Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Energetic alliance of tetrazole-1-oxides and 1,2,5-oxadiazoles†‡
Dennis
Fischer
,
Thomas M.
Klapötke
*,
Marius
Reymann
,
Jörg
Stierstorfer
and
Maurus B. R.
Völkl
Ludwig Maximilians University of Munich, Department of Chemistry, Energetic Materials Research, Butenandtstr. 5-13, D-81377 Munich, Germany. E-mail: tmk@cup.uni-muenchen.de
First published on 9th October 2014
Abstract
The connection of highly endothermic heterocycles with high nitrogen as well as oxygen content is a recent trend in the development of new energetic materials in order to increase densities and stabilities. Bis(1-hydroxytetrazolyl)furazane (9) and bis(1-hydroxytetrazolyl)furoxane (10) were synthesized for the first time from dicyanofurazane and dicyanofuroxane, respectively. Several nitrogen-rich compounds (e.g. ammonium and hydroxylammonium) and metal salts thereof were prepared. Most compounds were characterized by single crystal X-ray diffraction. In addition all compounds were analyzed by vibrational spectroscopy (IR and Raman), multinuclear NMR spectroscopy, elemental analysis and DSC measurements. The heats of formation of 4, 5, 15–16, 20 and 24 were calculated using the atomization method based on CBS-4M enthalpies. With these values and the experimental (X-ray) densities several detonation parameters such as the detonation pressure, velocity, energy and temperature were computed using the EXPLO5 code (V.5.05). In addition, the sensitivities towards impact, friction and electrical discharge were tested using the BAM drop hammer and friction tester as well as a small scale electrical discharge device.
Introduction
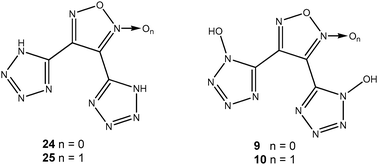
Research towards insensitive replacements for hexogen (RDX), octogen (HMX) and nitropenta (PETN) is still of particular interest in our and many other research groups worldwide. RDX has been identified as toxic and possibly carcinogenic.1 Several attempts to synthesize appropriate replacements for RDX have been made in the recent past using tetrazole oxides.2–4 The purpose of this study was to combine furazanes (1,2,5-oxadiazoles) and furoxanes (1,2,5-oxadiazole-2-oxides) with tetrazole oxides. The connection of highly endothermic heterocycles with high nitrogen as well as oxygen content is a recent trend in the development of new energetic materials in order to obtain powerful materials with great density and appropriate oxygen balance on the one hand, and perfect stability on the other hand. Tetrazoles (without N-oxide) have already been attached to furazanes5 and furoxanes.6 The resulting literature known compounds 24 and 25 as well as the new ones 9 and 10 are displayed in Fig. 1.Various nitrogen rich salts of 9 and 10 as well as metal salts were synthesized to investigate their properties as potential energetic ingredients. The resulting 3,4-(1-oxidotetrazolyl)-furoxanes and furazanes are capable and fairly stable compounds in their deprotonated form.
Results and discussion
Synthesis
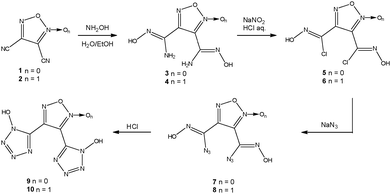
Compounds 9 and 10 were synthesised using a similar protocol from dicyanofurazane7 and dicyanofuroxane8 as depicted in Scheme 1.Hydroximoylamines 3 and 4 are made from the nitriles by exothermic addition of aqueous hydroxylamine in ethanol in about 85% yield. Hydroximoyl chlorides 5 and 6 were synthesized from 3 and 4 by diazotization in 15% HCl and subsequently extracted into diethyl ether. The reaction of 5 and 6 with sodium azide in aqueous ethanol affords hydroximoylazides 7 and 8, respectively, which are also extracted into diethyl ether. The dried ether phase was saturated with gaseous HCl at 0 °C and stirred for 24 h in order to close the desired aromatic tetrazole-oxide rings. Compounds 9 and 10 are obtained as slightly yellow sticky oils after removal of the ethereal HCl solution.
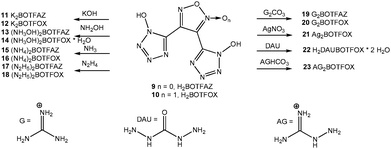
Salts of compounds 9 and 10 could be easily prepared by the addition of a base or the corresponding carbonates/bicarbonates to aqueous solutions of 9 and 10. The silver salt of 10 precipitated upon the addition of aqueous silver nitrate. An overview of the salts prepared in this work is given in Scheme 2.
Crystal structures
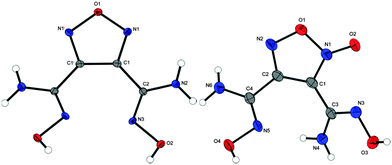
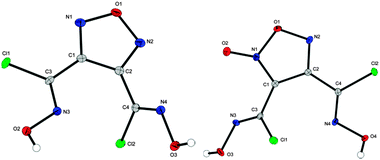
Single crystals for XRD of compounds 3–6, 11, 12, 14–17, 20 and 23 could be obtained during this work. Crystallographic data and parameters as well as CCDC numbers are given in Tables S1 and S2 in the ESI.‡In general, all bond lengths and angles were observed as expected and are comparable to similar crystal structures of furazanes,9 furoxanes,10 and tetrazole-oxides11 in the literature. Compound 3 crystallizes in the monoclinic space group C2/c with four molecules in the unit cell. The molecular unit is generated by C2 symmetry through atom O1 and bond C1–C1i. The density (1.667 g cm−3 at 100 K) of 3 is significantly smaller than that of the corresponding furazane 7 (1.780 g cm−3 at 173 K). Compound 4 crystallizes in the monoclinic space group P21/n. The molecular moieties are depicted in Fig. 2.
Hydroximoyl chlorides 5 and 6 crystallize in the monoclinic (P21/n) and orthorhombic (P212121) crystal systems with four molecules in the unit cell. The molecular units are shown in Fig. 3. The density of furazane (1.909 g cm−3 at 100 K) again is slightly lower than that of furoxane (1.949 g cm−3 at 100 K).
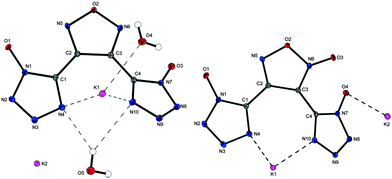
Crystal structures of both potassium salts were obtained. The structure of furazane 11, which crystallizes in the monoclinic space group P21/c, contains two crystal water molecules resulting in a lower density of 1.926 g cm−3 (at 100 K). For furoxane 12 (triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) a density of 2.156 g cm−3 at 100 K has been calculated. In both structures two rings (containing atoms C1 and C2/C3) are almost in plane while the third one is significantly deviated. Molecular moieties of 11 and 12 are depicted in Fig. 4.
) a density of 2.156 g cm−3 at 100 K has been calculated. In both structures two rings (containing atoms C1 and C2/C3) are almost in plane while the third one is significantly deviated. Molecular moieties of 11 and 12 are depicted in Fig. 4.
Compound 14·H2O could only be obtained in the crystalline form with inclusion of one crystal water molecule (Fig. 5). It crystallizes in the monoclinic space group P21 with a density of 1.794 g cm−3 at 173 K.
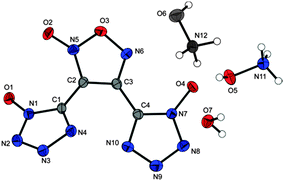
Bisammonium salt 15 crystallizes in the orthorhombic space group Pbca with a calculated density of 1.686 g cm−3 at 298 K. The corresponding bisammonium furoxane salt 16 crystallizes with a higher density of 1.748 g cm−3 (at 293 K) in the monoclinic space group P21/c. Both structures shown in Fig. 6 are dominated by strong hydrogen bonds involving all NH4+ protons.
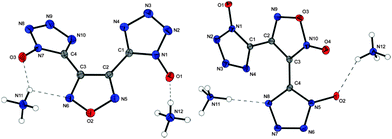
The products of deprotonation of 9 with hydrazine and different guanidinium bases have not been obtained as single crystals. The hydrazinium salt of 9 crystallizes in the triclinic (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) crystal system and with a density of 1.727 g cm−3 at 236 K without inclusion of crystal water. The molecular moiety is shown in Fig. 7.
) crystal system and with a density of 1.727 g cm−3 at 236 K without inclusion of crystal water. The molecular moiety is shown in Fig. 7.
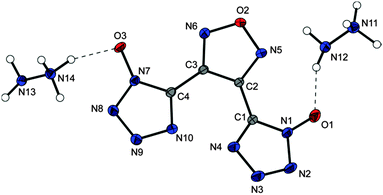 | ||
| Fig. 7 Molecular moiety of hydrazinium salt 17. Thermal ellipsoids are drawn at the 50% probability level. | ||
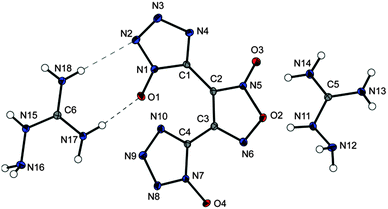
The bisguanidinium salt 20 (Fig. 8) crystallizes in the monoclinic space group C2/c and with a density of 1.739 g cm−3 at 100 K (Fig. 8).
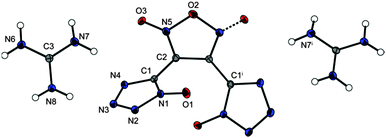 | ||
| Fig. 8 Molecular moiety of guanidinium salt 20. Thermal ellipsoids are drawn at the 50% probability level. Symmetry code: (i) 1 − x, y, 0.5 − z. | ||
In contrast to 20, the aminoguanidinium salt 23 shows a slightly lower crystal density of 1.692 g cm−3. The asymmetric unit of the monoclinic (P21/c) cell is shown in Fig. 9.
 | ||
| Fig. 9 Molecular moiety of aminoguanidinium salt 23. Thermal ellipsoids are drawn at the 50% probability level. | ||
NMR spectroscopy
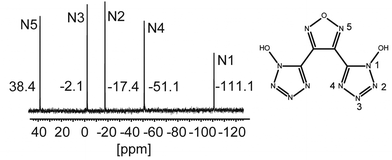
All 1H NMR and 13C NMR shifts of compounds 1–23 are gathered in Table 1. The 1H NMR spectra of 9 and 10 exhibit both a broad singlet at around 7 ppm although 10 has no C2V symmetry anymore. The oximes in compounds 3–8 are observed in low field regions from 10.33 to 13.00 ppm. In the case of the oxime and amide protons splitting of the signals of the furoxane compounds can be observed because of the lower symmetry. In the 13C NMR spectrum 9 exhibits two resonances at 142.1 ppm caused by furazane and 136.6 ppm caused by 1-hydroxytetrazole. 10 shows four resonances in the 13C NMR spectra because of the lower symmetry at 136.8 and 134.4 ppm for the tetrazole oxides and at 143.4 and 103.4 ppm for the furoxane ring. Upon deprotonation the furazane signal of 9 is shifted towards lower fields up to 148.7 ppm and the tetrazole-oxide signal is shifted towards higher fields down to 132.8 ppm. The same trend was observed for 10. Deprotonation led to a shift towards lower fields up to 149.4 and 107.7 ppm for furoxane but to a shift towards higher fields down to 133.2 and 130.3 ppm for the tetrazole-oxide resonances. The 15N NMR spectrum of 9 is depicted in Fig. 10. The chemical shifts are assigned to the particular nitrogen atoms.| C | 1H NMR shift [ppm] | 13C NMR shift [ppm] |
|---|---|---|
| 1 | — | 136.3, 106.7 |
| 2 | — | 134.7, 106.8, 105.0, 99.5 |
| 3 | 10.33, 6.20 | 148.7, 142.1 |
| 4 | 10.64, 10.08, 6.98, 6.08 | 151.6, 142.5, 139.8, 109.9 |
| 5 | 13.61 | 148.7, 122.9 |
| 6 | 13.78, 13.58 | 150.6, 124.8, 120.3, 110.2 |
| 7 | 12.85 | 147.1, 132.6 |
| 8 | 13.00, 12.75 | 149.4, 133.7, 130.4, 107.7 |
| 9 | 9.02 | 142.1, 136.6 |
| 10 | 6.67 | 143.4, 136.8, 134.4, 103.4 |
| 11 | — | 145.0, 132.8 |
| 12 | — | 147.7, 133.2, 130.3, 106.5 |
| 13 | 10.22 | 144.2, 133.9 |
| 14 | 10.27 | 146.4, 134.3, 131.5, 105.6 |
| 15 | 7.18 | 144.8, 133.1 |
| 16 | 7.22 | 147.2, 133.5, 130.7, 106.1 |
| 17 | 7.09 | 144.8, 133.1 |
| 18 | 7.16 | 147.1, 133.7, 130.9, 106.1 |
| 19 | 6.63 | 158.1, 144.2, 132.9 |
| 20 | 6.99 | 158.5, 146.9, 133.8, 130.9, 105.9 |
| 21 | — | 146.2, 133.2, 130.4, 105.4 |
| 22 | 6.36 | 158.4, 145.6, 135.1, 132.5, 105.0 |
| 23 | 8.70, 7.26, 6.90, 4.51 | 159.4, 147.0, 133.8, 130.9, 106.0 |
The carbon signals of the salts 11–23 are shifted to lower fields in comparison with their acids. No irregularities in the 1H NMR and 13C NMR shifts of the nitrogen-rich cations were observed.
Energetic properties
| ΔfH°(g,M,298) = H(M,298) − ΣH°(atoms,298) + ΣΔfH°(atoms,298) | (1) |
Sensitivities were measured using a BAM drop hammer, a BAM friction tester12 and a OZM electrostatic discharge device13 (see also the Experimental part, General methods).
Detonation parameters were calculated using the computer code EXPLO5.0514 using X-ray densities which were converted to room temperature values according to eqn (2). A coefficient of volume expansion15αv of 1.5 × 10−4 K−1 was used. The structures of 15 and 16 were already measured at room temperature. Further explanations are gathered in the ESI.‡
| ρ298K = ρT/(1 + αv(298 − T0)) | (2) |
Only the physicochemical properties of compounds 4, 5, 15–16, 20 and 23 are discussed since (i) they consist only of CHNO atoms and (ii) anhydrous crystal structures were obtained. The energetic parameters in comparison with RDX (cyclotrimethylene-trinitramine) are summarized in Table 2. All compounds investigated show improved sensitivities to RDX (IS 7.4 J, FS 120 N). Especially 20 is classified as insensitive towards impact and friction. The highest heat of formation was calculated for hydrazinium salt 17 (ΔfH°(s) = 947.5 kJ mol−1). For energetic materials it is more convenient to look for mass based enthalpies or energies. Also the highest mass based energy of formation value (ΔfU° 3245.4 kJ kg−1) was calculated for 17. The most important detonation parameters (heat of detonation, detonation temperature, pressure, velocity of detonation, and volume of detonation gases) were calculated with the EXPLO5.05 code and are summarized in Table 2. Based on these computations, compound 17 (8843 m s−1) has higher velocity of detonation than RDX (8763 m s−1). However, with respect to the synthetic expenditures and the assessment of all important energetic properties (sensitivities, stabilities and performance) probably none of the compounds will be used as an explosive filler by itself.
| 3 | 4 | 15 | 16 | 17 | 20 | 23 | RDX | |
|---|---|---|---|---|---|---|---|---|
| a Impact sensitivity (BAM drophammer (1 of 6)). b Friction sensitivity (BAM friction tester (1 of 6)). c Electrostatic discharge device (OZM research). d Nitrogen content. e Oxygen balance (Ω = (xO − 2yC − 1/2zH)M/1600). f Start of decomposition temperature from DSC (β = 5 °C). g From X-ray diffraction, values for 298 K were calculated with ρ298K = ρT/(1 + αv(298 − T)),15αv = 1.5 × 10−4 K−1. h Calculated enthalpy of formation. i Calculated energy of formation. j Energy of explosion. k Explosion temperature. l Detonation pressure. m Detonation velocity. n Volume of detonation gases (assuming only gaseous products). | ||||||||
| Formula | C4H6N6O3 | C4H6N6O4 | C4H8N12O3 | C4H8N12O4 | C4H10N14O3 | C6H12N16O4 | C6H14N18O4 | C3H6N6O6 |
| FW/g mol−1 | 186.13 | 202.13 | 272.18 | 288.18 | 302.21 | 372.26 | 402.29 | 222.12 |
| IS/Ja | >40 | 10 | 9 | 10 | 7 | 30 | 8 | 7.416 |
| FS/Nb | >360 | 240 | >360 | 240 | >360 | >360 | >360 | 12016 |
| ESD/Jc | >1.5 | 0.25 | 1.5 | 1 | 1.5 | n.d. | n.d. | 0.2 |
| N/%d | 45.15 | 41.58 | 61.75 | 58.32 | 64.89 | 60.20 | 62.67 | 37.84 |
| Ω CO2/%e | −68.76 | −55.40 | −52.90 | −44.41 | −52.94 | −60.17 | −59.65 | −21.61 |
| T Dec./°Cf | 198 | 180 | 259 | 234 | 211 | 197 | 165 | 205 |
ρ/g cm−3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g g |
1.668(100 K) | 1.781(173 K) | 1.727(236 K) | 1.739(100 K) | 1.692(100 K) | 1.858(90 K)17 | ||
| 1.64(298 K) | 1.75(298 K) | 1.686(293 K) | 1.748(293 K) | 1.71(298 K) | 1.69(298 K) | 1.64(298 K) | 1.806(298 K)18 | |
ΔfHm°/kJ mol−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h h |
150.2 | 159.3 | 625.6 | 621.7 | 947.5 | 638.3 | 885.4 | 66.616 |
ΔfU°/kJ kg−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i i |
907.1 | 886.4 | 2402.8 | 2260.2 | 3245.4 | 1820.8 | 2311.2 | 400.216 |
| EXPLO5.05: | ||||||||
−ΔExU°/kJ kg−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j j |
4713 | 5323 | 5122 | 5530 | 5779 | 4532 | 4884 | 6110 |
| T det/Kk | 3286 | 3631 | 3582 | 3841 | 3813 | 3193 | 3340 | 4224 |
| P CJ/kbarl | 229 | 287 | 279 | 313 | 318 | 261 | 261 | 351 |
V
Det./m s−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m m |
7727 | 8312 | 8364 | 8671 | 8843 | 8161 | 8224 | 8763 |
V
o/L kg−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n n |
720 | 719 | 769 | 772 | 793 | 764 | 782 | 739 |
Conclusions
From this combined experimental and theoretical study the following conclusions can be drawn.– The combination of furazanes or furoxanes with tetrazole-1-oxides is a suitable strategy in order to generate new triheterocyclic high-performing energetic materials due to their large positive heats of formation and appropriate densities.
– Generally the investigated furoxanes show mostly higher densities but lower thermal stabilities than the corresponding furazanes. Therefore, furazanes mostly are the better choice as energetic backbone heterocycles.
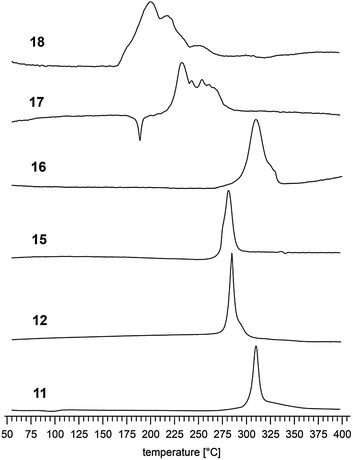
– The thermal stability of the tetrazole oxide anions attached to a furoxane or furazane ring is sufficient to reach decomposition temperatures above 200 °C.
Experimental part
For general methods, please see the ESI.‡Syntheses
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3162 (6), 1651 (71), 1592 (17), 1535 (35), 1513 (100), 1374 (43), 1282 (6), 1126 (6), 1040 (16), 984 (32), 956 (9), 923 (8), 826 (4), 761 (6), 488 (14) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 10.33, 6.20 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 148.7, 142.1 ppm.
(rel. int.): 3162 (6), 1651 (71), 1592 (17), 1535 (35), 1513 (100), 1374 (43), 1282 (6), 1126 (6), 1040 (16), 984 (32), 956 (9), 923 (8), 826 (4), 761 (6), 488 (14) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 10.33, 6.20 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 148.7, 142.1 ppm.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3463 (w), 3371 (w), 3309 (w), 1668 (m), 1647 (s), 1579 (s), 1539 (w), 1504 (m), 1418 (m), 1360 (m), 1311 (m), 1229 (w), 1082 (w), 1021 (w), 951 (s), 929 (s), 858 (w), 810 (m), 744 (vs), 688 (s) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3463 (w), 3371 (w), 3309 (w), 1668 (m), 1647 (s), 1579 (s), 1539 (w), 1504 (m), 1418 (m), 1360 (m), 1311 (m), 1229 (w), 1082 (w), 1021 (w), 951 (s), 929 (s), 858 (w), 810 (m), 744 (vs), 688 (s) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3372 (12), 1671 (39), 1651 (34), 1582 (13), 1542 (100), 1507 (12), 1421 (20), 1310 (14), 1232 (14), 1107 (10), 1066 (10), 1021 (10), 956 (9), 933 (11), 860 (6), 756 (10), 639 (6), 480 (24), 370 (5), 330 (11), 299 (5), 263 (6) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 10.64, 10.08, 6.98, 6.08 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 151.6, 142.5, 139.8, 109.9 ppm. EA (C4H6N6O4, 202.13 g mol−1) calc. (found): C 23.77 (23.99), H 2.99 (2.86), N 41.58 (41.45)%. IS: 10 J (<100 μm). FS: 240 N. ESD: 0.25 J.
(rel. int.): 3372 (12), 1671 (39), 1651 (34), 1582 (13), 1542 (100), 1507 (12), 1421 (20), 1310 (14), 1232 (14), 1107 (10), 1066 (10), 1021 (10), 956 (9), 933 (11), 860 (6), 756 (10), 639 (6), 480 (24), 370 (5), 330 (11), 299 (5), 263 (6) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 10.64, 10.08, 6.98, 6.08 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 151.6, 142.5, 139.8, 109.9 ppm. EA (C4H6N6O4, 202.13 g mol−1) calc. (found): C 23.77 (23.99), H 2.99 (2.86), N 41.58 (41.45)%. IS: 10 J (<100 μm). FS: 240 N. ESD: 0.25 J.
DSC (5 °C min−1): 115 °C (dec.). IR (ATR, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3502 (m), 3388 (m), 2991 (w), 2877 (w), 1731 (w), 1607 (m), 1561 (w), 1507 (w), 1499 (w), 1397 (m), 1390 (m), 1376 (m), 1359 (m), 1343 (m), 1265 (m), 1193 (w), 1094 (vw), 1057 (s), 1032 (s), 999 (s), 962 (s), 900 (s), 887 (vs), 863 (s), 818 (m), 795 (w), 749 (vw), 664 (m) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3502 (m), 3388 (m), 2991 (w), 2877 (w), 1731 (w), 1607 (m), 1561 (w), 1507 (w), 1499 (w), 1397 (m), 1390 (m), 1376 (m), 1359 (m), 1343 (m), 1265 (m), 1193 (w), 1094 (vw), 1057 (s), 1032 (s), 999 (s), 962 (s), 900 (s), 887 (vs), 863 (s), 818 (m), 795 (w), 749 (vw), 664 (m) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3397 (6), 2944 (9), 2245 (3), 1626 (9), 1611 (100), 1563 (14), 1509 (89), 1396 (16), 1374 (3), 1361 (2), 1273 (3), 160 (8), 1002 (3), 968 (4), 889 (18), 864 (2), 666 (29), 655 (6), 616 (5), 601 (7), 499 (7), 430 (13), 413 (3), 366 (4), 325 (7), 296 (9), 241 (19), 226 (10), 182 (8), 151 (10), 102 (55), 76 (11), 67 (6) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 13.61 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 148.7, 122.9 ppm. EA (C4H2N4O3Cl2, 224.99 g mol−1) calc. (found): C 21.35 (23.19), H 0.90 (1.53), N 24.90 (22.76)%.
(rel. int.): 3397 (6), 2944 (9), 2245 (3), 1626 (9), 1611 (100), 1563 (14), 1509 (89), 1396 (16), 1374 (3), 1361 (2), 1273 (3), 160 (8), 1002 (3), 968 (4), 889 (18), 864 (2), 666 (29), 655 (6), 616 (5), 601 (7), 499 (7), 430 (13), 413 (3), 366 (4), 325 (7), 296 (9), 241 (19), 226 (10), 182 (8), 151 (10), 102 (55), 76 (11), 67 (6) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 13.61 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 148.7, 122.9 ppm. EA (C4H2N4O3Cl2, 224.99 g mol−1) calc. (found): C 21.35 (23.19), H 0.90 (1.53), N 24.90 (22.76)%.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3258 (m), 3035 (w), 2981 (w), 2855 (w), 2361 (vw), 2325 (vw), 2132 (s), 1733 (w), 1614 (m), 1558 (vw), 1516 (w), 1445 (w), 1389 (m), 1340 (s), 1272 (s), 1223 (m), 1108 (vw), 1093 (w), 1026 (s), 976 (vs), 936 (s), 899 (m), 855 (s), 819 (w), 781 (vw), 753 (w), 668 (vw) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 12.85 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 147.1, 132.6 ppm.
(rel. int.): 3258 (m), 3035 (w), 2981 (w), 2855 (w), 2361 (vw), 2325 (vw), 2132 (s), 1733 (w), 1614 (m), 1558 (vw), 1516 (w), 1445 (w), 1389 (m), 1340 (s), 1272 (s), 1223 (m), 1108 (vw), 1093 (w), 1026 (s), 976 (vs), 936 (s), 899 (m), 855 (s), 819 (w), 781 (vw), 753 (w), 668 (vw) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 12.85 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 147.1, 132.6 ppm.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3404 (w), 2255 (w), 2128 (w), 1713 (w), 1660 (m), 1463 (m), 1344 (m), 1246 (m), 1197 (m), 1103 (m), 1053 (s), 1022 (s), 1005 (s), 982 (s), 922 (s), 895 (s), 819 (vs), 758 (s), 729 (s), 709 (m), 686 (m), 673 (m) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3404 (w), 2255 (w), 2128 (w), 1713 (w), 1660 (m), 1463 (m), 1344 (m), 1246 (m), 1197 (m), 1103 (m), 1053 (s), 1022 (s), 1005 (s), 982 (s), 922 (s), 895 (s), 819 (vs), 758 (s), 729 (s), 709 (m), 686 (m), 673 (m) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 2982 (8), 2940 (70), 2878 (14), 1618 (100), 1453 (10), 1387 (7), 1261 (30), 1205 (6), 1111 (5), 1011 (8), 907 (10), 764 (5), 736 (15), 711 (5), 455 (10), 92 (38) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 9.02 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 142.1, 136.6 ppm. 15N{1H} NMR (400 MHz, DMSO-d6, 25 °C), δ: 38.39, −2.1, −17.4, −51.1, −111.1 ppm.
(rel. int.): 2982 (8), 2940 (70), 2878 (14), 1618 (100), 1453 (10), 1387 (7), 1261 (30), 1205 (6), 1111 (5), 1011 (8), 907 (10), 764 (5), 736 (15), 711 (5), 455 (10), 92 (38) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 9.02 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 142.1, 136.6 ppm. 15N{1H} NMR (400 MHz, DMSO-d6, 25 °C), δ: 38.39, −2.1, −17.4, −51.1, −111.1 ppm.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3423 (w), 2460 (w), 1607 (vs), 1461 (w), 1402 (w), 1369 (m), 1301 (m), 1259 (m), 1223 (m), 1194 (w), 1135 (w), 1091 (w), 1000 (m), 965 (s), 816 (s), 762 (w), 744 (w), 728 (w), 696 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3423 (w), 2460 (w), 1607 (vs), 1461 (w), 1402 (w), 1369 (m), 1301 (m), 1259 (m), 1223 (m), 1194 (w), 1135 (w), 1091 (w), 1000 (m), 965 (s), 816 (s), 762 (w), 744 (w), 728 (w), 696 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 2997 (2), 2990 (2), 2982 (7), 2943 (44), 1612 (100), 1463 (12), 1309 (14), 1265 (35), 1227 (31), 1201 (6), 1138 (6), 1003 (12), 820 (8), 765 (9), 747 (13), 733 (14), 700 (7), 526 (7), 453 (10), 414 (6), 388 (6), 358 (8) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 6.67 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 143.4, 136.8, 134.4, 103.4 ppm.
(rel. int.): 2997 (2), 2990 (2), 2982 (7), 2943 (44), 1612 (100), 1463 (12), 1309 (14), 1265 (35), 1227 (31), 1201 (6), 1138 (6), 1003 (12), 820 (8), 765 (9), 747 (13), 733 (14), 700 (7), 526 (7), 453 (10), 414 (6), 388 (6), 358 (8) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 6.67 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 143.4, 136.8, 134.4, 103.4 ppm.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3552 (w), 3357 (m), 3242 (w), 1665 (w), 1635 (m), 1592 (m), 1574 (w), 1542 (w), 1471 (s), 1437 (m), 1407 (s), 1372 (m), 1362 (m), 1286 (s), 1239 (s), 1173 (w), 1118 (m), 1084 (w), 1033 (m), 1015 (w), 1000 (s), 983 (vs), 912 (s), 896 (m), 834 (w), 803 (w), 771 (m), 751 (w), 727 (w), 692 (w), 664 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3552 (w), 3357 (m), 3242 (w), 1665 (w), 1635 (m), 1592 (m), 1574 (w), 1542 (w), 1471 (s), 1437 (m), 1407 (s), 1372 (m), 1362 (m), 1286 (s), 1239 (s), 1173 (w), 1118 (m), 1084 (w), 1033 (m), 1015 (w), 1000 (s), 983 (vs), 912 (s), 896 (m), 834 (w), 803 (w), 771 (m), 751 (w), 727 (w), 692 (w), 664 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 1594 (26), 1575 (100), 1473 (6), 1374 (13), 1240 (11), 1176 (12), 1145 (8), 1121 (4), 1085 (3), 1017 (5), 1004 (3), 774 (5), 457 (5), 98 (13), 79 (5) cm−1. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 145.0, 132.8. EA (K2C4H4N10O5, 386.37 g mol−1) calc. (found): C 12.43 (13.60), H 1.04 (1.22), N 36.25 (36.72)%. MS (FAB+) m/z: 39.0 [K+], (FAB−) m/z: 237.2 [C4HN10O3−]. IS: 35 J (<100 μm), FS: >360 N. ESD: 1.5 J.
(rel. int.): 1594 (26), 1575 (100), 1473 (6), 1374 (13), 1240 (11), 1176 (12), 1145 (8), 1121 (4), 1085 (3), 1017 (5), 1004 (3), 774 (5), 457 (5), 98 (13), 79 (5) cm−1. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 145.0, 132.8. EA (K2C4H4N10O5, 386.37 g mol−1) calc. (found): C 12.43 (13.60), H 1.04 (1.22), N 36.25 (36.72)%. MS (FAB+) m/z: 39.0 [K+], (FAB−) m/z: 237.2 [C4HN10O3−]. IS: 35 J (<100 μm), FS: >360 N. ESD: 1.5 J.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3376 (w), 3142 (w), 3087 (w), 2841 (w), 2799 (w), 2652 (w), 2449 (w), 2357 (w), 2343 (w), 2167 (w), 2000 (w), 1799 (w), 1703 (w), 1670 (w), 1648 (w), 1609 (s), 1575 (s), 1546 (s), 1464 (m), 1450 (s), 1427 (s), 1421 (s), 1396 (s), 1370 (s), 1297(m), 1231 (vs), 1195 (w), 1167 (m), 1156 (w), 1144 (w), 1115 (w), 1095 (m), 1035 (w), 1017 (m), 988 (s), 964 (s), 879 (w), 836 (s), 792 (w), 767 (s), 754 (m), 731 (m), 711 (m), 705 (w), 693 (m), 682 (m), 654 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3376 (w), 3142 (w), 3087 (w), 2841 (w), 2799 (w), 2652 (w), 2449 (w), 2357 (w), 2343 (w), 2167 (w), 2000 (w), 1799 (w), 1703 (w), 1670 (w), 1648 (w), 1609 (s), 1575 (s), 1546 (s), 1464 (m), 1450 (s), 1427 (s), 1421 (s), 1396 (s), 1370 (s), 1297(m), 1231 (vs), 1195 (w), 1167 (m), 1156 (w), 1144 (w), 1115 (w), 1095 (m), 1035 (w), 1017 (m), 988 (s), 964 (s), 879 (w), 836 (s), 792 (w), 767 (s), 754 (m), 731 (m), 711 (m), 705 (w), 693 (m), 682 (m), 654 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 1616 (22), 1576 (100), 1549 (35), 1449 (11), 1403 (11), 1372 (3), 1299 (5), 1235 (24), 1195 (29), 1170 (19), 1158 (6), 1147 (6), 1098 (6), 1021 (6), 992 (18), 838 (8), 769 (16), 734 (3), 713 (3), 696 (7), 685 (3), 595 (4), 558 (5), 511 (13), 456 (13), 442 (5), 411 (5), 368 (6), 341 (3), 297 (2), 260 (4), 240 (4), 166 (34), 137 (46), 122 (24), 101 (36), 77 (27) cm−1. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 147.7, 133.2, 130.3, 106.5. MS (FAB+): 39.0 [K+], (FAB−): 253.1 [C4HN10O4−]. EA (K2C4N10O4, 330.30 g mol−1) calc. (found): C 14.55 (14.64), H 0.00 (0.00), N 42.41 (41.38)%. Found: C 14.64, H 0.00, N 41.38%. IS: 10 J (<100 μm). Friction tester: 48 N (<100 μm).
(rel. int.): 1616 (22), 1576 (100), 1549 (35), 1449 (11), 1403 (11), 1372 (3), 1299 (5), 1235 (24), 1195 (29), 1170 (19), 1158 (6), 1147 (6), 1098 (6), 1021 (6), 992 (18), 838 (8), 769 (16), 734 (3), 713 (3), 696 (7), 685 (3), 595 (4), 558 (5), 511 (13), 456 (13), 442 (5), 411 (5), 368 (6), 341 (3), 297 (2), 260 (4), 240 (4), 166 (34), 137 (46), 122 (24), 101 (36), 77 (27) cm−1. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 147.7, 133.2, 130.3, 106.5. MS (FAB+): 39.0 [K+], (FAB−): 253.1 [C4HN10O4−]. EA (K2C4N10O4, 330.30 g mol−1) calc. (found): C 14.55 (14.64), H 0.00 (0.00), N 42.41 (41.38)%. Found: C 14.64, H 0.00, N 41.38%. IS: 10 J (<100 μm). Friction tester: 48 N (<100 μm).
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3210 (w), 3043 (w), 2885 (w), 2663 (m), 1992 (w), 1623 (w), 1602 (w), 1497 (m), 1473 (s), 1434 (m), 1429 (m), 1404 (s), 1376 (w), 1361 (s), 1285 (s), 1245 (s), 1230 (s), 1197 (m), 1180 (m), 1126 (w), 1035 (w), 1009 (m), 1000 (s), 986 (vs), 894 (m), 878 (w), 773 (m), 748 (w), 694 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3210 (w), 3043 (w), 2885 (w), 2663 (m), 1992 (w), 1623 (w), 1602 (w), 1497 (m), 1473 (s), 1434 (m), 1429 (m), 1404 (s), 1376 (w), 1361 (s), 1285 (s), 1245 (s), 1230 (s), 1197 (m), 1180 (m), 1126 (w), 1035 (w), 1009 (m), 1000 (s), 986 (vs), 894 (m), 878 (w), 773 (m), 748 (w), 694 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 1606 (15), 1588 (100), 1477 (5), 1439 (2), 1376 (14), 1289 (2), 1249 (13), 1236 (4), 1183 (16), 1147 (8), 1128 (4), 1089 (5), 1012 (16), 903 (7), 776 (4), 750 (6), 686 (2), 556 (4), 462 (10), 46 (2), 349 (2), 309 (2), 98 (14) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 10.22 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 144.2, 133.9 ppm. EA (C4H8N12O5, 304.18 g mol−1) calc. (found): C 15.79 (16.39), H 2.65 (2.67), N 55.26 (54.23)%. IS: 7 J (<100 μm). FS: 216 N (<100 μm). ESD (<100 μm): 1 J.
(rel. int.): 1606 (15), 1588 (100), 1477 (5), 1439 (2), 1376 (14), 1289 (2), 1249 (13), 1236 (4), 1183 (16), 1147 (8), 1128 (4), 1089 (5), 1012 (16), 903 (7), 776 (4), 750 (6), 686 (2), 556 (4), 462 (10), 46 (2), 349 (2), 309 (2), 98 (14) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 10.22 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 144.2, 133.9 ppm. EA (C4H8N12O5, 304.18 g mol−1) calc. (found): C 15.79 (16.39), H 2.65 (2.67), N 55.26 (54.23)%. IS: 7 J (<100 μm). FS: 216 N (<100 μm). ESD (<100 μm): 1 J.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 2976 (m), 2709 (m), 1696 (w), 1625 (s), 1591 (s), 1559 (s), 1461 (s), 1426 (m), 1399 (m), 1376 (m), 1300 (m), 1232 (vs), 1187 (m), 1020 (m), 996 (s), 964 (s), 822 (m), 757 (m), 704 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 2976 (m), 2709 (m), 1696 (w), 1625 (s), 1591 (s), 1559 (s), 1461 (s), 1426 (m), 1399 (m), 1376 (m), 1300 (m), 1232 (vs), 1187 (m), 1020 (m), 996 (s), 964 (s), 822 (m), 757 (m), 704 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 2986 (13), 1630 (27), 1590 (100), 1567 (29), 1495 (6), 1463 (8), 1398 (14), 1300 (8), 1235 (15), 1212 (44), 1186 (6), 1137 (10), 1102 (5), 1018 (14), 1001 (33), 833 (4), 756 (8), 707 (3), 684 (3), 506 (4), 460 (7). 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 10.27 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 146.4, 134.3, 131.5, 105.6 ppm. EA (C4H10N12O7, 338.20 g mol−1) calc. (found): C 14.21 (14.30), H 2.98 (2.90), N 49.70 (48.43)%. IS: 10 J (100–500 μm). FS: 240 N (100–500 μm).
(rel. int.): 2986 (13), 1630 (27), 1590 (100), 1567 (29), 1495 (6), 1463 (8), 1398 (14), 1300 (8), 1235 (15), 1212 (44), 1186 (6), 1137 (10), 1102 (5), 1018 (14), 1001 (33), 833 (4), 756 (8), 707 (3), 684 (3), 506 (4), 460 (7). 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 10.27 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 146.4, 134.3, 131.5, 105.6 ppm. EA (C4H10N12O7, 338.20 g mol−1) calc. (found): C 14.21 (14.30), H 2.98 (2.90), N 49.70 (48.43)%. IS: 10 J (100–500 μm). FS: 240 N (100–500 μm).
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3134 (w), 3000 (w), 2881 (w), 2796 (w), 1665 (w), 1604 (w), 1594 (w), 1469 (m), 1440 (s), 1405 (s), 1366 (s), 1283 (s), 1229 (vs), 1181 (w), 1133 (w), 1122 (m), 1031 (m), 1014 (w), 1003 (m), 983 (s), 905 (s), 889 (m), 765 (w), 748 (s), 731 (w), 716 (w), 696 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3134 (w), 3000 (w), 2881 (w), 2796 (w), 1665 (w), 1604 (w), 1594 (w), 1469 (m), 1440 (s), 1405 (s), 1366 (s), 1283 (s), 1229 (vs), 1181 (w), 1133 (w), 1122 (m), 1031 (m), 1014 (w), 1003 (m), 983 (s), 905 (s), 889 (m), 765 (w), 748 (s), 731 (w), 716 (w), 696 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 1606 (31), 1594 (100), 1482 (4), 1373 (11), 1235 (20), 1182 (16), 1136 (12), 1123 (4), 1015 (6), 1005 (3), 906 (7), 767 (4), 750 (7), 612 (3), 461 (10), 305 (2), 163 (5), 129 (6), 102 (42). 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 7.18 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 144.8, 133.1 ppm. EA (C4H8N12O3, 272.19 g mol−1) calc. (found): C 17.65 (17.93), H 2.96 (2.95), N 61.75 (61.00)%. IS: 9 J (<100 μm). FS: >360 N (<100 μm). ESD (<100 μm): 1.5 J.
(rel. int.): 1606 (31), 1594 (100), 1482 (4), 1373 (11), 1235 (20), 1182 (16), 1136 (12), 1123 (4), 1015 (6), 1005 (3), 906 (7), 767 (4), 750 (7), 612 (3), 461 (10), 305 (2), 163 (5), 129 (6), 102 (42). 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 7.18 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 144.8, 133.1 ppm. EA (C4H8N12O3, 272.19 g mol−1) calc. (found): C 17.65 (17.93), H 2.96 (2.95), N 61.75 (61.00)%. IS: 9 J (<100 μm). FS: >360 N (<100 μm). ESD (<100 μm): 1.5 J.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3166 (w), 3010 (w), 2892 (w), 2801 (w), 1622 (s), 1583 (m), 1555 (m), 1462 (s), 1426 (s), 1399 (s), 1374 (m), 1297 (m), 1228 (vs), 1181 (w), 1020 (w), 996 (m), 964 (s), 832 (s), 760 (m), 744 (w), 733 (w), 706 (w), 688 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3166 (w), 3010 (w), 2892 (w), 2801 (w), 1622 (s), 1583 (m), 1555 (m), 1462 (s), 1426 (s), 1399 (s), 1374 (m), 1297 (m), 1228 (vs), 1181 (w), 1020 (w), 996 (m), 964 (s), 832 (s), 760 (m), 744 (w), 733 (w), 706 (w), 688 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3031 (3), 1622 (29), 1585 (100), 1556 (20), 1398 (10), 1297 (4), 1229 (14), 1207 (28), 1182 (4), 1134 (9), 1102 (4), 1025 (4), 999 (8), 832 (3), 761 (4), 711 (2), 687 (2), 553 (2), 500 (4). 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 7.22 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 147.2, 133.5, 130.7, 106.1 ppm. EA (C4H8N12O4, 288.18 g mol−1) calc. (found): C 16.67 (16.88), H 2.80 (2.82), N 58.32 (56.18)%. IS: 10 J (<100 μm). FS: 240 N (<100 μm). ESD (<100 μm): 1 J.
(rel. int.): 3031 (3), 1622 (29), 1585 (100), 1556 (20), 1398 (10), 1297 (4), 1229 (14), 1207 (28), 1182 (4), 1134 (9), 1102 (4), 1025 (4), 999 (8), 832 (3), 761 (4), 711 (2), 687 (2), 553 (2), 500 (4). 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 7.22 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 147.2, 133.5, 130.7, 106.1 ppm. EA (C4H8N12O4, 288.18 g mol−1) calc. (found): C 16.67 (16.88), H 2.80 (2.82), N 58.32 (56.18)%. IS: 10 J (<100 μm). FS: 240 N (<100 μm). ESD (<100 μm): 1 J.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3323 (w), 3187 (w), 2839 (m), 2710 (m), 2640 (m), 1604 (m), 1537 (m), 1471 (m), 1422 (w), 1402 (s), 1374 (w), 1361 (m), 1285 (s), 1232 (m), 1222 (s), 1173 (w), 1141 (m), 1115 (s), 1093 (s), 1078 (s), 1009 (w), 999 (m), 982 (s), 962 (vs), 897 (s), 874 (m), 768 (w), 758 (m), 747 (m), 731 (w), 716 (w), 697 (w), 689 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3323 (w), 3187 (w), 2839 (m), 2710 (m), 2640 (m), 1604 (m), 1537 (m), 1471 (m), 1422 (w), 1402 (s), 1374 (w), 1361 (m), 1285 (s), 1232 (m), 1222 (s), 1173 (w), 1141 (m), 1115 (s), 1093 (s), 1078 (s), 1009 (w), 999 (m), 982 (s), 962 (vs), 897 (s), 874 (m), 768 (w), 758 (m), 747 (m), 731 (w), 716 (w), 697 (w), 689 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3326 (3), 3188 (4), 1609 (57), 1587 (100), 1473 (8), 1376 (15), 1235 (16), 1174 (18), 1150 (9), 1137 (5), 1087 (7), 1004 (7), 963 (10), 901 (9), 772 (7), 749 (5), 620 (3), 464 (14), 171 (4), 141 (5), 104 (9), 92 (16). 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 7.09 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 144.8, 133.1 ppm. EA (C4H10N14O3, 302.21 g mol−1) calc. (found): C 15.90 (16.36), H 3.34 (3.27), N 64.89 (64.45)%. IS: 7 J (<100 μm). FS: >360 N (<100 μm). ESD (<100 μm): 1.5 J.
(rel. int.): 3326 (3), 3188 (4), 1609 (57), 1587 (100), 1473 (8), 1376 (15), 1235 (16), 1174 (18), 1150 (9), 1137 (5), 1087 (7), 1004 (7), 963 (10), 901 (9), 772 (7), 749 (5), 620 (3), 464 (14), 171 (4), 141 (5), 104 (9), 92 (16). 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 7.09 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 144.8, 133.1 ppm. EA (C4H10N14O3, 302.21 g mol−1) calc. (found): C 15.90 (16.36), H 3.34 (3.27), N 64.89 (64.45)%. IS: 7 J (<100 μm). FS: >360 N (<100 μm). ESD (<100 μm): 1.5 J.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3563 (w), 3461 (w), 3344 (m), 3331 (m), 3285 (m), 2833 (m), 2725 (m), 2606 (m), 2105 (m), 1613 (s), 1580 (s), 1551 (s), 1512 (s), 1455 (s), 1426 (s), 1413 (s), 1391 (s), 1371 (s), 1345 (m), 1292 (m), 1230 (s), 1108 (m), 1088 (s), 1018 (m), 984 (s), 956 (s), 942 (vs), 820 (s), 773 (m), 760 (s), 746 (m), 732 (m), 704 (m), 688 (m), 679 (m) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3563 (w), 3461 (w), 3344 (m), 3331 (m), 3285 (m), 2833 (m), 2725 (m), 2606 (m), 2105 (m), 1613 (s), 1580 (s), 1551 (s), 1512 (s), 1455 (s), 1426 (s), 1413 (s), 1391 (s), 1371 (s), 1345 (m), 1292 (m), 1230 (s), 1108 (m), 1088 (s), 1018 (m), 984 (s), 956 (s), 942 (vs), 820 (s), 773 (m), 760 (s), 746 (m), 732 (m), 704 (m), 688 (m), 679 (m) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 1613 (19), 1583 (100), 1554 (12), 1457 (4), 1396 (9), 1295 (3), 1237 (11), 1209 (30), 1181 (7), 1152 (7), 1137 (7), 1096 (7), 1018 (4), 989 (11), 946 (4), 764 (10), 734 (3), 707 (3), 692 (3), 556 (4), 500 (7), 456 (11), 439 (5), 366 (3), 324 (6), 295 (2), 159 (14), 110 (46), 88 (45) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 7.16 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 147.1, 133.7, 130.9, 106.1 ppm. MS (FAB+): 33.0 [N2H5+], (FAB−): 253.1 [C4HN10O4−]. EA (C4H10N14O4, 318.21 g mol−1) calc. (found): C 15.10 (15.56), H 3.17 (3.24), N 61.62 (59.96)%. IS: 5 J (<100 μm). FS: 96 N (<100 μm). ESD (<100 μm): 0.5 J.
(rel. int.): 1613 (19), 1583 (100), 1554 (12), 1457 (4), 1396 (9), 1295 (3), 1237 (11), 1209 (30), 1181 (7), 1152 (7), 1137 (7), 1096 (7), 1018 (4), 989 (11), 946 (4), 764 (10), 734 (3), 707 (3), 692 (3), 556 (4), 500 (7), 456 (11), 439 (5), 366 (3), 324 (6), 295 (2), 159 (14), 110 (46), 88 (45) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 7.16 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 147.1, 133.7, 130.9, 106.1 ppm. MS (FAB+): 33.0 [N2H5+], (FAB−): 253.1 [C4HN10O4−]. EA (C4H10N14O4, 318.21 g mol−1) calc. (found): C 15.10 (15.56), H 3.17 (3.24), N 61.62 (59.96)%. IS: 5 J (<100 μm). FS: 96 N (<100 μm). ESD (<100 μm): 0.5 J.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3360 (s), 3206 (m), 3119 (s), 2813 (w), 1691 (w), 1656 (vs), 1591 (m), 1582 (m), 1469 (s), 1432 (w), 1404 (s), 1363 (m), 1291 (s), 1237 (s), 1140 (m), 1126 (m), 1040 (m), 1001 (m), 987 (s), 899 (m), 882 (m), 770 (w), 750 (w), 696 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3360 (s), 3206 (m), 3119 (s), 2813 (w), 1691 (w), 1656 (vs), 1591 (m), 1582 (m), 1469 (s), 1432 (w), 1404 (s), 1363 (m), 1291 (s), 1237 (s), 1140 (m), 1126 (m), 1040 (m), 1001 (m), 987 (s), 899 (m), 882 (m), 770 (w), 750 (w), 696 (w) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3370 (2), 3229 (9), 1597 (9), 1578 (100), 1470 (5), 1368 (19), 1243 (9), 1180 (16), 1151 (6), 1128 (4), 1099 (7), 1010 (24), 906 (5), 777 (6), 752 (2), 542 (6), 462 (10), 293 (6), 136 (2), 106 (2) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 6.63 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 158.1, 144.2, 132.9 ppm. EA (C6H12N16O3, 356.27 g mol−1) calc. (found): C 20.23 (19.56), H 3.40 (3.63), N 62.90 (59.68)%. IS: >40 J (<100 μm). FS: >360 N (<100 μm). ESD (<100 μm): 1.5 J.
(rel. int.): 3370 (2), 3229 (9), 1597 (9), 1578 (100), 1470 (5), 1368 (19), 1243 (9), 1180 (16), 1151 (6), 1128 (4), 1099 (7), 1010 (24), 906 (5), 777 (6), 752 (2), 542 (6), 462 (10), 293 (6), 136 (2), 106 (2) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 6.63 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 158.1, 144.2, 132.9 ppm. EA (C6H12N16O3, 356.27 g mol−1) calc. (found): C 20.23 (19.56), H 3.40 (3.63), N 62.90 (59.68)%. IS: >40 J (<100 μm). FS: >360 N (<100 μm). ESD (<100 μm): 1.5 J.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3428 (s), 3342 (s), 3161 (s), 2793 (m), 2202 (w), 1999 (w), 1640 (vs), 1590 (s), 1553 (s), 1458 (m), 1423 (s), 1400 (m), 1335 (s), 1303 (s), 1243 (s), 1226 (s), 1180 (s), 1134 (m), 1106 (m), 1089 (m), 1026 (m), 1010 (m), 986 (s), 964 (s), 846 (w), 818 (s), 763 (s), 735 (s), 726 (s), 700 (m), 692 (m) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3428 (s), 3342 (s), 3161 (s), 2793 (m), 2202 (w), 1999 (w), 1640 (vs), 1590 (s), 1553 (s), 1458 (m), 1423 (s), 1400 (m), 1335 (s), 1303 (s), 1243 (s), 1226 (s), 1180 (s), 1134 (m), 1106 (m), 1089 (m), 1026 (m), 1010 (m), 986 (s), 964 (s), 846 (w), 818 (s), 763 (s), 735 (s), 726 (s), 700 (m), 692 (m) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3267 (2), 1623 (42), 1594 (100), 1558 (40), 1460 (22), 1434 (12), 1338 (3), 1307 (27), 1234 (9), 1210 (64), 1183 (7), 1136 (15), 1109 (8), 1092 (5), 1028 (7), 1009 (69), 989 (18), 967 (3), 823 (8), 761 (12), 729 (8), 704 (9), 592 (4), 564 (11), 529 (14), 494 (11), 448 (14), 421 (14), 368 (23), 286 (7), 230 (26), 163 (26) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 6.99 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 158.5, 146.9, 133.8, 130.9, 105.9 ppm. MS (FAB+): 60.1 [CH6N3+], 373.1 [M + H+], (FAB−): 253.1 [C4HN10O4−]. EA (C6H12N16O4, 372.26 g mol−1) calc. (found): C 19.36 (19.74), H 3.25 (3.22), N 60.20 (59.93)%. IS: 30 J (100–500 μm). FS: 360 N (100–500 μm). ESD (<100 μm): 1.5 J.
(rel. int.): 3267 (2), 1623 (42), 1594 (100), 1558 (40), 1460 (22), 1434 (12), 1338 (3), 1307 (27), 1234 (9), 1210 (64), 1183 (7), 1136 (15), 1109 (8), 1092 (5), 1028 (7), 1009 (69), 989 (18), 967 (3), 823 (8), 761 (12), 729 (8), 704 (9), 592 (4), 564 (11), 529 (14), 494 (11), 448 (14), 421 (14), 368 (23), 286 (7), 230 (26), 163 (26) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 6.99 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 158.5, 146.9, 133.8, 130.9, 105.9 ppm. MS (FAB+): 60.1 [CH6N3+], 373.1 [M + H+], (FAB−): 253.1 [C4HN10O4−]. EA (C6H12N16O4, 372.26 g mol−1) calc. (found): C 19.36 (19.74), H 3.25 (3.22), N 60.20 (59.93)%. IS: 30 J (100–500 μm). FS: 360 N (100–500 μm). ESD (<100 μm): 1.5 J.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3365 (w), 3155 (w), 1628 (s), 1584 (s), 1462 (s), 1432 (s), 1397 (s), 1372 (m), 1302 (m), 1231 (vs), 1185 (m), 1092 (w), 1027 (w), 990 (m), 967 (s), 818 (s), 765 (s), 747 (m), 726 (m), 696 (m), 677 (m) cm−1. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 146.2, 133.2, 130.4, 105.4 ppm. MS (DEI+): 107.0 [Ag+]. EA (Ag2C4H2N10O5, 485.86 g mol−1) calc. (found): C 9.89 (10.09), H 0.41 (0.41), N 28.83 (28.47)%. IS: 3 J (<100 μm). FS: 16 N (<100 μm).
(rel. int.): 3365 (w), 3155 (w), 1628 (s), 1584 (s), 1462 (s), 1432 (s), 1397 (s), 1372 (m), 1302 (m), 1231 (vs), 1185 (m), 1092 (w), 1027 (w), 990 (m), 967 (s), 818 (s), 765 (s), 747 (m), 726 (m), 696 (m), 677 (m) cm−1. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 146.2, 133.2, 130.4, 105.4 ppm. MS (DEI+): 107.0 [Ag+]. EA (Ag2C4H2N10O5, 485.86 g mol−1) calc. (found): C 9.89 (10.09), H 0.41 (0.41), N 28.83 (28.47)%. IS: 3 J (<100 μm). FS: 16 N (<100 μm).
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3288 (w), 2964 (m), 2683 (m), 2133 (w), 1693 (m), 1618 (s), 1575 (s), 1557 (s), 1454 (s), 1427 (m), 1396 (m), 1377 (m), 1297 (m), 1233 (vs), 1180 (m), 1106 (w), 1014 (w), 990 (m), 965 (s), 826 (s), 750 (s), 734 (m), 678 (m) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3288 (w), 2964 (m), 2683 (m), 2133 (w), 1693 (m), 1618 (s), 1575 (s), 1557 (s), 1454 (s), 1427 (m), 1396 (m), 1377 (m), 1297 (m), 1233 (vs), 1180 (m), 1106 (w), 1014 (w), 990 (m), 965 (s), 826 (s), 750 (s), 734 (m), 678 (m) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 1618 (20), 1586 (100), 1557 (20), 1453 (2), 1400 (11), 1298 (6), 1248 (9), 1209 (33), 1180 (5), 1145 (8), 1104 (5), 1016 (6), 993 (19), 830 (7), 769 (7), 736 (3), 711 (3), 691 (3), 591 (2), 502 (8), 453 (8), 407 (3), 670 (3), 242 (6), 154 (31), 102 (47) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 6.36 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 158.4, 145.6, 135.1, 132.5, 105.0. MS (FAB−): 253.1 [C4HN10O4−], EA (C5H12N14O7, 380.24 g mol−1) calc. (found): C 15.79 (16.23), H 3.18 (2.99), N 51.57 (51.47)%. IS: 40 J (<100 μm). FS: 216 N (<100 μm). ESD (<100 μm): 1.5 J.
(rel. int.): 1618 (20), 1586 (100), 1557 (20), 1453 (2), 1400 (11), 1298 (6), 1248 (9), 1209 (33), 1180 (5), 1145 (8), 1104 (5), 1016 (6), 993 (19), 830 (7), 769 (7), 736 (3), 711 (3), 691 (3), 591 (2), 502 (8), 453 (8), 407 (3), 670 (3), 242 (6), 154 (31), 102 (47) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 6.36 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 158.4, 145.6, 135.1, 132.5, 105.0. MS (FAB−): 253.1 [C4HN10O4−], EA (C5H12N14O7, 380.24 g mol−1) calc. (found): C 15.79 (16.23), H 3.18 (2.99), N 51.57 (51.47)%. IS: 40 J (<100 μm). FS: 216 N (<100 μm). ESD (<100 μm): 1.5 J.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3424 (w), 3359 (m), 3303 (m), 3241 (m), 3101 (w), 1668 (vs), 1620 (s), 1585 (m), 1560 (s), 1455 (m), 1428 (m), 1400 (m), 1363 (m), 1301 (m), 1238 (s), 1227 (s), 1193 (m), 1168 (m), 1095 (m), 1077 (m), 1056 (m), 1024 (w), 1009 (w), 990 (m), 961 (s), 910 (s), 823 (s), 771 (m), 758 (s), 732 (m), 710 (m) cm−1. Raman (1064 nm, 400 mW, 25 °C),
(rel. int.): 3424 (w), 3359 (m), 3303 (m), 3241 (m), 3101 (w), 1668 (vs), 1620 (s), 1585 (m), 1560 (s), 1455 (m), 1428 (m), 1400 (m), 1363 (m), 1301 (m), 1238 (s), 1227 (s), 1193 (m), 1168 (m), 1095 (m), 1077 (m), 1056 (m), 1024 (w), 1009 (w), 990 (m), 961 (s), 910 (s), 823 (s), 771 (m), 758 (s), 732 (m), 710 (m) cm−1. Raman (1064 nm, 400 mW, 25 °C), ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (rel. int.): 3363 (6), 3263 (5), 1622 (31), 1588 (100), 1562 (30), 1456 (9), 1430 (6), 1397 (7), 1365 (5), 1303 (15), 1215 (69), 1170 (10), 1138 (14), 1107 (10), 1025 (6), 1011 (5), 992 (33), 966 (14), 824 (13), 773 (8), 752 (9), 735 (5), 711 (3), 687 (2), 624 (4), 591 (4), 557 (10), 501 (16), 460 (11), 434 (6), 405 (2), 375 (12), 342 (3), 285 (3), 260 (6), 231 (20), 155 (49), 138 (64), 127 (60), 100 (77), 89 (91) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 8.70, 7.26, 6.90, 4.51 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 159.4, 147.0, 133.8, 130.9, 106.0 ppm. MS (FAB+): 75.1 [CH7N4+], 403.2 [M + H+], (FAB−): 253.1 [C4HN10O4−]. EA (C6H14N18O4, 402.29 g mol−1) calc. (found): C 17.91 (18.17), H 3.51 (3.47), N 62.67 (61.54)%. IS: 8 J (<100 μm). FS: 360 N (<100 μm).
(rel. int.): 3363 (6), 3263 (5), 1622 (31), 1588 (100), 1562 (30), 1456 (9), 1430 (6), 1397 (7), 1365 (5), 1303 (15), 1215 (69), 1170 (10), 1138 (14), 1107 (10), 1025 (6), 1011 (5), 992 (33), 966 (14), 824 (13), 773 (8), 752 (9), 735 (5), 711 (3), 687 (2), 624 (4), 591 (4), 557 (10), 501 (16), 460 (11), 434 (6), 405 (2), 375 (12), 342 (3), 285 (3), 260 (6), 231 (20), 155 (49), 138 (64), 127 (60), 100 (77), 89 (91) cm−1. 1H NMR (270 MHz, DMSO-d6, 25 °C), δ: 8.70, 7.26, 6.90, 4.51 ppm. 13C{1H} NMR (270 MHz, DMSO-d6, 25 °C), δ: 159.4, 147.0, 133.8, 130.9, 106.0 ppm. MS (FAB+): 75.1 [CH7N4+], 403.2 [M + H+], (FAB−): 253.1 [C4HN10O4−]. EA (C6H14N18O4, 402.29 g mol−1) calc. (found): C 17.91 (18.17), H 3.51 (3.47), N 62.67 (61.54)%. IS: 8 J (<100 μm). FS: 360 N (<100 μm).
Acknowledgements
Financial support of this work by the Ludwig-Maximilian University of Munich (LMU), the U.S. Army Research Laboratory (ARL) under grant no. W911NF-09-2-0018, the Armament Research, Development and Engineering Center (ARDEC) under grant no. W911NF-12-1-0467, and the Office of Naval Research (ONR) under grant nos. ONR.N00014-10-1-0535 and ONR.N00014-12-1-0538 is gratefully acknowledged. The authors acknowledge collaborations with Dr Mila Krupka (OZM Research, Czech Republic) in the development of new testing and evaluation methods for energetic materials and with Dr Muhamed Suceska (Brodarski Institute, Croatia) in the development of new computational codes to predict the detonation and propulsion parameters of novel explosives. We are indebted to and thank Drs Betsy M. Rice and Brad Forch (ARL, Aberdeen, Proving Ground, MD) for many inspired discussions. We also thank Mr Stefan Huber for sensitivity measurements.Notes and references
- G. A. Parker, G. Reddy and M. A. Major, Int. J. Toxicol., 2006, 25(5), 373–378 CrossRef CAS PubMed.
- N. Fischer, D. Fischer, T. M. Klapötke, D. G. Piercey and J. Stierstorfer, J. Mater. Chem., 2012, 22, 20418–20422 RSC.
- D. Fischer, T. M. Klapötke, D. G. Piercey and J. Stierstorfer, Chem. – Eur. J., 2013, 19, 4602–4613 CrossRef CAS PubMed.
- M. Göbel, K. Karaghiosoff, T. M. Klapötke, D. G. Piercey and J. Stierstorfer, J. Am. Chem. Soc., 2010, 132, 17216–17226 CrossRef PubMed.
- T. I. Godovikova, S. K. Vorontsova, L. D. Konyushkin, S. I. Firgang and O. A. Rakitin, Russ. Chem. Bull., 2010, 58, 406–409 CrossRef PubMed.
- H. Huang, Z. Zhou, L. Liang, J. Song, K. Wang, D. Cao, C. Bian, W. Sun and M. Xue, Z. Anorg. Allg. Chem., 2012, 638, 392 CrossRef CAS.
- C. Grundmann, Chem. Ber., 1964, 97, 575–878 CrossRef CAS.
- C. Grundmann, G. W. Nickel and R. K. Bansal, Justus Liebigs Ann. Chem., 1975, 6, 1029–1050 CrossRef.
- S. M. Aldoshin, Z. G. Aliev, A. A. Astratev, T. K. Goncharov, D. V. Dashko, Yu. M. Milekhin, A. I. Stepanow and N. I. Shishow, Zh. Strukt. Khim., 2013, 54, 399 Search PubMed (J. Struct. Chem.).
- H. Huang, Z. Zhou, L. Liang, J. Song, K. Wang, D. Cao, W. Sun, C. Bian and M. Xue, Chem. – Asian J., 2012, 7, 707 CrossRef CAS PubMed.
- D. Fischer, T. M. Klapötke and J. Stierstorfer, Chem. – Eur. J., 2013, 19, 4602 CrossRef CAS PubMed.
- (a) Test methods according to the UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria, fourth revised edition, United Nations Publication, New York and Geneva 2003; (b) http://www.bam.de ; (c) http://www.reichel-partner.de .
- http://www.ozm.cz .
- (a) M. Sućeska, Calculation of Detonation Parameters by EXPLO5 Computer Program, Mater. Sci. Forum, 2004, 465–466, 325 CrossRef; (b) M. Sućeska, Calculation of the Detonation Properties of C–H–N–O Explosives, Propellants, Explos., Pyrotech., 1991, 16, 197 CrossRef.
- C. Xue, J. Sun, B. Kang, Y. Liu, X. Liu, G. Song and Q. Xue, Propellants, Explos., Pyrotech., 2010, 35, 333–338 CrossRef CAS.
- R. Mayer, J. Köhler and A. Homburg, Explosives, Wiley VCH, Weinheim, 5th edn, 2002 Search PubMed.
- P. Hakey, W. Ouellette, J. Zubieta and T. Korter, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, E64, 1428 Search PubMed.
- C. S. Choi and E. Prince, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1972, 28, 2857 CrossRef CAS.
Footnotes |
| † Dedicated to Dr. Klaus Römer on the occasion of his 75th birthday. |
| ‡ Electronic supplementary information (ESI) available: X-ray diffraction data, computations, and general experimental methods. CCDC 988403–988414. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4nj01351d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |