11′b have growth inhibition in the 80–90% range.
FRAP values of majority of the heterocycles are around 225 (mmol per 100 g), indicating that they are moderate in anti-oxidant power. However, compound 10b revealed remarkable activity.
The antiproliferative activity of 7–11 was evaluated against a panel of representative human tumor cell lines including A549 (lung), HeLa (cervix), SW1573 (lung), T-47D (breast) and WiDr (colon), using the SRB assay.28 The experimental GI50 values are summarized in Table 4 and compared to those of cisplatin, etoposide and camptothecin after 48 h of treatment. Taken as a whole, pyrrolo-fused-indoles 7–8 was the most active class of compounds, with activity against all cell lines tested. In this particular context, compounds 7–8 showed more activity against HeLa cells, with GI50 values in the range 3.1–14 μM and comparable to those of cisplatin (2.0 μM) or etoposide (3.3 μM).
| Compound | Antiproliferative activity (GI50)a | ||||
|---|---|---|---|---|---|
| Cell line (origin) | |||||
| A549 (lung) | HeLa (cervix) | SW1573 (lung) | T-47D (breast) | WiDr (colon) | |
| [A]: cisplatin, [B]: etoposide, [C]: camptothecin.a Values are given in μM and are means of two to four experiments; standard deviation is given in parentheses.b One experiment was done. | |||||
| 7a | >100 | 3.3 (±0.4) | 68 (±45) | 67 (±47) | 66 (±48) |
| 7b | 61 (±18) | 5.7 (±1.6) | 92 (±11) | 84 (±29) | 76 (±37) |
| 7c | >100 | 3.9 (±1.2) | 53 (±26) | 66 (±49) | 62 (±54) |
| 7d | 41b | 8.3 (±3.2) | 29 (±0.2) | 26 (±6.5) | 22 (±4.0) |
| 7e | 32 (±8.8) | 9.9 (±5.2) | 32 (±2.3) | 25 (±5.8) | 23 (±4.1) |
| 8a | 33 (±3.0) | 14 (±1.4) | 25 (±3.3) | 27 (±4.8) | 24 (±1.7) |
| 8b | 43 (±19) | 3.4 (±0.7) | 33 (±4.6) | 29 (±16.0) | 28 (±10) |
| 8c | 47 (±19) | 3.6 (±0.3) | 41 (±4.3) | 37 (±13.0) | 35 (±7.8) |
| 8d | 28 (±6.9) | 13 (±2.8) | 27 (±3.5) | 25 (±9.4) | 27 (±4.3) |
| 8e | 40 (±20) | 3.1 (±0.4) | 32 (±2.5) | 26 (±4.7) | 24 (±8.9) |
| 9c | >100 | 41 (±30) | >100 | 54 (±21) | >100 |
| 9d | >100 | 48 (±36) | >100 | 57 (±43) | 89 (±15) |
| 9e | >100 | >100 | >100 | >100 | >100 |
| 10a | >100 | >100 | >100 | >100 | >100 |
| 11a | 89 (±16) | 33 (±6.8) | >100 | 53 (±14) | 73 (±39) |
| 11′a | >100 | 84 (±21) | >100 | >100 | >100 |
| 11b | 18 (±14) | 9.5 (±7.1) | 17 (±1.9) | 15 (±7.3) | 14 (±2.4) |
| [A] | — | 2.0 (±0.3) | 3.0 (±0.4) | 15 (±2.3) | 26 (±5.3) |
| [B] | — | 3.3 (±1.6) | 14 (±1.5) | 22 (±5.5) | 23 (±3.1) |
| [C] | — | 0.6 (±0.4) | 0.25 (±0.12) | 2.0 (±0.5) | 1.8 (±0.7) |
No significant differences were observed between methyl and ethyl ester derivatives. In contrast, the presence of a benzyl group (9) or a morpholine substituent (10) on the pyrrol nitrogen produced a severe loss in activity. From the pyrrolo-fused-isoquinolines 11 obtained in our investigations, the best results of antiproliferative activity were obtained for adduct 11b, which showed active against all the cell lines with GI50 values in the range 9.5–18 μM. This is a relevant result, since the class of adduct correlates to selectivity towards cancer cell lines.
Analysing N-fused indoles derived from amino acid esters, structurally, it reveals that methyl at pyrolidine nitrogen confers heterocycles with enhanced resistivity against Clostridium tetani and Escherichia coli bacteria, when carbpropoxy moiety is present at carbon next to this nitrogen. Pyrrolidin with morpholine or ethyl moieties at nitrogen in combination with carbmethoxy group also had a similar effect against these bacteria. Carbbutoxy moiety, on the other hand, had very less effect on activity, irrespective of substituent present at pyrrolidine nitrogen. Antiproliferative activity, nevertheless, seemed to be altered very less taking any ester component with N-methyl/N-ethyl pyrrolidine ring, against HeLa (cervix) cell lines. In addition, N-ethyl pyrrolidine with any ester component had similar effect against Widr(colon) cell lines. The N-fused indoles derived from tetrahydreoisoquinolines showed remarkable bioactivities as well. In general, heterocycles derived from electron releasing methoxy substituted-tetrahyderoisoquinoline are excellent in antibacterial, antitubercular, antioxidant and antiproliferative activities, compared to the ones derived from simple tetrahydro isoquinoline.
Conclusions
Thus, we synthesized N-fused indole and isoquinoline derivatives as new bioactive compounds via 1,3-dipolar cycloaddition reaction, using an ionic liquid, TEAA as inexpensive, environmentally friendly and recyclable reaction medium. The resistance power of 7c, 7d, 8a and 10a at least against one of the bacterial species studied is highest resembling chloramphenicol in activity with MIC 62.5 μg L−1. The antiproliferative activity of 11b, on the other hand, was found to be excellent against all cell lines, with GI50 values lying in the 9.5–18 μM range, comparable to standard drugs used. Besides, 10b, with the FRAP value of 225 (mmol per 100 g), showed excellent ferric reducing anti-oxidant power.Experimental
Materials and methods
All solvents and reagents were used as supplied from commercial sources. The recorded melting points are uncorrected. IR spectra were recorded in KBr on a Shimadzu FT-IR 8401 spectrometer and are reported in wave numbers (cm−1). 1H NMR and 13C NMR spectra were recorded on a Bruker Avance 400 spectrometer operating at 400 MHz for 1H NMR and 100 MHz for 13C NMR as solutions in CDCl3, unless otherwise indicated. Chemical shifts are reported as parts per million (ppm, d) and referenced to the residual protic solvent. Coupling constants are reported in Hertz (Hz). Splitting patterns are designated as s, singlet; d, doublet; t, triplet; q, quartet; br, broad; m, multiplet. The degree of substitution (C, CH, CH2, and CH3) was determined by the DEPT-135 method. The ESI mass spectra were measured on a Shimadzu LCMS-2010 spectrometer. Elemental analysis (% C, H, N) was carried out using a Perkin-Elmer 2400 series-II elemental analyzer (Perkin-Elmer, USA). TLC was performed on Merck 60 F254 precoated silica plates, spots were detected either by UV (254 nm, 366 nm) or dipping into a permanganate [KMnO4 (3 g), K2CO3 (20 g), NaOH (5 mL, 5% in H2O), H2O (300 mL)] or an anisaldehyde solution [3% p-methoxybenzaldehyde and 1% H2SO4 in MeOH] or 2,4-dinitro phenyl hydrazine solution [2,4-DNP (12 g), conc. H2SO4 (6 mL), water (8 mL), EtOH (20 mL)] followed by heating.General procedure for the synthesis of N-fused indoles and isoquinolines
A mixture of an aldehyde 1 (1 equiv.) and an acyclic secondary amine 2–5 (1 equiv.) or isoquinoline 6a–b (1 equiv.) in 2 mL of ionic liquid TEAA in a round bottom flask was heated at 100 °C and completion of the reaction was confirmed through TLC. The reaction mass was cooled to room temperature and poured into ice species. The oily product thus emulsified was then extracted with three 10 mL of diethyl ether portions. It yielded crude products in quantitative amounts upon removal of ether. Finally, the product was purified by column chromatography using a 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 n-hexane-ethyl acetate mixture as an eluent. The yields were in the 75–85% range. TEAA was recovered quantitatively by heating the aqueous layer-left after the ether extraction of the product-under the reduced presser at 80 °C. The recovered ionic liquid can be used again for the same. It was noticed that TEAA can be recycled at least four-times with its unaltered efficiency.
10 n-hexane-ethyl acetate mixture as an eluent. The yields were in the 75–85% range. TEAA was recovered quantitatively by heating the aqueous layer-left after the ether extraction of the product-under the reduced presser at 80 °C. The recovered ionic liquid can be used again for the same. It was noticed that TEAA can be recycled at least four-times with its unaltered efficiency.
Spectroscopy data of compounds (7–11)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS (m/z): 304.97 (M)+, anal. calcd for C16H17ClN2O2: C, 63.05; H, 5.62; N, 9.19; found: C, 63.35; H, 5.27; N, 9.42.
O). ESI-MS (m/z): 304.97 (M)+, anal. calcd for C16H17ClN2O2: C, 63.05; H, 5.62; N, 9.19; found: C, 63.35; H, 5.27; N, 9.42.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS (m/z): 319.10 (M)+, anal. calcd for C17H19ClN2O2: C, 64.05; H, 6.01; N, 8.79; found: C, 64.21; H, 6.17; N, 8.62.
O). ESI-MS (m/z): 319.10 (M)+, anal. calcd for C17H19ClN2O2: C, 64.05; H, 6.01; N, 8.79; found: C, 64.21; H, 6.17; N, 8.62.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS (m/z): 332.92 (M)+, anal. calcd for C18H21ClN2O2: C, 64.96; H, 6.36; N, 8.42; found: C, 64.75; H, 6.47; N, 8.62.
O). ESI-MS (m/z): 332.92 (M)+, anal. calcd for C18H21ClN2O2: C, 64.96; H, 6.36; N, 8.42; found: C, 64.75; H, 6.47; N, 8.62.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS (m/z): 332.94 (M)+, anal. calcd for C18H21N2O2, C, 64.96; H, 6.36; N, 8.42; found: C, 64.88; H, 6.25; 8.34.
O). ESI-MS (m/z): 332.94 (M)+, anal. calcd for C18H21N2O2, C, 64.96; H, 6.36; N, 8.42; found: C, 64.88; H, 6.25; 8.34.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). ESI-MS (m/z): 347.04 (M)+, anal. calcd for C19H23ClN2O2: C, 65.79; H, 6.68; N, 8.08; found: C, 65.55; H, 6.57; N, 8.22.
O). ESI-MS (m/z): 347.04 (M)+, anal. calcd for C19H23ClN2O2: C, 65.79; H, 6.68; N, 8.08; found: C, 65.55; H, 6.57; N, 8.22.
![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 3.35 (1H, m, another of –NCH2CH3), 3.78 (4H, m, 3H of –COOCH3, and 1H of H-3a), 3.96 (2H, m, 1H of H-2, and 1H of H-4), 4.18 (1H, dd, J = 10, 8.4 Hz, H-4′), 4.82 (1H, d, J = 8 Hz, H-10b), 7.20 (3H, m, Ar–H), 7.60 (1H, dd, J = 7.6, 1.6 Hz, H-9); 13C NMR (100 MHz, CDCl3): δ 13.8 (–NCH2CH3), 35.9 (C-3), 44.2 (–NCH2CH3), 45.0 (C-3a), 50.5 (C-4), 51.3 (CH3), 63.9 (C-2), 64.1 (C-10b), 97.7 (C-10), 110.0 (C-6), 118.5, 120.0, 122.1, 130.1, 131.8, 139.4 (Ar–C), 173.8 (C
CH3), 3.35 (1H, m, another of –NCH2CH3), 3.78 (4H, m, 3H of –COOCH3, and 1H of H-3a), 3.96 (2H, m, 1H of H-2, and 1H of H-4), 4.18 (1H, dd, J = 10, 8.4 Hz, H-4′), 4.82 (1H, d, J = 8 Hz, H-10b), 7.20 (3H, m, Ar–H), 7.60 (1H, dd, J = 7.6, 1.6 Hz, H-9); 13C NMR (100 MHz, CDCl3): δ 13.8 (–NCH2CH3), 35.9 (C-3), 44.2 (–NCH2CH3), 45.0 (C-3a), 50.5 (C-4), 51.3 (CH3), 63.9 (C-2), 64.1 (C-10b), 97.7 (C-10), 110.0 (C-6), 118.5, 120.0, 122.1, 130.1, 131.8, 139.4 (Ar–C), 173.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 319.10 (M)+, anal. calcd for C17H19ClN2O2: C, 64.05; H, 6.01; N, 8.79; found: C, 63.88; H, 6.23; N, 8.94.
O); ESI-MS (m/z): 319.10 (M)+, anal. calcd for C17H19ClN2O2: C, 64.05; H, 6.01; N, 8.79; found: C, 63.88; H, 6.23; N, 8.94.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 332.82 (M)+, anal. calcd for C18H21ClN2O2: C, 64.96; H, 6.36; N, 8.42; found: C, 64.86; H, 6.53; N, 8.74.
O); ESI-MS (m/z): 332.82 (M)+, anal. calcd for C18H21ClN2O2: C, 64.96; H, 6.36; N, 8.42; found: C, 64.86; H, 6.53; N, 8.74.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 346.87 (M)+, anal. calcd for C19H23ClN2O2: C, 65.79; H, 6.68; N, 8.08; found: C, 65.89; H, 6.43; N, 7.89.
O); ESI-MS (m/z): 346.87 (M)+, anal. calcd for C19H23ClN2O2: C, 65.79; H, 6.68; N, 8.08; found: C, 65.89; H, 6.43; N, 7.89.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 346.00 (M)+, anal. calcd for C19H23ClN2O2: C, 65.79; H, 6.68; N, 8.08; found: C, 65.88; H, 6.73; N, 8.16.
O); ESI-MS (m/z): 346.00 (M)+, anal. calcd for C19H23ClN2O2: C, 65.79; H, 6.68; N, 8.08; found: C, 65.88; H, 6.73; N, 8.16.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 360.88 (M)+, anal. calcd for C20H25ClN2O2: C, 66.56; H, 6.98; N, 7.76; found: C, 66.78; H, 6.54; N, 7.98.
O); ESI-MS (m/z): 360.88 (M)+, anal. calcd for C20H25ClN2O2: C, 66.56; H, 6.98; N, 7.76; found: C, 66.78; H, 6.54; N, 7.98.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 380.87 (M)+, anal. calcd for C22H21ClN2O2: C, 69.38; H, 5.56; N, 7.36; found: C, 69.54; H, 5.68; N, 7.42.
O); ESI-MS (m/z): 380.87 (M)+, anal. calcd for C22H21ClN2O2: C, 69.38; H, 5.56; N, 7.36; found: C, 69.54; H, 5.68; N, 7.42.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 395.30 (M)+, anal. calcd for C23H23ClN2O2: C, 69.95; H, 5.87; N, 7.09; found: C, 69.74; H, 5.68; N, 7.23.
O); ESI-MS (m/z): 395.30 (M)+, anal. calcd for C23H23ClN2O2: C, 69.95; H, 5.87; N, 7.09; found: C, 69.74; H, 5.68; N, 7.23.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 408.92 (M)+, anal. calcd for C24H25ClN2O2: C, 70.49; H, 6.16; N, 6.85; found: C, 70.54; H, 6.38; N, 6.72.
O); ESI-MS (m/z): 408.92 (M)+, anal. calcd for C24H25ClN2O2: C, 70.49; H, 6.16; N, 6.85; found: C, 70.54; H, 6.38; N, 6.72.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 408.90 (M)+, anal. calcd for C24H25ClN2O2: C, 70.49; H, 6.16; N, 6.85; found: C, 70.54; H, 6.28; N, 6.73.
O); ESI-MS (m/z): 408.90 (M)+, anal. calcd for C24H25ClN2O2: C, 70.49; H, 6.16; N, 6.85; found: C, 70.54; H, 6.28; N, 6.73.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 422.95 (M)+, anal. calcd for C25H27ClN2O2: C, 70.99; H, 6.43; N, 6.62; found: C, 70.64; H, 6.58; N, 6.92.
O); ESI-MS (m/z): 422.95 (M)+, anal. calcd for C25H27ClN2O2: C, 70.99; H, 6.43; N, 6.62; found: C, 70.64; H, 6.58; N, 6.92.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 404.02 (M)+, anal. calcd for C21H26ClN3O3: C, 62.45; H, 6.49; N, 10.40; found: C, 62.64; H, 6.38; N, 10.02.
O); ESI-MS (m/z): 404.02 (M)+, anal. calcd for C21H26ClN3O3: C, 62.45; H, 6.49; N, 10.40; found: C, 62.64; H, 6.38; N, 10.02.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 417.90 (M)+, anal. calcd for C22H28ClN3O3: C, 63.22; H, 6.75; N, 10.05; found: C, 63.54; H, 6.58; N, 10.22.
O); ESI-MS (m/z): 417.90 (M)+, anal. calcd for C22H28ClN3O3: C, 63.22; H, 6.75; N, 10.05; found: C, 63.54; H, 6.58; N, 10.22.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 430.90 (M)+, anal. calcd for C23H30ClN3O3: C, 63.95; H, 7.00; N, 9.73; found: C, 63.74; H, 6.78; N, 9.96.
O); ESI-MS (m/z): 430.90 (M)+, anal. calcd for C23H30ClN3O3: C, 63.95; H, 7.00; N, 9.73; found: C, 63.74; H, 6.78; N, 9.96.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 430.94 (M)+, anal. calcd for C23H30ClN3O3: C, 63.95; H, 7.00; N, 9.73; found: C, 63.86; H, 6.81; N, 9.86.
O); ESI-MS (m/z): 430.94 (M)+, anal. calcd for C23H30ClN3O3: C, 63.95; H, 7.00; N, 9.73; found: C, 63.86; H, 6.81; N, 9.86.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS (m/z): 446.10 (M)+, anal. calcd for C24H32ClN3O3: C, 64.63; H, 7.23; N, 9.42; found: C, 64.74; H, 7.48; N, 9.76.
O); ESI-MS (m/z): 446.10 (M)+, anal. calcd for C24H32ClN3O3: C, 64.63; H, 7.23; N, 9.42; found: C, 64.74; H, 7.48; N, 9.76.
Acknowledgements
We sincerely express our thanks to Head, Department of Chemistry, Sardar Patel University, for providing necessary research facilities. One of the authors, TRS, specially thanks the UGC, New Delhi, India, for providing financial assistance under the UGC Scheme of RFSMS. We thank DST, New Delhi, in general, and the PURSE central facility for mass analysis (vide sanction letter DO. No. SR/59/Z-23/2010/43 dated 16th march 2011). J.M.P. thanks the EU Research Potential (FP7-REGPOT-2012-CT2012-31637-IMBRAIN), the European Regional Development Fund (FEDER), and the Spanish Instituto de Salud Carlos III (PI11/00840) for financial support. G.B.P. thanks Fundación CajaCanarias for a postgraduate grant.Notes and references
- (a) F. Nakataubo, A. J. Cocuzza, D. E. Keeley and Y. Kishi, J. Am. Chem. Soc., 1977, 99, 4835–4836 CrossRef; (b) F. Nakatsubo, T. Fukuyama, A. J. Cocuzza and Y. Kishi, J. Am. Chem. Soc., 1977, 99, 8115–8116 CrossRef CAS; (c) Y. J. Kishi, Nat. Prod., 1979, 42, 549–568 CrossRef CAS; (d) L. Hao, Y. Pan, T. Wang, M. Lin, L. Chen and Z. P. Zhan, Adv. Synth. Catal., 2010, 352, 3215–3222 CrossRef CAS; (e) S. E. Wolkenberg and D. L. Boger, Chem. Rev., 2002, 102, 2477–2496 CrossRef CAS PubMed; (f) D. Crich and A. Banerjee, Acc. Chem. Res., 2007, 40, 151–161 CrossRef CAS PubMed; (g) S. W. Pelletier, John Wiley & Sons, New York, 1988, vol. 6, pp. 1–74; (h) G. R. Allen, J. F. Poletto and M. J. Weiss, J. Am. Chem. Soc., 1964, 86, 3877–3878 CrossRef CAS; (i) E. O. M. Orlemans, W. Verboom, M. W. Scheltinga, D. N. Reinhoudt, P. Lelieveld, H. H. Fiebig, B. R. Winterhalter, J. A. Double and M. C. Bibby, J. Med. Chem., 1989, 32, 1612–1620 CrossRef CAS; (j) J. J. Vepsäläinen, S. Auriola, M. Tukiainen, N. Ropponen and J. C. Callaway, Planta Med., 2005, 71, 1053–1057 Search PubMed; (k) U. Galm, M. H. Hager, S. G. Van Lanen, J. Ju, J. S. Thorson and B. Shen, Chem. Rev., 2005, 105, 739–758 CrossRef CAS PubMed.
- (a) I. S. Marcos, R. F. Moro, I. Costales, P. Basabe and D. Díez, Nat. Prod. Rep., 2013, 30, 1509–1526 RSC; (b) M. Somei and F. Yamada, Nat. Prod. Rep., 2005, 22, 73–103 RSC; (c) X. Yu, X. Xie and S. M. Li, Appl. Microbiol. Biotechnol., 2011, 92, 737–748 CrossRef CAS PubMed; (d) Modern Alkaloids: Structure, Isolation, Synthesis, and Biology-, ed. F. Fattorusso and O. T. Scafati, Wiley-VCH, 2008 Search PubMed; (e) Marine Pharmacognosy: Trends and Applications-, ed. S. M. Kim, CRC Press, 2012 Search PubMed.
- (a) S. T. Crooke and A. W. Prestayko, Academic Press, New York, 1981, vol. 3, p. 49; (b) S. Alcaro, F. Ortuso and R. S. Coleman, J. Med. Chem., 2002, 45, 861–870 CrossRef CAS PubMed; (c) N. Zein, W. Solomon, K. L. Colson and D. R. Schroeder, Biochemistry, 1995, 34, 11591–11597 CrossRef CAS; (d) W. A. Remers and R. T. Dorr, in In Alkaloids Chemical and Biological Perspectives, ed. S. W. Pelletier, John Wiley & Sons, New York, 1988, vol. 6, pp. 1–74 Search PubMed; (e) R. S. Coleman, C. H. Burk, A. Navarro, R. W. Brueggemeier and E. S. Diaz-Cruz, Org. Lett., 2002, 4, 3545–3548 CrossRef CAS PubMed.
- (a) W. Verboom, D. N. Reinhoudt, B. H. M. Lammerink, E. O. M. Orlemans, F. C. J. M. Van Veggel and P. Lelieveld, Anti-Cancer Drug Des., 1987, 2, 271–277 CAS; (b) W. Verboom, E. O. M. Orlemans, M. W. Scheltinga, D. N. Reinhoudt, P. Lelieveld, H. H. Fiebig, B. R. Winterhalter, J. A. Double and M. C. Bibby, J. Med. Chem., 1989, 32, 1612–1620 CrossRef; (c) M. Maliepaard, N. J. de Mol, L. H. M. Janssen, J. C. Hoogvliet, W. van der Neut, W. Verboom and D. N. Reinhoudt, J. Med. Chem., 1993, 36, 2091–2097 CrossRef CAS.
- (a) L. S. Fernandez, M. F. Jobling, K. T. Andrews and V. M. Avery, Phytother. Res., 2008, 22, 1409–1412 CrossRef PubMed; (b) D. H. Dethe, R. D. Erande and A. Ranjan, J. Am. Chem. Soc., 2011, 133, 2864–2867 CrossRef CAS PubMed; (c) L. S. Fernandez, M. S. Buchanan, A. R. Carroll, Y. J. Feng, R. J. Quinn and V. M. Avery, Org. Lett., 2009, 11, 329–332 CrossRef CAS PubMed; (d) L. S. Fernandez, M. L. Sykes, K. T. Andrews and V. M. Avery, Int. J. Antimicrob. Agents, 2010, 36, 275–279 CrossRef CAS PubMed; (e) R. Vallakati and J. A. May, J. Am. Chem. Soc., 2012, 134, 6936–6939 CrossRef CAS PubMed; (f) R. M. Zeldin and F. D. Toste, Chem. Sci., 2011, 2, 1706–1709 RSC.
- (a) C. V. Suneel Kumar, V. G. Puranik and C. V. Ramana, Chem. – Eur. J., 2012, 18, 9601–9611 CrossRef PubMed; (b) Q. Yin and S. L. You, Chem. Sci., 2011, 2, 1344–1348 RSC; (c) P. Patel and C. V. Ramana, Org. Biomol. Chem., 2011, 9, 7327–7334 RSC; (d) A. Karadeolian and M. A. Kerr, Angew. Chem., Int. Ed., 2010, 49, 1133–1135 CrossRef CAS PubMed; (e) J. F. Liu, Z. Y. Jiang, R. R. Wang, Y. T. Zeng, J. J. Chen, X. M. Zhang and Y. B. Ma, Org. Lett., 2007, 9, 4127–4129 CrossRef CAS PubMed; (f) A. Karadeolian and M. A. Kerr, J. Org. Chem., 2010, 75, 6830–6841 CrossRef CAS PubMed; (g) X. Zhang, T. Mu, F. Zhan, L. Ma and G. Liang, Angew. Chem., Int. Ed., 2011, 50, 6164–6166 CrossRef CAS PubMed.
- J. J. Vepsäläinen, S. Auriola, M. Tukiainen, N. Ropponen and J. C. Callaway, Planta Med., 2005, 71, 1053–1057 Search PubMed.
- (a) A. R. Battersby, R. Binks and R. Huxtable, Tetrahedron Lett., 1968, 9, 6111–6115 CrossRef; (b) J. Lundstrom, in The Alkaloids, ed. A. Brossi, Academic, New York, NY, 1983, vol. 21, p. 312 Search PubMed; (c) K. W. Bentley, Nat. Prod. Rep., 2003, 20, 342–365 RSC; (d) S. H. Chung, J. Yook, B. J. Min, J. Y. Lee, Y. S. Lee and C. Jin, Arch. Pharmacal Res., 2000, 23, 353–359 CrossRef CAS.
- R. F. Wang, X. W. Yang, C. M. Ma, S. Q. Cai, J. J. Nong and Y. Shoyama, Heterocycles, 2004, 63, 1443–1448 CrossRef CAS.
- L. Xiang, D. Xing, W. Wang, R. Wang, Y. Ding and L. Du, Phytochemistry, 2005, 66, 2595–2601 CrossRef CAS PubMed.
- (a) X. J. Zhang, Y. B. Ji, Z. Y. Qu, J. Ch. Xia and L. Wang, Chin. J. Microecol., 2002, 14, 277–280 Search PubMed; (b) K. Chan, M. W. Islam, M. Kamil, R. Radhakrishnan, M. N. M. Zakaria, M. Habibullah and A. Attas, J. Ethnopharmacol., 2000, 73, 445–451 CrossRef CAS; (c) O. Parry, F. Okwuasaba and C. Ejike, J. Ethnopharmacol., 1987, 19, 247–253 CrossRef CAS; (d) A. N. Rashed, F. U. Afifi and A. M. Disi, J. Ethnopharmacol., 2003, 88, 131–136 CrossRef CAS.
- Q. Zhang, G. Tu, Y. Zhao and T. Cheng, Tetrahedron, 2002, 58, 6795–6798 CrossRef CAS.
- (a) S. Zhang, X. Zhao, W. Kao and J. Wang, U.S. Pat., US 566604, 1997Chem. Abstr.1997126P305533m Search PubMed; (b) P. Sonnet, P. Dallemagne, J. Guillon, C. Enguehard, S. Stiebing, J. Tanguy, R. Bureau, S. Rault, P. Auvray, S. Moslemi, P. Sourdaine and G. E. Seralini, Bioorg. Med. Chem., 2000, 8, 945–955 CrossRef CAS; (c) W. K. Anderson and R. H. Mach, J. Heterocycl. Chem., 1990, 27, 1025–1030 CrossRef CAS.
- (a) C. J. Fong, J. Addo, M. Dukat, C. Smith, N. A. Mitchell, K. Herrick-Davis, M. Teitler and R. A. Glennon, Bioorg. Med. Chem. Lett., 2002, 12, 155–158 CrossRef; (b) M. Bos, F. Jenck, J. R. Martin, J. L. Moreau, V. Mutel, A. J. Sleight and U. Widmer, Eur. J. Med. Chem., 1997, 32, 253–261 CrossRef CAS.
- (a) R. Peters, P. Waldmeier and A. Joncour, Org. Process Res. Dev., 2005, 9, 508–512 CrossRef CAS; (b) J. S. Shiue and J. M. Fang, Chem. Commun., 1993, 1277–1278 RSC; (c) M. Ishikura, W. Ida and K. Yanada, Tetrahedron, 2006, 62, 1015–1024 CrossRef CAS PubMed.
- (a) S. Caddick, K. Aboutayab, K. Jenkins and R. I. West, J. Chem. Soc., Perkin Trans. 1, 1996, 675–682 RSC; (b) M. L. Bennasar, T. Roca and F. Ferrando, Org. Lett., 2004, 6, 759–762 CrossRef CAS PubMed; (c) S. F. Wang and C. P. Chuang, Tetrahedron Lett., 1997, 38, 7597–7598 CrossRef CAS; (d) E. Z. Frederick and G. H. Patrick, J. Org. Chem., 1993, 58, 2168–2773 Search PubMed.
- K. Tsuboike, D. J. Guerin, S. M. Mennen and S. J. Miller, Tetrahedron, 2004, 60, 7367–7374 CrossRef CAS PubMed.
- D. T. Richard, G. O. Stephane, A. R. Robert, J. J. G. Edward and J. R. Paul, J. Am. Chem. Soc., 2000, 122, 1215–1216 CrossRef.
- (a) M. B. Egle, B. Gianluigi, L. R. Concetta, P. Daniele, P. Tullio, T. Alberto and Z. Gaetano, J. Org. Chem., 2000, 65, 8924–8932 CrossRef PubMed; (b) S. Kathiravan and R. Raghunathan, Synlett, 2010, 952–954 CAS.
- (a) S. Majumder and P. J. Bhuyan, Tetrahedron Lett., 2012, 53, 762–764 CrossRef CAS PubMed; (b) W. Zhang, Y. Lu and S. Geib, Org. Lett., 2005, 7, 2269–2272 CrossRef CAS PubMed; (c) M. Ghandi, A. Taheri, A. H. Bozcheloei, A. Abbasi and R. Kia, Tetrahedron, 2012, 68, 3641–3648 CrossRef CAS PubMed; (d) M. Bakthadoss, N. Sivakumar, G. Sivakumar and G. Murugan, Tetrahedron Lett., 2008, 49, 820–823 CrossRef CAS PubMed; (e) N. Sirisha and R. Raghunathan, Tetrahedron Lett., 2010, 51, 2515–2518 CrossRef CAS PubMed; (f) S. Kathiravan, D. Vijayarajan and R. Raghunathan, Tetrahedron Lett., 2010, 51, 3065–3070 CrossRef CAS PubMed; (g) E. M. Beccalli, G. Broggini, C. L. Rosa, D. Passarella, T. Pilati, A. Terraneo and G. Zecchi, J. Org. Chem., 2000, 65, 8924–8932 CrossRef CAS PubMed; (h) N. J. Parmar, B. R. Pansuriya, H. A. Barad, R. Kant and V. K. Gupta, Bioorg. Med. Chem. Lett., 2012, 22, 4075–4079 CrossRef CAS PubMed; (i) N. J. Parmar, B. R. Pansuriya, B. M. Labana, R. Kant and V. K. Gupta, RSC Adv., 2013, 3, 17527–17539 RSC.
- (a) I. Coldham and R. Hufton, Chem. Rev., 2005, 105, 2765–2810 CrossRef CAS PubMed; (b) M. Poornachandran and R. Raghunathan, Tetrahedron Lett., 2005, 46, 7197–7200 CrossRef CAS PubMed; (c) S. Kathiravan and R. Raghunathan, Synlett, 2010, 952–954 CAS; (d) G. B. Jones, C. J. Moody, A. Padwa and J. M. Kassirb, J. Chem. Soc., Perkin Trans. 1, 1991, 1721–1727 RSC; (e) Stereochemistry of carbon compounds, ed. E. L. Eliel, Tata McGraw-Hill Publishing Co. Ltd., New Delhi, 1975, reprint 2003, p. 274 Search PubMed.
- (a) J. M. Khurana and D. Magoo, Tetrahedron Lett., 2009, 50, 7300–7303 CrossRef CAS PubMed; (b) D. Chaturvedi, Curr. Org. Synth., 2011, 8, 438–471 CrossRef CAS; (c) J. R. Harjani, S. J. Nara and M. M. Salunkhe, Tetrahedron Lett., 2002, 43, 1127–1130 CrossRef CAS; (d) V. V. Namboodiri and R. S. Varma, Chem. Commun., 2002, 342–343 RSC; (e) R. Sheldon, Chem. Commun., 2001, 2399–2407 RSC.
- (a) I. Coldham, B. C. Dobson, A. I. Franklin and S. R. Fletcher, Tetrahedron Lett., 2007, 48, 873–875 CrossRef CAS PubMed; (b) S. Kathiravan, D. Vijayarajan and R. Raghunathan, Tetrahedron Lett., 2010, 51, 3065–3070 CrossRef CAS PubMed; (c) M. Bakthadoss, N. Sivakumar, G. Sivakumar and G. Murugan, Tetrahedron Lett., 2008, 49, 820–823 CrossRef CAS PubMed; (d) S. Purushothaman, R. Prasanna, P. Niranjana, R. Raghunathan, S. Nagaraj and R. Rengasamy, Bioorg. Med. Chem. Lett., 2010, 20, 7288–7291 CrossRef CAS PubMed.
- (a) J. F. Dubreuil and J. P. Bazureau, Tetrahedron Lett., 2000, 41, 7351–7355 CrossRef CAS; (b) C. P. Frizzo, D. P. Moreira and M. A. P. Martins, in In Ionic Liquids Applications in Heterocyclic Synthesis, Ionic Liquids Applications and Perspectives, ed. K. Alexander, InTech, 2011, ISBN 978-953-307-248-7 DOI:10.5772/15343; (c) A. Hazra, Y. P. Bharitkar, A. Maity, S. Mondal and N. B. Mondal, Tetrahedron Lett., 2013, 54, 4339–4342 CrossRef CAS PubMed.
- (a) A. J. Speziale and E. G. Jaworski, J. Org. Chem., 1960, 25, 728–732 CrossRef CAS; (b) J. L. Moore, S. M. Taylor and V. A. Soloshonok, ARKIVOC, 2005, 287–292 CrossRef CAS.
- V. J. Majo and P. T. Perumal, J. Org. Chem., 1996, 61, 6523–6525 CrossRef CAS PubMed.
- (a) S. Kanemasa, K. Sakamoto and O. Tsuge, Bull. Chem Soc. Jpn., 1989, 62, 1960 CAS; (b) A. F. Khlebnikov, M. S. Novikov, R. R. Kostikov and J. Kopl, Russ. J. Org. Chem., 2005, 41, 1341–1348 CrossRef CAS.
- P. O Miranda, J. M. Padrón, J. I. Padrón, J. Villar and V. S. Martín, ChemMedChem, 2006, 1, 323–329 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 960921 and 1018001. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4nj02308k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |