Open Access Article
Open Access ArticleStudies of the complexation behavior of tetramorpholinylo-PNP-lariat ether with Ag(I), Ca(II), Cd(II), Cu(II) and Pb(II) using Electrospray Ionization Mass Spectrometry
Natalia
Gutowska
*a,
Beata
Pasternak
b,
Piotr
Seliger
a and
Grzegorz
Andrijewski
a
aDepartment of Inorganic and Analytical Chemistry, Faculty of Chemistry, University of Lodz, Tamka 12, 91-403 Łódź, Poland. E-mail: natalia_gutowska@wp.pl
bLaboratory of Molecular Spectroscopy, Faculty of Chemistry, University of Lodz, Tamka 12, 91-403 Łódź, Poland
First published on 17th December 2014
Abstract
In this publication the cationic metal complexes of tetramorpholinylo-PNP-lariat ether have been studied using electrospray ionization mass spectrometry (ESI-MS). The tandem mass spectra (MS/MS) of these complexes have also been tested to evaluate the stability of the different types of the complexes formed. As occurred, all selected metal cations form the complexes with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with the investigated ligand. Only silver ions create a “sandwich” type complex. Furthermore, the divalent cations form complexes with the nitrate anion adduct. In the case of Ca(II), Cd(II) and Pb(II) we also observed another type of the species with an additional water molecule attached to the parent complex.
1 stoichiometry with the investigated ligand. Only silver ions create a “sandwich” type complex. Furthermore, the divalent cations form complexes with the nitrate anion adduct. In the case of Ca(II), Cd(II) and Pb(II) we also observed another type of the species with an additional water molecule attached to the parent complex.
Introduction
Electrospray ionization mass spectrometry (ESI-MS)1–4 is currently an effective tool for analysis of a wide variety of noncovalent complexes,5–16 such as those formed in host–guest chemistry. Many studies show that equilibrium distribution of complexes in solution is reflected in the intensities of host–guest complexes observed in the ESI mass spectra.17–23 ESI-MS analysis enables us to directly determine the complexes formed in the solution, as well as to study the equilibrium states, carry out quantitative analysis of stability constants or evaluate the binding selectivities.24–39 This method is considered to be a “soft ionization” process (involving the transfer of solution ions into the gas phase), and therefore typically yields molecular ions with little or no fragmentation at all. The simplicity of spectra obtained using this technique is of great benefit. Conventional techniques such as infrared spectroscopy, X-ray diffraction and NMR have several drawbacks. The use of ESI-MS for studying metal–ligand interactions and metal complexes is readily recognized. MS is more sensitive than conventional techniques and opens up the possibility of screening the complex samples. Moreover, MS enables simultaneous monitoring of the response of ligand-exchange reactions, additionally obtaining chemical information about the specific compound. Furthermore, ions generated using the electrospray ionization process can be easily fragmented using techniques such as tandem mass spectrometry (MS/MS). In this method, an ion (called the precursor ion) is selected on the basis of the first stage of MS measurements and activated to produce fragment ions which are then analysed in the second stage of MS.40,41 ESI-MS can provide important information concerning the structure, stoichiometry, and metal oxidation state of dissolved metal complexes.42,43Macrocyclic ligands built on a cyclotriphosphazene ring are compounds which bind cyclophosphazene chemistry with macrocyclic chemistry. The combination of these two fields gives the compounds which have a high possibility for the modification of their structure through the nucleophilic substitution reaction, polycondensation or creation of possible binding centres for cations, anions or neutral species. In this way, the design of various compounds with potential practical use can be realized. Lariat ethers are derived from the group of crown ethers having a side chain attached to the crown moiety by the so-called pivot atoms (C, N or P). The side arms contain atoms or groups with a lone pair of electrons that can cooperate with the heteroatom's electrons from the macrocyclic ring, thus providing a three-dimensional coordination of the guest cation.44–46
Three types of lariat ethers are known depending on the atom to which the side arm is attached: C- (carbon-pivot lariat ethers),47–49 N- (nitrogen-pivot lariat ethers)50,51 or P- (phosphorus-pivot lariat ethers).52 The object of the presented publications is the P-pivot lariat ether derivative. This compound is derived from the group of reactive crown ethers which was formed by incorporation of a chloro-substituted cyclotriphosphazene unit into the macrocyclic polyether skeleton to give mono- and bis-cyclosubstituted derivatives.53 By substitution of the reactive chloride substituents with various nucleophiles we can obtain a whole series of PNP-lariat ethers. There are known PNP-lariat ether derivatives substituted with different amines54–58 and sodium arylates.59,60 These compounds exhibit very good abilities toward complexation. Due to the presence of the binding sites in the form of both oxygen and nitrogen donor atoms, these ligands form stable complexes with alkali metals and alkaline earth metal cations as well as transition metal cations.61,62 Macrocyclic derivatives of cyclotriphosphazenes and their complexes are recognized as compounds of proven antitumor activity63 as well as anti-AIDS activity.64 Anti-proliferative activity may be modified by the formation of complexes with metal ions. These types of compounds form selective bindings with silver ions.65,66 Additionally, recent investigations showed that silver compounds exhibit antibacterial properties.67 It has been proved that the thio-substituted PNP-lariat ethers are commonly used to remove highly toxic heavy metal pollutants and for recovery of silver from industrial wastes.68 Complexing abilities of these compounds give the possibility of modelling biological systems, in which sulphur–organic compounds are involved.69,70 The PNP-crown amino ethers can be potentially useful as “pH-controlled active ion carriers” in liquid membranes.71 However, bis-PNP-lariat ethers were found to behave as efficient ion carriers for heavy metal (Zn2+, Cd2+, Pb2+) transport carriers across polymer inclusion membranes, in particular for lead(II), due to the formation of “sandwich” type complexes with the macrocyclic compounds.72
In the present paper we report the ESI-MS studies of the tetramorpholinylo-PNP-lariat ether complexes with Ca(II), Ag(I), Cd(II), Cu(II) and Pb(II) ions. The tandem mass spectra (MS/MS) of these complexes were also examined to evaluate the stability of the different complex types. The tetramorpholinylo-PNP-lariat ether has a few structural units with the potential ability to take part in the binding of metal cations: the polyether oxygen donors of the macrocyclic PNP-crown skeleton, the endocyclic nitrogen atom of the cyclotriphosphazene ring and the exocyclic nitrogen atoms and oxygen donor atoms of the morpholinyl substituents. This ligand is capable of complexing “hard” and “soft” cations.
Experimental
Synthesis
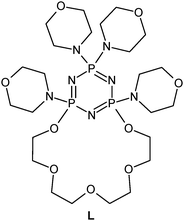
The synthesis of tetramorpholinylo-PNP-lariat ether has been previously reported.65 The investigated ligand is presented in Fig. 1.Materials
AgNO3 (Sigma Aldrich) was used as received. Nitrates of Ca(II), Cd(II), Cu(II) and Pb(II) were purchased from POCh Gliwice and used without further purification.Methanol (HPLC-grade) was purchased from J.T. Baker (POCh Gliwice) and used without purification.
Stock solutions of the ligand and metals salts in methanol (10−3 M each) were prepared prior to dilution to 10−4 M for mass spectrometric investigation (ligand/metal salt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In each case freshly prepared solutions were used.
1). In each case freshly prepared solutions were used.
Instrumental methods
ESI-MS and MS/MS were recorded using a Varian 500-MS LC ion-trap mass spectrometer (Palo Alto, CA, USA). The whole sample was introduced into ESI-MS source by continuous infusion by means of the instrument syringe pump at a rate of 10 μl min−1. The ESI-source was operated at 5.00 kV and the capillary heater was set to 300 °C. The cone voltage was set within the range of 40–260 V. Scanning was performed from m/z = 100 to 1500. For fragmentation experiments, mass-selected monoisotopic molecular ions were isolated in the ion trap and collisionally activated using helium damping gas present in the mass analyser as a collision gas. The experiments were performed in positive ion-mode.Results and discussion
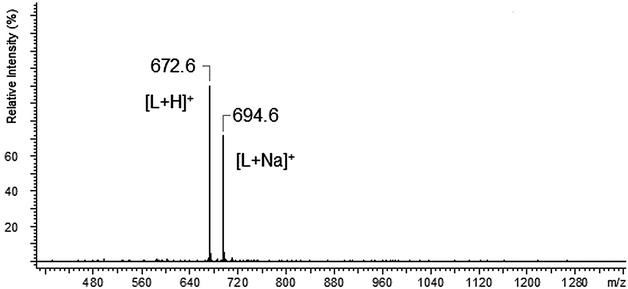
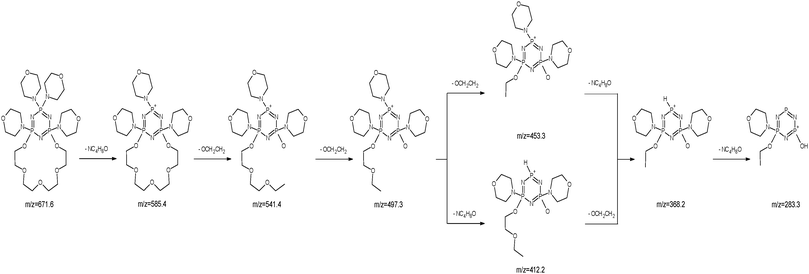
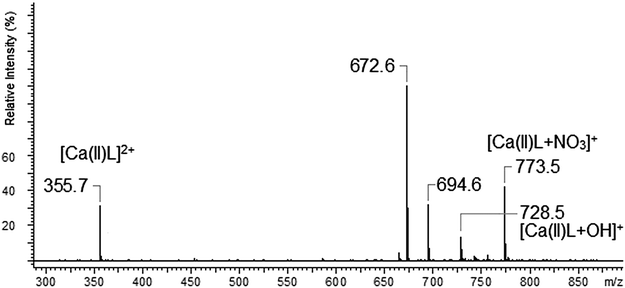
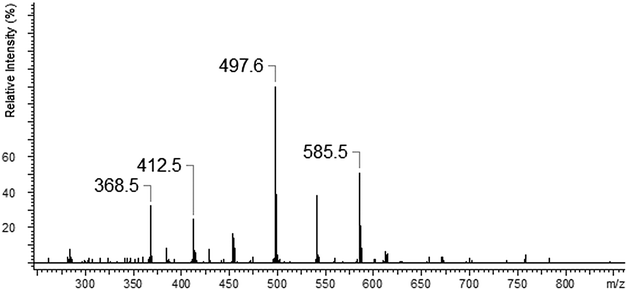
The ESI-MS spectrum of L revealed the presence of the protonated molecule, at m/z 672.6, and a less abundant ion corresponding to the Na+ adduct of the parent compound Fig. 2. ESI-MS fragmentation analysis of L is shown on Scheme 1. In the first step we observed the loss of morpholinyl substituent (m/z = 585.4), probably from the exomacrocyclic P atom. Such a behaviour for a tetrapyrrolidinyl cyclophosphazene derivative was described previously.62 There was also whole substituent loss observed without the cleavage of the ring. In the following steps the loss of successive fragments of the polyether chain was noticed. After detachment of the two –OCH2CH2 fragments we observed two concurrent fragmentation patterns. The first involved a stepwise loss of one morpholinyl substituent (m/z = 412.2) and the subsequent –OCH2CH2 group (m/z = 368.2). The second one showed two major fragment ions with m/z = 453.3 due to the loss of the –OCH2CH2 moiety and with m/z = 368.2 caused by the loss of the morpholinyl substituent. In the last stage splitting-off of the third morpholinyl substituent is observed.The ESI mass spectra of the mixture of L with metal ions (Ca, Ag, Cd, Cu and Pb) indicate the presence of many different forms of coordination compounds. The experiments were done using nitrate salts because the stability constants of the investigated ligand with silver(I) ions (with nitrate as counter ions) had been reported earlier in the literature.65 The resulting complexes were presented in Table 1. As occurs from the analysis of mass spectra – all the metal ions (apart from the calcium ion) are bound with the endocyclic nitrogen atom. The nitrogen atom built-in the structure of the polyether represents a “soft” electron donor place to which the “soft” transition metals cations as well as heavy metals cations are preferably bound. The oxygen atoms from crown ether moiety are “hard” donors having the affinity toward “hard” cations of I and II group of the periodic table of elements.73
| Metal salt | Ions (m/z) |
|---|---|
| AgNO3 | [Ag(I)L]+ (778.5) |
| [Ag(I)L2]+ (1449.7) | |
| Ca(NO3)2 | [Ca(II)L]2+ (355.7) |
| [Ca(II)L + NO3]+ (773.5) | |
| [Ca(II)L + OH]+ (728.5) | |
| Cu(NO3)2 | [Cu(I)L]+ (734.6) |
| [Cu(II)L]2+ (367.3) | |
| [Cu(II)L + NO3]+ (796.5) | |
| Cd(NO3)2 | [Cd(II)L]2+ (392.8) |
| [Cd(II)L + NO3]+ (847.6) | |
| [Cd(II)L + H2O]+ (802.5) | |
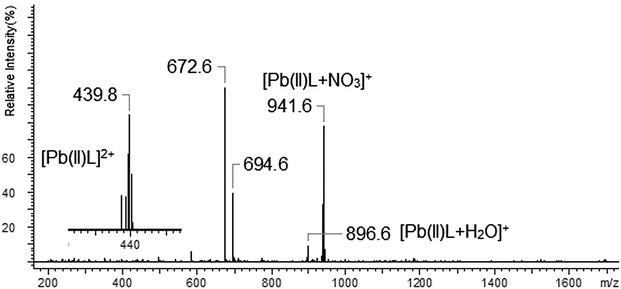
| Pb(NO3)2 | [Pb(II)L]2+ (439.8) |
| [Pb(II)L + NO3]+ (941.6) | |
| [Pb(II)L + H2O]+ (896.6) | |
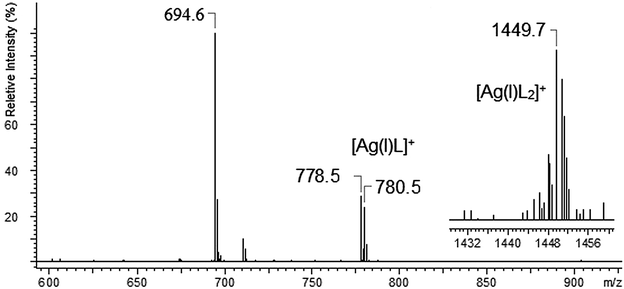
The mass spectrum of L with AgNO3 was dominated by the [Ag(I)L]+ complex. Among other investigated metal ions only the silver ion form a “sandwich” type complex with two molecules of the ligand (peak at m/z = 1449.7). The interesting fact about the system with the Ag(I) ion was no signal of the uncomplexed ligand (no peak at m/z = 672.6), what probably may confirm the very strong binding of silver ions by the investigated ligand. The complexes of AgL and AgL2 type obtained during the ESI-MS measurements are in accordance with the results obtained using the potentiometric method.65 Divalent cations form complexes with the nitrate anion or with water molecule attached to parent compound, such behaviour was observed for Cd(II), Pb(II) and Ca(II) ions. For the complexes with divalent cations, the loss of one counter ion (NO3−) seems to be the predominated ionization process. The ESI-MS spectra of L with Cd(II), Cu(II) and Pb(II) show the presence of [Cd(II)L]2+, [Cu(II)L]2+ and [Pb(II)L]2+ complexes. The relative intensity of these complexes was very low (RI < 5%). The situation is different in the case of calcium ions – because in this case the formed [Ca(II)L]2+ complex exhibits much higher relative abundance of 31%.
In the case of the copper ion due to the measurement conditions we observed the reduction process Cu(II) → Cu(I). This type of process is a phenomenon observed in ESI74–77 and is usually not an electrochemical redox reaction. This process can be explained as an electron transfer between a Cu complex and a solvent molecule in the gas phase.
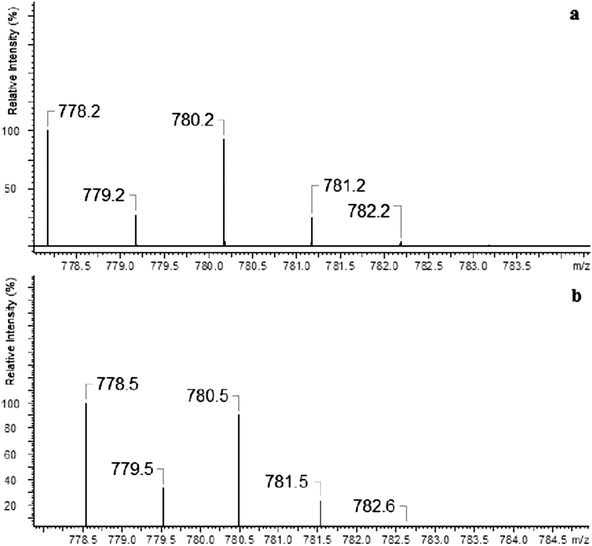
In the case of silver ions we observed two types of complexes [Ag(I)L]+ and [Ag(I)L2]+ – Fig. 3. The ESI-MS for [Ag(I)L]+ show two main peaks at m/z 778.5 and 780.5, which are expanded in Fig. 4(b), and compared well with the corresponding calculated (Fig. 4(a)) isotope pattern. The isotope patterns in Fig. 4 are consistent with a molecular cation containing one silver atom and exhibits the natural intensity ratio for silver isotopes.
 | ||
| Fig. 4 Comparison of the theoretical isotope pattern calculation (a) for [Ag(I)L]+ with the one observed experimentally (b). | ||
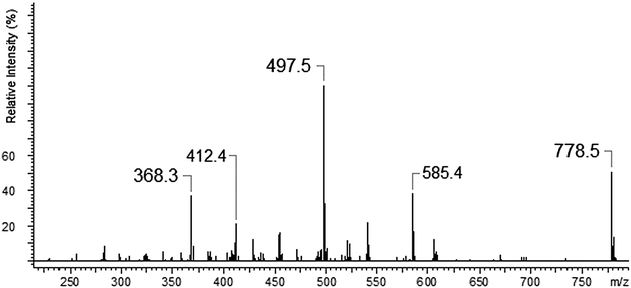
We made the fragmentation of the peak m/z = 778.5. The fragmentation pattern for [Ag(I)L]+ shows the same fragmentation pattern as in the case of the ligand Fig. 5. This suggests that binding of silver ions by the ligand molecule is reasonably strong and complex formed does not completely disintegrate under the influence of the ionization process.
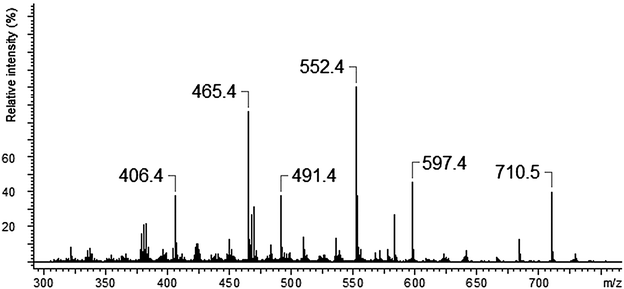
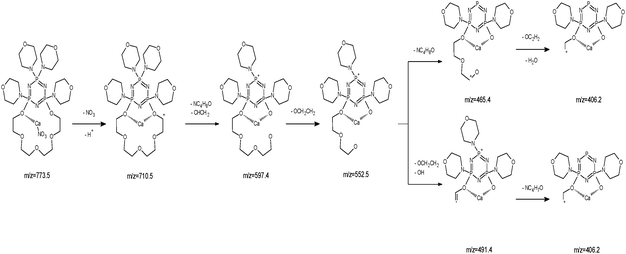
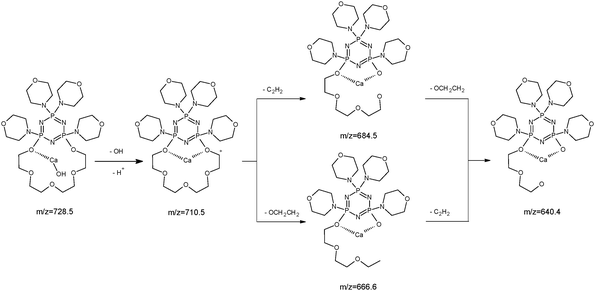
The ESI-MS of an equimolar mixture of L and Ca(NO3)2 shows three types of complexes: [Ca(II)L]2+, [Ca(II)L + NO3]+ and [Ca(II)L + OH]+ (Fig. 6). The most abundant peak at m/z 773.5 is the one corresponding to [Ca(II)L + NO3]+ complex. The ESI-MS/MS spectrum of [Ca(II)L + NO3]+ complex is shown in Fig. 7. For this complex we observed the loss of nitrate anion and one proton (m/z = 710.5). The MS/MS fragmentation analysis of calcium complex revealed at the first stage the loss of one morpholinyl substituent (probably from the exomacrocyclic P atom) and –(CHCH2) group (m/z = 597.4) Then we saw an intense peak at m/z 552.5, corresponding to the loss of fragment of the polyether chain –(OCH2CH2). From this step two concurrent fragmentation paths were observed. In the first one – two less abundant peaks at m/z = 465.4 and m/z = 406.2, corresponding to the loss of second morpholinyl group from exomacrocyclic P atom and another fragment of the crown ether ring and H2O molecule. The second fragmentation pattern involves a stepwise loss of –(OCH2CH2) and OH moiety to an ion with m/z 491.4, followed by losses of morpholinyl substituent to an ion with m/z 406.2. These two fragmentation patterns are presented in Scheme 2. The region within the peak at m/z 406.2, which is expanded in Fig. 8(b), compare well with the corresponding calculated (Fig. 8(a)) isotope pattern.
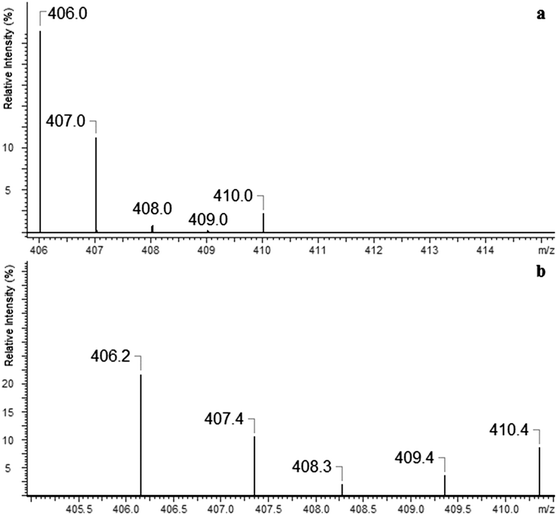 | ||
| Fig. 8 Comparison of the theoretical isotope pattern calculation (a) for calcium complex at m/z = 406.2 with the experimentally observed pattern (b). | ||
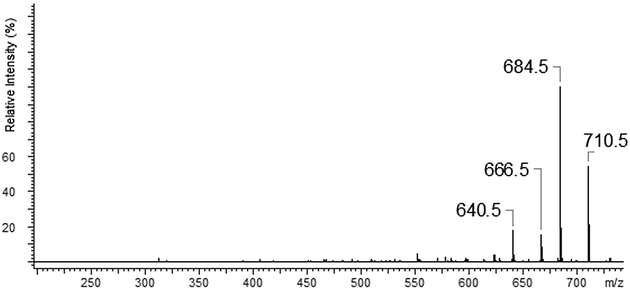
Additionally the fragmentation of the [Ca(II)L + OH]+ complex was checked (Fig. 9). In this case we also noticed two fragmentation patterns. At the beginning we observed the loss of –OH fragment as H2O molecule (18 Da) to an ion with m/z = 710.5. The most intense peak m/z 684.5 is connected with the loss of –(C2H2) group which was followed by the loss of 44 Da –(OCH2CH2). The less intense signal (m/z = 666.6) is originated from the loss of –(OCH2CH2) and that with m/z 640.4 due to subsequent loss of –(C2H2). The proposal of the fragmentation pattern is presented in Scheme 3.
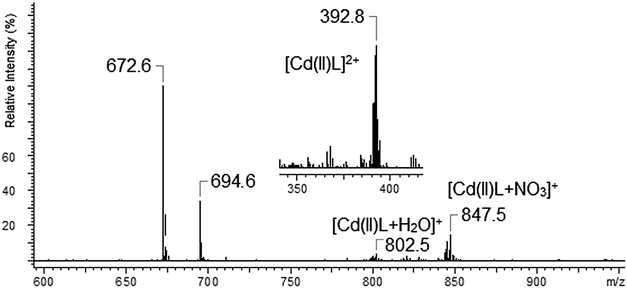
The ESI-MS spectra of L and Cd(NO3)2 show three types of complexes: [Cd(II)L]2+, [Cd(II)L + NO3]+ and [Cd(II)L + H2O]+ (Fig. 10). We made the fragmentation of the peak m/z = 847.6 and we observed the same fragmentation pattern as in the case of the free ligand Fig. 11. This fact proves that the cadmium(II) complex is very unstable structure. A similar situation was observed during the fragmentation of the [Cd(II)L + H2O]+ complex.
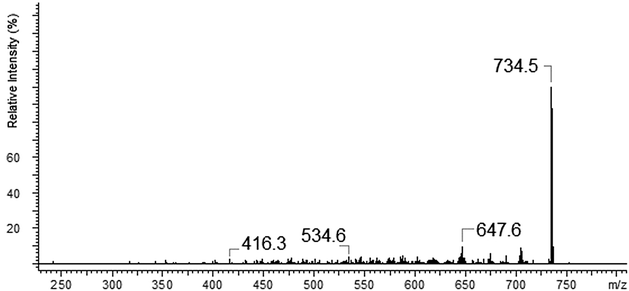
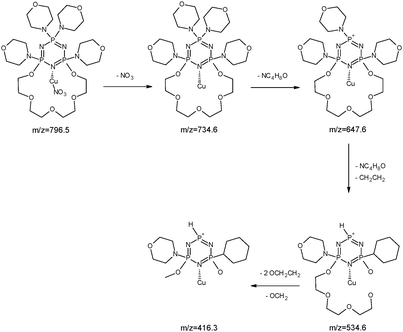
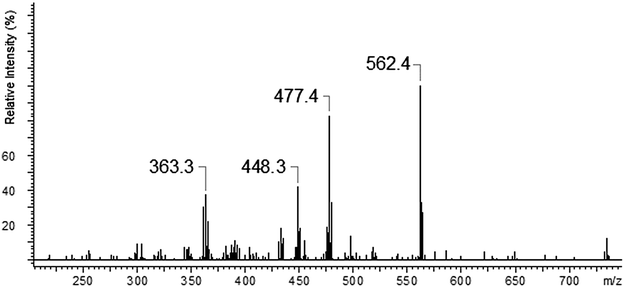
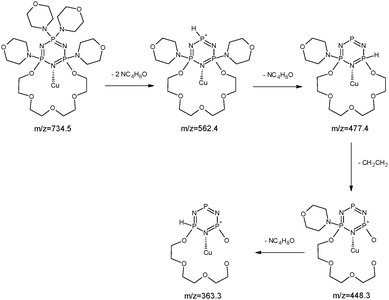
The copper ions create three types of complexes: [Cu(I)L]+, [Cu(II)L]2+ and [Cu(II)L + NO3]+ (Fig. 12). The MS/MS fragmentation pattern for the [Cu(II)L + NO3]+ complex (m/z = 796.5) shows the loss of –NO3 to an ion with m/z = 734.6 (Fig. 13). During the next step the loss of the morpholinyl group with m/z = 647.6 was noticed. Then we observed the loss of another morpholinyl substituent together with the –(CH2CH2) group with m/z = 534.6. In the last step the splitting-off of the fragments of the polyether moiety was observed (Scheme 4). Fragmentation of [Cu(I)L]+ with m/z 734.6 (Fig. 14) proceeds with the loss of two morpholinyl substituents (m/z = 562.4). The most abundant fragment observed at m/z = 477.4 was formed due to the loss of another morpholinyl group. Then the losses of 28 Da –(CH2CH2) peaks with m/z = 448.3 and with m/z = 363.2 due to subsequent loss of the last morpholinyl group were observed. This fragmentation pattern is shown on Scheme 5. Analysing the ionic radii of Cu(I) and the probable ligand cavity size we can notice that the copper ion fits well into crown moiety. Crown size (170–220 pm) and copper(I) ion (182 pm)78 are comparable, while the copper(II) ion (144 pm) is much smaller hence almost whole fragment of the polyether chain decay is observed.
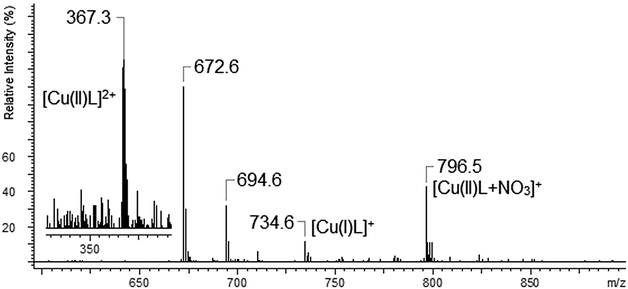 | ||
| Fig. 12 The mass spectrum of L with Cu(NO3)2 (the attached fragment of Cu(II)L spectrum had RI < 5%). | ||
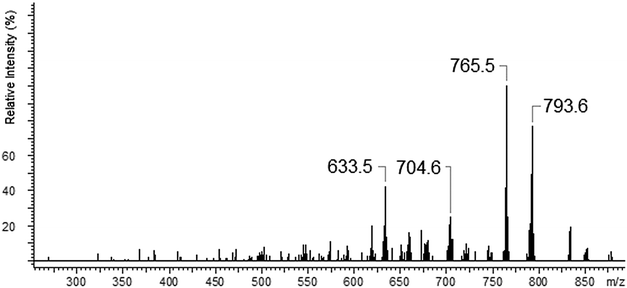
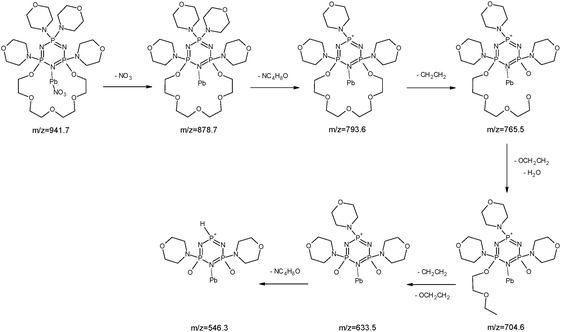
In the case of lead(II) ions we observed three types of complexes: [Pb(II)L]2+, [Pb(II)L + NO3]+ and [Pb(II)L + H2O]+ (Fig. 15). The fragmentation pattern of [Pb(II)L + NO3]+ shows (as in the case of copper derivative) the loss of –NO3 group to an ion with m/z = 878.7. Then the loss of one morpholinyl substituent giving the peak at m/z = 793.6 was observed. The most intense peak at m/z = 765.5 is related to the fragment which remained after –(CH2CH2) loss. The further decomposition was realized through the stepwise splitting-off almost all parts of the polyether moiety (m/z = 633.5). In the final step the morpholinyl group was detached giving the peak at m/z = 546.3 (Fig. 16), (Scheme 6).
Conclusion
Electrospray ionization mass spectrometry was used to probe the complexation behaviour of tetramorpholinyl-PNP-lariat ether with a wide range of metals ions. All selected metal cations form the complexes with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with the investigated ligand. Only silver ions create the “sandwich” type complex ([Ag(I)L2]+). Furthermore divalent cations form complexes with the nitrate anion adduct. In the case of Ca(II), Cd(II) and Pb(II) we noticed also another type of complex species where water molecules are involved in the formation of the molecular ion observed during the measurements: [Ca(II)L + OH]+, [Cd(II)L + H2O]+ and [Pb(II)L + H2O]+. Additionally, as far as copper ions are concerned, what we observed during the ionisation process was the reduction of Cu(II) → Cu(I) and formation of the complexes with both copper(I) and copper(II) ions. As we noticed from the fragmentation pattern, the complex with the cadmium ion is unstable under the measurement conditions. Calcium, copper and lead forms of complexes in the MS/MS fragmentation pattern of [M(II)L + NO3]+ show the loss of the –NO3 anion. In the case of tandem MS/MS for all investigated complexes – at the beginning we always observed the loss of the morpholinyl substituent and subsequent splitting-off of the polyether chain. Breaking of the chain and loss of the –(CH2CH2) and –(OCH2CH2) group fragments were observed in the literature in case of common crown ethers derivatives.79 Only in the case of copper(I) we observed the loss of four morpholinyl substituents. This fact suggests that the copper(I) is strongly accommodated inside the polyether ring. The obtained results suggest that ESI-MS technique seems to be a promising tool for characterization of complexes which are existing in the solution, despite the differences in the phase of the measurement conditions.
1 stoichiometry with the investigated ligand. Only silver ions create the “sandwich” type complex ([Ag(I)L2]+). Furthermore divalent cations form complexes with the nitrate anion adduct. In the case of Ca(II), Cd(II) and Pb(II) we noticed also another type of complex species where water molecules are involved in the formation of the molecular ion observed during the measurements: [Ca(II)L + OH]+, [Cd(II)L + H2O]+ and [Pb(II)L + H2O]+. Additionally, as far as copper ions are concerned, what we observed during the ionisation process was the reduction of Cu(II) → Cu(I) and formation of the complexes with both copper(I) and copper(II) ions. As we noticed from the fragmentation pattern, the complex with the cadmium ion is unstable under the measurement conditions. Calcium, copper and lead forms of complexes in the MS/MS fragmentation pattern of [M(II)L + NO3]+ show the loss of the –NO3 anion. In the case of tandem MS/MS for all investigated complexes – at the beginning we always observed the loss of the morpholinyl substituent and subsequent splitting-off of the polyether chain. Breaking of the chain and loss of the –(CH2CH2) and –(OCH2CH2) group fragments were observed in the literature in case of common crown ethers derivatives.79 Only in the case of copper(I) we observed the loss of four morpholinyl substituents. This fact suggests that the copper(I) is strongly accommodated inside the polyether ring. The obtained results suggest that ESI-MS technique seems to be a promising tool for characterization of complexes which are existing in the solution, despite the differences in the phase of the measurement conditions.
Notes and references
- M. Yamashita and J. B. Fenn, J. Phys. Chem., 1984, 88, 4451 CrossRef CAS.
- R. B. Cole, J. Mass Spectrom., 2000, 35, 763–772 CrossRef CAS.
- J. B. Fenn, M. Mann, C. K. Meng, S. F. Wong and C. M. Whitehouse, Mass Spectrom. Rev., 1990, 9, 37–70 CrossRef CAS.
- Electrospray Ionization Mass Spectrometry: Fundamentals, Instrumentation and Applications, ed. R. B. Cole, Wiley-Interscience, New York, NY, 1997, pp. 1–577 Search PubMed.
- J. A. Loo, Int. J. Mass Spectrom., 2000, 200, 175–186 CrossRef CAS.
- C. A. Schalley, Int. J. Mass Spectrom., 2000, 194, 11–39 CrossRef CAS.
- J. M. Daniel, S. D. Friess, S. Rajagopalan, S. Wendt and R. Zenobi, Int. J. Mass Spectrom., 2002, 216, 1–27 CrossRef CAS.
- T. D. Veenstra, Biophys. Chem., 1999, 79, 63–79 CrossRef CAS.
- P. B. Armentrout, C. A. Austin and M. T. Rodgers, J. Phys. Chem., 2014, 118, 8088–8097 CrossRef CAS PubMed.
- J. S. Brodbelt, Int. J. Mass Spectrom., 2000, 200, 57–69 CrossRef CAS.
- N. Shen, R. Marshall Pope and D. V. Dearden, Int. J. Mass Spectrom., 2000, 195/196, 639–652 CrossRef CAS.
- R. Frański and B. Gierczyk, J. Am. Soc. Mass Spectrom., 2010, 21, 545–549 CrossRef PubMed.
- R. Frański, Rapid Commun. Mass Spectrom., 2009, 23, 3488–3491 CrossRef PubMed.
- A. Tsuda, H. Moriwaki and T. Oshima, J. Chem. Soc., Perkin Trans. 2, 1999, 1235–1240 RSC.
- T. Ly and R. R. Julian, J. Am. Soc. Mass Spectrom., 2006, 17, 1209–1215 CrossRef CAS PubMed.
- S. F. Ralph, M. M. Sheil, L. A. Rodney, J. Geue and A. M. Sargeson, J. Chem. Soc., Dalton Trans., 1996, 4417–4424 RSC.
- D.-S. Young, H.-Y. Hung and L. K. Liu, J. Mass Spectrom., 1997, 32, 432–437 CrossRef CAS.
- S. M. Blair, J. S. Brodbelt, G. M. Reddy and A. P. Marchand, J. Mass Spectrom., 1998, 33, 721–728 CrossRef CAS.
- E. C. Kempen, J. S. Brodbelt, R. A. Bartsch, Y. Jang and J. S. Kim, Anal. Chem., 1999, 10, 5494–5500 Search PubMed.
- E. C. Kempen, J. S. Brodbelt and M. Reyzer, Struct. Chem., 1999, 10, 213–220 CrossRef.
- M. Reyzer, J. S. Brodbelt, A. P. Marchand, Z. Chen, Z. Huang and I. N. N. Namboothiri, Int. J. Mass Spectrom., 2001, 200, 57–69 Search PubMed.
- S. M. Blair, J. S. Brodbelt, A. P. Marchand, H.-S. Chong and S. Alidhodzic, J. Am. Soc. Mass Spectrom., 2000, 11, 884–891 CrossRef CAS.
- V. B. Di Marco and G. G. Bombi, Mass Spectrom. Rev., 2006, 25, 347–379 CrossRef CAS PubMed.
- A. Chapeaurouge, L. Bigler, A. Schafer and S. Bienz, J. Am. Soc. Mass Spectrom., 1995, 6, 207 CrossRef CAS.
- K. Wang and R. B. Cole, Org. Mass Spectrom., 1994, 29, 419 CrossRef.
- K. Wang and G. W. Gokel, J. Org. Chem., 1996, 61, 4693–4697 CrossRef CAS PubMed.
- S. M. Blair, J. S. Brodbelt, A. P. Marchand, K. A. Kumar and H. S. Chong, Anal. Chem., 2000, 72, 2433–2445 CrossRef CAS.
- S. M. Blair, E. C. Kempen and J. S. Brodbelt, J. Am. Soc. Mass Spectrom., 1998, 9, 1049–1059 CrossRef CAS.
- D.-S. Young, H.-Y. Hung and L. K. Liu, Rapid Commun. Mass Spectrom., 1997, 11, 769–773 CrossRef CAS.
- E. Leize, A. Jaffrezic and A. Van Dorsselaer, J. Mass Spectrom., 1996, 31, 537–544 CrossRef CAS.
- S. Williams, S. M. Blair, J. S. Brodbelt, X. Huang and R. A. Bartsch, Int. J. Mass Spectrom., 2001, 212, 389–401 CrossRef CAS.
- C. L. Scherman and J. S. Brodbelt, Anal. Chem., 2003, 75, 1828–1836 CrossRef.
- J. B. Nicoll and D. V. Dearden, Int. J. Mass Spectrom., 2001, 204, 171–183 CrossRef CAS.
- T. Oshima, F. Matsuda, K. Fukushima, H. Tamura, G. Matsubayashi and R. Arakawa, J. Chem. Soc., Perkin Trans. 2, 1998, 145–148 RSC.
- R. A. Bartsch and M. D. Eley, Tetrahedron, 1996, 52, 8979–8988 CrossRef CAS.
- W. Z. Shou and R. F. Browner, Anal. Chem., 1999, 71, 2265–3373 CrossRef.
- J. G. Krabbe, A. R. de Boer, G. van der Zwan, H. Lingeman, W. M. A. Niessen and H. Irth, J. Am. Soc. Mass Spectrom., 2007, 18, 707–713 CrossRef CAS PubMed.
- A. Ohki, J. Ping Lu, X. Huang and R. A. Bartsch, Anal. Chem., 1994, 66, 4332–4336 CrossRef CAS.
- S. M. Williams, J. S. Brodbelt and R. A. Bartsch, J. Am. Soc. Mass Spectrom., 2003, 14, 1215–1228 CrossRef CAS.
- K. Vékey, J. Chromatogr. A, 2001, 921, 227–236 CrossRef.
- O. Núñez, E. Moyano and M. T. Galceran, Trends Anal. Chem., 2005, 24, 683–703 CrossRef PubMed.
- A. R. S. Ross, M. G. Ikonomon, J. A. Thompson and K. I. Orians, Anal. Chem., 1998, 70, 2225–2235 CrossRef CAS PubMed.
- X. Yu, M. Wojciechowski and C. Fenselau, Anal. Chem., 1993, 65, 1355–1359 CrossRef CAS.
- G. W. Gokel, Chem. Soc. Rev., 1992, 39, 39–47 RSC.
- G. W. Gokel, Crown Ethers and Cryptand, University of Miami Coral Gables, Florida, USA, 1991 Search PubMed.
- K. Brandt, I. Porwolik and M. Siwy, Wiadomości chemiczne, Chemia supramolekularna, 1997, pp. 91–117 Search PubMed.
- D. M. Dishong, C. J. Diamond, M. I. Cinoman and G. W. Gokel, J. Am. Chem. Soc., 1983, 105, 586 CrossRef CAS.
- D. M. Goli, D. M. Dishing, C. J. Diamond and G. W. Gokel, Tetrahedron Lett., 1982, 23(50), 5243–5246 CrossRef CAS.
- D. B. White, K. A. Arnold and G. W. Gokel, Tetrahedron Lett., 1987, 28(16), 1749–1752 CrossRef.
- R. A. Schultz, D. B. White, D. M. Dishing, K. A. Arnold and G. W. Gokel, J. Am. Chem. Soc., 1985, 107, 6659 CrossRef CAS.
- D. M. Dishing, C. J. Diamond, M. I. Cinoman and G. W. Gokel, J. Am. Chem. Soc., 1983, 105, 586 CrossRef.
- K. Brandt, T. Kupka, J. Drozd, J. C. van de Grampel, A. Meetsma and A. P. Jekel, Inorg. Chim. Acta, 1995, 228(2), 187–192 CrossRef CAS.
- K. Brandt, I. Porwolik, T. Kupka, R. A. Shaw and D. B. Davies, J. Org. Chem., 1995, 60, 7433–7438 CrossRef CAS.
- I. Porwolik-Czomperlink, K. Brandt, T. A. Clayton, D. B. Davies, R. J. Eaton and R. A. Shaw, Inorg. Chem., 2002, 41(19), 4944–4951 CrossRef PubMed.
- S. Besli, Inorg. Chem. Commun., 2005, 8, 449–452 CrossRef CAS PubMed.
- K. Brandt, I. Porwolik-Czomperlink, M. Siwy, T. Kupka, R. A. Shaw, D. B. Davies and R. A. Bartsch, J. Inclusion Phenom. Macrocyclic Chem., 1999, 35, 281–289 CrossRef CAS.
- K. Brandt, I. Porwolik, M. Siwy, T. Kupka, R. A. Shaw, D. B. Davies, M. B. Hursthouse and G. D. Sykara, J. Am. Chem. Soc., 1997, 119, 1143–1144 CrossRef CAS.
- D. B. Davies, T. A. Clayton, R. J. Eaton, R. A. Shaw, A. Egan, M. B. Hursthouse, G. D. Sykara, I. Porwolik-Czomperlik, M. Siwy and K. Brandt, J. Am. Chem. Soc., 2000, 122, 12447–12457 CrossRef CAS.
- R. A. Bartsch, E. K. Lee, S. Chun, N. Elkarim, K. Brandt, I. Porwolik-Czomperlink, M. Siwy, D. Lach and J. Silverring, J. Chem. Soc., Perkin Trans. 2, 2002, 442–448 RSC.
- K. Brandt, I. Porwolik, T. Kupka, A. Olejnik, R. A. Shaw and D. B. Davies, J. Org. Chem., 1995, 60, 7433–7438 CrossRef CAS.
- P. Seliger, G. Andrijewski, M. Siwy and D. Sęk, Pol. J. Chem., 2009, 83, 581–588 CAS.
- K. Brandt, P. Seliger, A. Grzejdziak, T. J. Bartczak, R. Kruszyński, D. Lach and J. Silberring, Inorg. Chem., 2001, 40, 3704–3710 CrossRef CAS PubMed.
- M. Siwy, D. Sęk, B. Kaczmarczyk, I. Jaroszewicz, A. Nasulewicz, M. Pelczyńska, D. Nevozhay and A. Opolski, J. Med. Chem., 2006, 49, 806–810 CrossRef CAS PubMed.
- K. Brandt, R. Kruszyński, T. J. Bartczak and I. Porwolik-Czomperlink, Inorg. Chim. Acta, 2001, 322, 138–144 CrossRef CAS.
- P. Seliger, N. Sołtys, G. Andrijewski and M. Siwy, New J. Chem., 2012, 36, 2607–2612 RSC.
- N. Sołtys, P. Seliger, G. Andrijewski and M. Siwy, RSC Adv., 2013, 3, 25351–25359 RSC.
- S. A. Cotton, The Chemistry of Precious Metals, Blackie Academic & Professional, an Imprint of Chapman & Hall, London UK, 1997, p. 325 Search PubMed.
- R. Kruszyński, M. Siwy, I. Porwolik-Czomperlink and A. Trzesowska, Inorg. Chim. Acta, 2006, 359, 649–658 CrossRef PubMed.
- T. Nabeshima, Coord. Chem. Rev., 1996, 148, 151 CrossRef CAS.
- V. V. Litvinova and A. V. Anisimov, Khim. Geterotsikl. Soedin., 1999, 1587 Search PubMed.
- M. Penso, A. Maia, V. Lupi and G. Tricarico, Tetrahedron, 2011, 67, 2096–2102 CrossRef CAS PubMed.
- C. A. Kozlowski and J. Kozlowska, J. Membr. Sci., 2009, 326, 215–221 CrossRef CAS PubMed.
- in Crown ethers and analogs, ed. S. Patai and Z. Rappoport, Wiley, Chichester, 1989 Search PubMed.
- L. Gianelli, V. Amendola, L. Fabbrizzi, P. Pallavicini and G. G. Mellerio, Rapid Commun. Mass Spectrom., 2001, 15, 2347–2353 CrossRef CAS.
- H. Lavanant, H. Virelizier and Y. Hoppilliard, J. Am. Soc. Mass Spectrom., 1998, 9, 1217–1221 CrossRef CAS.
- D. Schroder, T. Weiske and H. Schwarz, Int. J. Mass Spectrom., 2002, 219, 729 CrossRef CAS.
- A. Tintaru, L. Charles, P. Milko, J. Roithova and D. Schroder, J. Phys. Org. Chem., 2009, 22, 229 CrossRef CAS.
- The Elements, ed. J. Emsley, Clarendon Press, Oxford, 2 edn, 1991 Search PubMed.
- H. Gleispach, H. J. Leis, C. Erk and M. Bulut, J. Inclusion Phenom. Mol. Recognit. Chem., 1996, 24, 163–173 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |