Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
HSA carbonylation with methylglyoxal and the binding/release of copper(II) ions
Ana Z.
Penezić
,
Vesna B.
Jovanović
,
Ivan D.
Pavićević
,
Jelena M.
Aćimović
and
Ljuba M.
Mandić†
*
Department of Biochemistry, Faculty of Chemistry, University of Belgrade, Studentski trg 12-16, Belgrade 11158, Serbia. E-mail: ljmandic@chem.bg.ac.rs; Tel: +381 11 333 66 76
First published on 24th August 2015
Abstract
The potential of carbonylation with methylglyoxal to alter HSA's binding affinity for copper(II) ions and its influence on the release of copper(II) ions from copper–HSA complexes were studied. The affinity of HSA to coordinate copper(II) decreased upon carbonylation of the Cys34-SH group. Carbonylation of copper–HSA complexes caused a decrease in Cys34-SH content, conformational changes and the release of copper(II) ions. The ratio between the percentage of reduction in the Cys34-SH group content and the percentage of release of copper(II) from complexes is 2.12 ± 0.28. Because the same ratio (1.96 ± 0.36) was obtained upon oxidation of the Cys34-SH group (with no changes in HSA conformation), the binding/release of copper (II) by HSA depended mainly on the redox state of the Cys34-SH group. The contents of Cys34-SH and HSA-bound copper(II) ions in the diabetic group (0.457 ± 0.081 mol SH per mol HSA, 10.7 ± 0.01 mmol per mol HSA, resp.) were significantly lower (p < 0.01) compared to the control group (0.609 ± 0.027 mol SH per mol HSA; 13.4 ± 0.01 mmol per mol HSA, resp.). Very strong correlations between the values for HSA-SH and glycated haemoglobin, HbA1c, (R = −0.803, p < 0.01), and between the values for the HSA-bound copper(II) content and HSA-SH content (R = 0.841, p < 0.002) were found in the diabetic group. Thus, HSA carbonylation leads to decrease in HSA-SH content and to the impairment of its copper(II) binding capacity that could contribute to further enhancement of oxidative and carbonyl stress in diabetes (as well as in other diseases with carbonyl stress).
1 Introduction
Human serum albumin (HSA) is the most abundant serum protein, with several important physiological functions.1,2 Besides regulation of oncotic pressure, it also serves as a transporter of metal ions (Cu, Zn, Fe, Co, Ni etc.), fatty acids, cholesterol, hormones, drugs and bile pigments. HSA contains two binding sites for copper ions, one with high affinity, located at the N-terminus (NT, with 1 pM affinity), and second positioned at the interface of domains I and II (multi-metal binding site, MBS).3,4 Although ceruloplasmin represents a major pool of copper in plasma, HSA, due to its high concentration, is considered to be its second largest pool. About 15% of all blood copper is bound to HSA as Cu(II).4 Copper(II) ion levels are reported to be increased in diabetes and some other pathological states,5,6 and any unbound copper(II) ions in circulation may undergo Fenton reaction causing oxidative stress by the formation of free radicals.7Oxidative and/or carbonyl stress are believed to play an important role in pathogenesis of various diseases e.g. uraemia,8 renal failure, diabetes mellitus9,10etc. as well as in genesis of secondary complications in diabetic patients involving microangiopathies and cardio-vascular complications.11
HSA has 35 Cys residues, 34 of them being involved in 17 intramolecular disulfide bridges, and one, Cys-34, that is redox active. With normal serum concentration between 35 and 50 g L−1, and 70–80% of its Cys34 in the reduced/sulfhydryl form, HSA represents the predominant serum protein and a major plasma antioxidant.1,2,12 Antioxidant properties of HSA depend on nucleophilic properties of Cys34 as well as on copper-binding ability.2,13 Both of these attributes may become impaired when HSA is exposed to increased glycation, leading to protein modification, formation of advanced glycated end-products (AGEs) and protein cross-linking.14–18
Diabetes mellitus is one of the most prevalent chronic diseases, affecting around 360 million people worldwide,19 of which ∼90–95% are categorized as type 2.20 Hyperglycemia in diabetes provokes Maillard reaction, formation of Schiff bases, and Amadori products, and finally leads to generation of AGEs.21
Methylglyoxal (MG) represents a naturally occurring α-oxoaldehyde, generated either non-enzymatic, or from the spontaneous decomposition of triose phosphates, by autoxidation of carbohydrates, and glucose degradation, or by several minor metabolic pathways including the Maillard reaction and lipid peroxidation. The formation rate of MG in normal systems is 120 μmol per day, but several studies have shown that this rate in diabetes is increased by 5- to 6-fold.22,23 Because of its high reactivity (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold more reactive than glucose24), MG represents a potent modifying agent of proteins14,18 and nucleic acids.25
000-fold more reactive than glucose24), MG represents a potent modifying agent of proteins14,18 and nucleic acids.25
Thus, studies related to diabetic pathology reveal the existence of oxidative stress in these patients, decreased content of the Cys34 thiol group,26 elevated levels of serum MG and copper(II) ions, especially in type 2 diabetics.27,28 Recent studies regarding MG as modifying agent of HSA (in vitro and in diabetes), and the ability of glycated HSA to bind of copper(II) ions reported different opposite results.29,30 Besides decrease in HSA-SH group content, carbonylation with MG leads to conformational changes in HSA molecules,31 which could also influence HSA copper binding. Therefore, the goal of this study was to determine the potential of MG, as a modifying agent, to alternate HSA's binding affinity for copper(II) ions, i.e. the potential of reaction of carbonylation to release copper(II) ions from copper–HSA complexes. The changes in the Cys34 thiol group content and in the content of copper(II) ions bound to HSA, and their ratios, as well as the changes in conformation of HSA and copper–HSA complexes during carbonylation in vitro (with MG) and in diabetes type 2, were monitored. Deciphering the effect of HSA modification with MG on its ability to bind and sequester copper(II) ions in circulation could prove useful in treating secondary complications in diabetic patients.
2 Experimental
2.1. Chemicals and instrumentation
All chemicals were purchased from Merck (Darmstadt, Germany) and Sigma-Aldrich Chemie (Steinheim, Germany) unless otherwise noted. The 20% solution of HSA (96% purity, containing 0.40 mol SH per mol HSA) was purchased from Baxter (Vienna, Austria).Spectrophotometric measurements were performed using a Beckman DU-50 spectrophotometer (Fullerton, CA, USA). Fluorescence spectra were obtained on a Fluoromax-4 Jobin Yvon (Horiba Scientific, Japan) spectrofluorimeter.
2.2. Serum samples
Blood samples were collected from patients with type 2 diabetes who were hospitalized due to poor metabolic control (HbA1c >10.0%) and healthy volunteers of appropriate age and sex. Informed consent was sought from all participants. Blood was allowed to clot at room temperature, and serum was separated by centrifugation (4000g, 10 min) and used immediately for HSA isolation.2.3. Isolation of HSA
HSA was isolated from serum by ammonium sulphate (AS) precipitation using a two-step protocol following the method of Jovanovic et al.32 Briefly, a stock solution of saturated AS (pH 7.4) was added to the serum sample until a concentration of 54% AS was reached. The precipitated proteins were removed by centrifugation at 5000g for 10 min and the supernatant containing HSA was collected. In the second step, a stock solution of saturated AS (pH 7.4) was added to the supernatant up to a final concentration of 70% AS. The precipitated HSA was separated by centrifugation at 5000g for 10 min, and the HSA pellet was resuspended in 0.1 M sodium phosphate buffer (pH 7.4). To eliminate AS, HSA solution was further diluted by the same buffer and concentrated by ultra-filtration (Ultracel-10K, Millipore). The obtained HSA solution was used for further analyses.2.4. Thiol quantification
Serum total free thiol groups (protein and nonprotein, i.e., total thiol content) and HSA-SH content were determined spectrophotometrically according to a modified Ellman's method.33 DTNB reagent (5,5′-dithiobis-(2-nitrobenzoic acid), 100 μL of 2 mM solution) was mixed with equal volumes of sample and 1 M Tris buffer (pH 8.0) and brought up to 1000 μL with water. Absorbance was measured after 30 min at room temperature at 412 nm against the sample and reagent blanks. The concentration of thiols was calculated by using the molar extinction coefficient (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 mol L−1 cm−1). Values were expressed in mol L−1 for serum and mol SH per mol HSA.
600 mol L−1 cm−1). Values were expressed in mol L−1 for serum and mol SH per mol HSA.
2.5. HSA–copper(II) content
This was quantified by using bathocuproinedisulfonic acid disodium salt (BCDS) as the chelator. The BCDS–Cu(I) complex exhibits a maximum of absorbance at 480 nm. Absorbance was recorded after mixing the samples with ascorbate (800 μM); in order to reduce Cu(II) to Cu(I) and BCDS (400 μM) against the sample and reagent blanks. Measurements were performed in triplicate and at room temperature. The concentration of copper(II) ions was calculated using a calibration curve prepared for CuSO4 in PBS (5–200 μM, R = 0.9997, P < 0.0001). Values were expressed in mol per mol HSA.2.6. Preparation of highly reduced HSA (mercapto-HSA)
Commercial HSA contains 0.4 mol SH group per mol HSA, so for experiments in which the HSA-SH content needed to be higher than 0.4 mol-SH per mol HSA, commercial HSA was reduced with dithiothreitol. Prior to the reduction, the content of the HSA-SH group was determined. An appropriate amount of HSA was mixed with dithiothreitol at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (molar content of oxidized thiol group
1 (molar content of oxidized thiol group![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dithiothreitol) for 1 h at 37 °C in 0.1 M sodium phosphate buffer, pH 7.4. Subsequently, dithiothreitol was washed away from HSA with 0.1 M sodium phosphate, pH 7.4, using an Ultracel-30K device (Millipore, USA). After this treatment, the HSA-SH content was 0.89 mol SH group per mol HSA.
dithiothreitol) for 1 h at 37 °C in 0.1 M sodium phosphate buffer, pH 7.4. Subsequently, dithiothreitol was washed away from HSA with 0.1 M sodium phosphate, pH 7.4, using an Ultracel-30K device (Millipore, USA). After this treatment, the HSA-SH content was 0.89 mol SH group per mol HSA.
2.7. Preparation of in vitro carbonylated HSA
Carbonylated HSA was prepared by incubating 0.5 mM HSA with MG (10 mM) at 37 °C for 24 h in PBS (pH = 7.4). Following incubation, samples were washed with PBS from any unreacted MG using an Ultracel-30K Millipore ultrafiltration device.2.8. Preparation of HSA–Cu(II) complexes
An aliquot of CuSO4 solution in 10 mM PBS (pH = 7.4) was added to HSA samples (0.5 mM) in order to obtain HSA–Cu(II) complexes (0.05, 0.1 and 0.2 mol of copper(II) per mol HSA for Complexes I, II and III resp.). The mixture was incubated for 40 min at 4 °C in the dark (according to Gryzunov et al.34). Any unbound copper(II) ions were removed by washing the samples five times with PBS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, vol/vol; 10 mM, pH = 7.4) using an Ultracel-30K device (Millipore, USA).
15, vol/vol; 10 mM, pH = 7.4) using an Ultracel-30K device (Millipore, USA).
2.9. HSA assay
HSA concentration was measured by a Biuret reaction,35 using a HSA standard curve (concentration range from 1 to 100 g L−1, R = 0.999).2.10. Fluorescence spectroscopy
The protein concentration used for fluorescence measurements was 2 μM. The spectra were recorded in the wavelength range of 300 to 450 nm following excitation at 295 nm using a quartz cell (1 cm path length) and slit widths (4 nm). Each spectrum was the average of two scans and the respective blanks of PBS were used for the correction of all fluorescence spectra.2.11. Native PAGE
Native-PAGE (polyacrylamide gel electrophoresis) was performed according to the manufacturer's recommendations using a Hoeffer SE 260 electrophoretic unit (San Francisco, CA, USA), and densitometric analyses of gel by using ImageJ software.2.12. Statistical analysis
Data are expressed as mean values ± standard deviation from at least three different experiments. Statistical significances were determined by using Student's t-test (P values less than 0.05 were considered statistically significant), and statistical correlation by determining Pearson's correlation coefficient.3 Results and discussion
3.1. The copper(II) binding affinity of carbonylated HSA
HSA is a metal ion transporter having one high-affinity binding site for copper(II) ions located on the N-terminus. Contradictory results have been observed for copper(II) binding capacity in the amino terminus binding site of glycated HSA.2 Having in mind that unbound copper(II) ions can undergo Fenton/Haaber Weiss reaction leading to free radical production, a decrease in HSA copper binding affinity/capacity would contribute to the development of oxidative stress. Therefore, it is of interest to investigate the copper binding affinity of carbonylated HSA.In order to investigate the influence of HSA carbonylation on copper(II) binding affinity, mercapto-HSA (with 0.879 mol SH per mol HSA) was pre-incubated with 10 mM MG for 24 h at 37 °C. The obtained carbonylated HSA (HSA-MG; with the thiol group content of 0.587 mol SH per mol HSA), as well as each mercapto and commercial HSA (0.400 mol SH per mol HSA) were incubated with three different concentrations of copper(II) ions (0.05, 0.10 and 0.20 mol of copper(II) per mol HSA). These Cu(II) concentrations were used because we wanted to have one (nearly) physiological, one slightly elevated and one supra-physiological saturation in order to be able to relate to physiological and pathological conditions during carbonylation with MG, and see if there is a significant difference in Cu(II) binding capacity of HSA. The content of bound copper in thus formed copper–HSA complexes I, II and III (resp.) was determined (Table 1). The mercapto-HSA sample (with 0.879 mol SH per mol HSA) is able to bind all available copper(II) ions. On the other hand, commercial HSA with 0.400 mol SH per mol HSA binds 14.4%, 15.6% and 29.9% less copper(II) ions than mercapto-HSA. These results suggested that the copper binding capacity of HSA is positively correlated with HSA-SH content, which is in accordance with the results of Zhang and Wilcox36 who found that both in vitro and in vivo Cu(II) ions preferently bind to albumin with reduced Cys34. The redox state of Cys34 was found to affect the chemical environment of His3, located ∼20 A away37 included in copper coordination besides the N-terminal amine and the first two deprotonated amides.
| Copper–HSA complex | The content of Cu(II) ions bound to | Decrease in the content of Cu(II) ions bound to HSA (%) | |||
|---|---|---|---|---|---|
| Mercapto-HSA (mol per mol HSA ± SD) | Commercial HSA (mol per mol HSA ± SD) | HSA-MG (mol per mol HSA ± SD) | Commercial HSA vs. mercapto-HSA | HSA-MGavs. mercapto-HSA | |
| a HSA-MG, HSA carbonylated with methylglyoxal. | |||||
| I | 0.0512 ± 0.0035 | 0.0438 ± 0.0034 | 0.0434 ± 0.0013 | 14.4 | 15.2 |
| II | 0.0959 ± 0.0038 | 0.0809 ± 0.0021 | 0.0810 ± 0.0021 | 15.6 | 15.5 |
| III | 0.2025 ± 0.0042 | 0.1420 ± 0.0064 | 0.1655 ± 0.0019 | 29.9 | 18.2 |
In comparison to mercapto-HSA, carbonylated HSA samples (with 0.587 mol SH per mol HSA) bind copper(II) ions with reduced capacity (15.2%, 15.5% and 18.2% resp.) (Table 1).
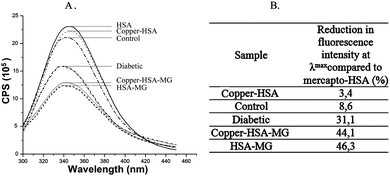
This capacity reduction could be the consequence of decrease in the thiol group content, and also of the conformational changes in HSA (as Lys and Arg residues are also targeted during protein modification with MG).15 The changes in the three-dimensional structure were confirmed by recording the fluorescence emission spectra (Fig. 1).
Due to carbonylation of the HSA molecule, the quenching of internal fluorescence (originating from Trp214 after excitation of HSA molecule at 295 nm) at λem = 346 nm by 46% compared to the unmodified HSA was observed. The differences in fluorescence intensity of HSA and HSA-MG do not arise from changes in their secondary structure (far-UV CD spectra are not shown).
These results confirmed alterations in the capacity of HSA with low HSA-SH content to bind to copper(II) ions. The reaction of carbonylation leads to decrease in HSA copper(II) binding affinity, having important implications considering the involvement of free copper ions in the development of oxidative stress in diabetes (and other diseases with carbonyl stress).
3.2. Does carbonylation of the Cys34 thiol group influence the release of copper(II) from copper–HSA complexes?
To answer this question, mercapto-HSA (with 0.879 mol SH per mol HSA) was first pre-incubated with three concentrations of copper(II) ions (0.05, 0.10 and 0.20 mol of copper(II) per mol HSA). The obtained copper–HSA complexes (I, II and III), as well as mercapto-HSA were subsequently incubated (carbonylated) with 10 mM MG for 24 h at 37 °C (copper–HSA I-MG, copper–HSA II-MG, copper–HSA III-MG and mercapto-HSA-MG). The content of HSA-SH groups and the content of HSA bound copper(II) ions in all the samples were determined (Table 2).| Sample | HSA-SH content following 24 h incubation (mol SH per mol HSA) | Decrease in HSA-SH content compared to mercapto-HSA (%) | Content of HSA bound Cu(II) ion (mol per mol HSA) | Decrease in Cu(II) ion content (%) | |
|---|---|---|---|---|---|
| 0 h | 24 h | ||||
| Mercapto-HSA | 0.800 ± 0.046 | 8.9 | — | — | — |
| Copper–HSA I | 0.818 ± 0.030 | 6.9 | 0.0511 ± 0.0035 | 0.0496 ± 0.0035 | 2.9 |
| Copper–HSA II | 0.788 ± 0.030 | 10.3 | 0.0979 ± 0.0038 | 0.0920 ± 0.0015 | 6.0 |
| Copper–HSA III | 0.746 ± 0.028 | 15.1 | 0.2025 ± 0.0042 | 0.1855 ± 0.0032 | 8.4 |
| Mercapto-HSA-MG | 0.587 ± 0.049 | 33.2 | — | — | — |
| Copper–HSA I-MG | 0.600 ± 0.021 | 31.7 | 0.0511 ± 0.0035 | 0.0432 ± 0.0042 | 15.5 |
| Copper–HSA II-MG | 0.586 ± 0.041 | 33.3 | 0.0979 ± 0.0038 | 0.0814 ± 0.0033 | 16.8 |
| Copper–HSA III-MG | 0.538 ± 0.060 | 38.8 | 0.2025 ± 0.0042 | 0.1675 ± 0.0019 | 17.3 |
The incubation of mercapto-HSA for 24 h at 37 °C, leads to decrease in HSA-SH content for almost 9%, caused by aerobic oxidation of the Cys34 free thiol group. The decrease in the thiol group content obtained for copper–HSA complexes I, II and III was different (6.9%, 10.3% and 15.1% resp.).
These changes implied the existence of the correlation between the thiol group content and the amount of bound copper ions. In order to perceive if this decrease is caused by binding of copper(II) ions (during preparation of complexes I, II and III) or, is the consequence of aerobic oxidation, or both, the content of HSA-SH groups of copper–HSA complexes obtained by incubation of mercapto-HSA with five copper concentrations (0.05, 0.08, 0.10, 0.16 and 0.20 mol Cu(II) per mol HSA) during 40 min, was tested (Table 3).
| Content of Cu(II) ions incubated with mercapto-HSA (mol per mol HSA) | Content of HSA-SH groups after incubation of mercapto-HSA with Cu(II) ions (mol per mol HSA) | Decrease in the HSA-SH group content | |
|---|---|---|---|
| (mol SH per mol HSA) | (%) | ||
| 0.05 | 0.828 ± 0.003 | 0.051 ± 0.003 | 5.8 |
| 0.08 | 0.806 ± 0.005 | 0.073 ± 0.006 | 8.3 |
| 0.10 | 0.802 ± 0.010 | 0.077 ± 0.011 | 8.8 |
| 0.16 | 0.779 ± 0.004 | 0.100 ± 0.003 | 11.4 |
| 0.20 | 0.759 ± 0.008 | 0.120 ± 0.008 | 13.7 |
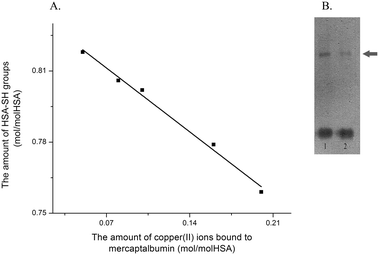
These data showed that loading HSA with copper(II) ions leads to decrease in Cys34 free thiol group content, and that this decrease is proportional to the concentration of copper(II) ions (a Pearson's correlation coefficient R = 0.996, Fig. 2A).
Comparison of thiol group changes obtained after 40 min (complex time preparation, Table 3) and 24 h (incubation time, Table 2) showed no significant differences (5.8%, 8.8% and 13.7% vs. 6.9%, 10.3% and 15.1%). Thus, it could be concluded that binding of copper(II) ions to HSA leads to decrease in the Cys-thiol group content. These results suggest that copper(II) ions, during the course of forming complexes with HSA molecules, could cause the oxidation of Cys34 (and thus affect the redox state of HSA34). Densitometric analyses of gel obtained by native-PAGE of mercapto-HSA and copper–HSA complex II (Fig. 2B) showed an increase of 10% in the intensity of the dimer band in copper–HSA complex II, compared to the dimer band present in mercapto-HSA. This percent corresponds to the percent of decrease in the thiol group content obtained after 24 h of incubation (10.3%), suggesting that copper(II) ions cause oxidation of free Cys34-thiol groups into a disulfide bridge formed between two HSA molecules.
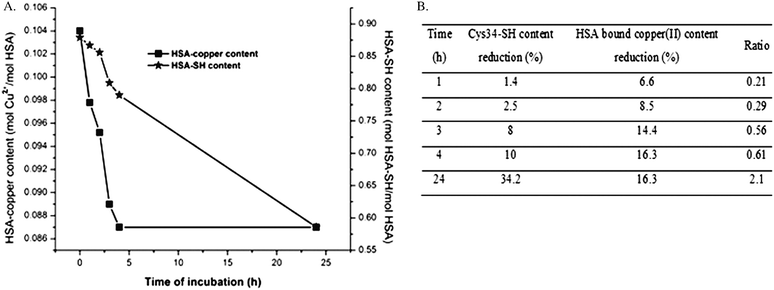
The carbonylation of mercapto-HSA (HSA-SH content 0.879 mol per mol HSA, control) with MG for 24 h caused a decrease in HSA-SH content of 33.2% (Table 2), which is in accordance with the previously published data.15 A similar decrease in the content of HSA-SH groups was obtained for copper–HSA complexes (I-MG, II-MG and III-MG: 31.7%, 33.3% and 38.8%, resp.). However, when the decrease in HSA-SH content caused by the preparation of the copper–HSA complexes (I, II and III), is taken into account, it can be noticed that the percentage of HSA-SH groups which react with MG is lower (24.8%, 23% and 23.6%, resp.). The decrease in the Cys34-thiol group content resulted in the reduction of HSA-bound copper(II) ion content of 15.5%, 16.8% and 17.3% resp. (Table 2). Thus, copper(II) ions are released from HSA molecules during their carbonylation with MG. In order to test if carbonylation of the Cys34 free thiol group is the underlying cause for the release of copper(II) ions from copper–HSA complexes during incubation with MG, the HSA-bound copper(II) and the Cys34-SH group contents were measured in aliquots taken from the incubation mixture (Fig. 3A). The time course curve of Cys34-SH group carbonylation is similar to the copper releasing curve. The release of copper(II) ions occurred in the first three to four hours of the incubation of HSA–copper(II) complex with MG. The ratios between the percentage of reductions (Cys34-SH group content/HSA bound copper) upon HSA carbonylation were in the range from 0.21 to 2.1 (Fig. 3B).
These results show that similar to copper binding, the release of copper(II) from copper–HSA complexes during carbonylation is strongly dependant on the redox state of the Cys34-thiol group. In addition, it should be underlined that the percentage of decrease in HSA bound copper content, obtained during copper binding capacity investigations of carbonylated HSA-MG (15.2%, 15.5% and 18.2% resp., Table 1) and the percentage of copper release from copper–HSA-MG complexes during carbonylation (15.5%, 16.8% and 17.3% resp.) are almost equal. This result would be expected if decrease in thiol group content was considered to be the only cause of observed HSA binding capacity changes, as in both experiments the same concentration of MG was used. Nevertheless, since Cu(II) ion forms strong tetragonal complexes with biological nitrogen ligands (which is important for fast exchange of ligands in terms of intracellular transfers of this metal38) the observed release of copper(II) ions bound to HSA could also be the consequence of HSA conformational changes due to carbonylation. The conformational changes in HSA-MG and copper–HSA-MG obtained by florescence spectroscopy, i.e. quenching of internal fluorescence at λem 346 by 46.3% and 44.1% (resp.) are nearly identical in comparison to unmodified mercapto-HSA (Fig. 1). The ratio between the percentage of reduction in the Cys34 thiol group content due to carbonylation of copper–HSA complexes and the percentage of release of copper from complexes is 2.12 ± 0.28 (Table 4). The value of this ratio, obtained when oxidation of the thiol group (1.96 ± 0.36) occurs, is almost equal to the value obtained after carbonylation of the thiol group with MG. Because the HSA conformation and Cys34-SH accessibility39 are changed during carbonylation, but not after HSA-SH oxidation (Fig. 1), these results indicate that the binding/release of copper (II) ions by HSA depends mainly on the redox state of the free thiol group. Thus, if the Cys34 residue becomes carbonylated with MG, the copper(II) binding capacity of HSA reduces, and copper(II) ions are released from the complex copper–HSA-MG. The increase in flux of MG and the other reactive dicarbonyl compounds (glyoxal and 3-deoxyglucosone) occurring during carbonyl stress (in diabetes, Alzheimer's disease, renal failure, liver cirrhosis, anemia, uremia, and atherosclerosis)40 could lead to the Cys34 side chain carbonylation and therefore to the decrease in HSA-SH and HSA-bound copper contents. This, also, implicates the question of the correlation between these two parameters under real physiological conditions.
| Thiol group | Decrease in HSA-SH content (%) | Decrease in the Cu(II) ion content (%) | Ratio | Ratio mean value |
|---|---|---|---|---|
| Oxidation | 6.9 | 2.9 | 2.38 | 1.96 ± 0.36 |
| 10.3 | 6.0 | 1.71 | ||
| 15.1 | 8.4 | 1.80 | ||
| Carbonylation | 24.8 | 12.9 | 1.92 | 2.12 ± 0.28 |
| 23.0 | 11.5 | 2.00 | ||
| 23.6 | 9.7 | 2.44 | ||
3.3. Changes in the HSA-SH group and HSA-bound copper(II) content in diabetic patients
To perceive the above given conclusion, sera and HSA of 11 patients with diabetes type 2 and 10 healthy persons were analyzed in order to determine the contents of total serum thiols, total serum copper(II), HSA-SH groups, and HSA-bound copper(II) (Table 5).| Diabetic patients | Control | |
|---|---|---|
| *p < 0.05, compared to control group; **p < 0.01, compared to control group. | ||
| n | 11 | 10 |
| HbA1c (%) | 10.25 ± 1.52* | 5.59 ± 0.53 |
| Total serum Cu2+ (μM) | 34.3 ± 8.1 | 28.5 ± 1.7 |
| Total serum-SH (mM) | 0.330 ± 0.059** | 0.427 ± 0.037 |
| HSA-SH (mol SH per mol HSA) | 0.457 ± 0.081** | 0.609 ± 0.027 |
| Copper–HSA (mmol per mol HSA) | 10.7 ± 0.01** | 13.4 ± 0.01 |
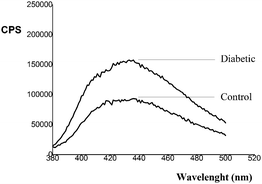
HbA1c content in the diabetic group was significantly higher (p < 0.05) compared to the control group. In the HSA sample isolated from the serum of diabetic persons (diabetic), the quenching of internal fluorescence at λem = 346 nm (originating from Trp214 after excitation of the HSA molecule at 295 nm) by 23%, compared to the HSA of healthy person (control), was observed (Fig. 1). Thus, HSA glycation in hyperglycemia leads to change in HSA conformation. In addition, AGEs show fluorescence after excitation at λexc higher than 290 nm, i.e. they have characteristic excitation at wavelengths in the range of 328 to 370 nm and fluorescence emission from 378 to 440 nm. The monitoring of the fluorescence at λeksc/λem = 365/44041 or 370/43042 was suggested as an indicator of the protein glycation level. Fluorescence emission spectra of HSA samples isolated from the serum of healthy (control) and diabetic persons (Fig. 4), recorded in the wavelength range of 380 to 500 nm following excitation at 365 nm, are the approval that HSA modification occurred.
The total serum copper(II) ion content in diabetic patients was higher than in the control group, which is in accordance with the results of several studies.43,44 In contrast, the total serum thiol content was lower. The HSA-SH group content in the diabetic group is 24.9% lower compared to the control group, which is in accordance with the results from in vitro experiments and with our previously reported HSA-thiol group content decrease in diabetes.32 This difference is statistically significant (p < 0.01). As it was expected, based on in vitro experiments, the content of HSA-bound copper(II) ions in the diabetic group was also significantly lower (p < 0.01) in comparison to the control group. Decreased levels of HSA-bound copper(II) ions in diabetes were also found by Guerin-Dubourg et al.30 When the ratio between the percentages of decrease in the Cys34-SH group content and HSA bound copper content in the diabetic group were compared to the control, the values from 0.51 to 2.54 were obtained (almost identical to the above given in vitro results).
There is a negative correlation between the values for HSA-SH content and the HbA1c fraction, as well as for total serum thiol content and HbA1c fraction (R = −0.803, p < 0.01; R = −0.716, p < 0.05 resp.) in the diabetic group. In contrast, very good positive correlation (R = 0.841, p < 0.002) between the HSA-SH group contents and values of HSA-bound copper(II) ions was found in the diabetic group. These results confirm our hypothesis that modification of the HSA molecule in patients with diabetes type 2 causes a decrease in the Cys34 thiol group content, leading to the impairment of its copper(II) binding capacity. The increase of the free copper(II) ions in serum could contribute to the increase of reactive oxygen species.2 Free copper(II) can react with hydrogen peroxide (via the Fenton reaction) leading to the formation of hydroxyl radicals 60 times faster than iron.2 Free copper(II) ions increase glucose autoxidation, causing formation of the shorter-chain reactive carbonyl compounds,45 and acceleration of alpha-oxoaldehyde formation from early glycation products.46 On the other hand, as the HSA-SH group constitutes an important redox regulator in extracellular compartments,47 its decrease due to carbonylation leads also to the decrease of HSA antioxidative potential.48 Thus, carbonylation of HSA-SH leads to consequences that cause further enhancement of oxidative and carbonyl stress.
4 Conclusions
Overall, the reaction of HSA carbonylation in vitro (with MG) and in vivo (diabetes in which increasing flux of carbonyl species occurs) leads to decrease of its copper(II) binding affinity, i.e. to the release of copper(II) ions from copper–HSA complexes in an extent which depends mainly on the redox state of the Cys34 free thiol group. The decrease of the HSA-SH content and increase of the free copper ions in serum could contribute to further enhancement of oxidative and carbonyl stress.Acknowledgements
The Ministry of Education, Science and Technological Development of Serbia supported this work with Grant No. 172049. The authors acknowledge support of the FP7 RegPot project FCUB ERA GA No. 256716. The EC does not share responsibility for the content of the article. The authors would like to thank Dr Vesna Dimitrijevic Sreckovic from the Institute of Endocrinology, Diabetes, and Metabolic Diseases, Clinical Centre of Serbia for kindly providing the samples.References
-
T. Peters, Jr, All About Albumin: Biochemistry, Genetics, and Medical Applications, Academic Press, Inc., San Diego, California, 1996 Search PubMed
.
- M. Roche, P. Rondeau, N. Ranjan Singh, E. Tarnus and E. Bourdon, FEBS Lett., 2008, 582, 1783–1787 CrossRef CAS PubMed
.
- M. Rozga, M. Sokolowska, A. M. Protas and W. Bal, JBIC, J. Biol. Inorg. Chem., 2007, 12, 913–918 CrossRef CAS
.
- W. Bal, M. Sokolowska, E. Kurowska and P. Faller, Biochim. Biophys. Acta, 2013, 1830, 5444–5455 CrossRef CAS PubMed
.
- R. M. Walter, J. Y. Uriu-Hare, K. Lewis Olin, M. H. Oster, B. D. Anawalt, J. W. Critchfield and C. L. Keen, Diabetes Care, 1991, 14, 1050–1056 CrossRef
.
- T. Gul Kazi, H. I. Afridi, N. Kazi, M. Khan Jamali, M. Bilal Arain, N. Jalbani and G. Abbas Kandhro, Biol. Trace Elem. Res., 2008, 122, 1–18 CrossRef PubMed
.
- M. E. Letelier, S. Sánchez-Jofré, L. Peredo-Silva, J. Cortés-Troncoso and P. Aracena-Parks, Chem.-Biol. Interact., 2010, 188, 220–227 CrossRef CAS PubMed
.
- M. L. Wratten, L. Sereni and C. Tetta, Renal Failure, 2001, 23, 563–571 CrossRef CAS PubMed
.
- K. Oettl, V. Stadlbauer, F. Petter, J. Greilberger, C. Putz-Bankuti, S. Hallstrom, C. Lacknerc and R. E. Stauberb, Biochim. Biophys. Acta, 2008, 1782, 469–473 CrossRef CAS PubMed
.
- Z. Rasheed and R. Ali, Life Sci., 2006, 79, 2320–2328 CrossRef CAS
.
- C. G. Schalkwijk and C. D. A. Stehouwer, Clin. Sci., 2005, 109, 143–159 CrossRef CAS PubMed
.
- P. Rondeau and E. Bourdon, Biochimie, 2010, 93, 645–658 CrossRef PubMed
.
- P. Faure, L. Troncy, M. Lecomte, N. Wiernsperger, M. Lagarde, D. Ruggiero and S. Halimi, Diabetes Metab., 2005, 31, 169–177 CAS
.
- T. W. C. Lo, M. E. Westwood, A. C. McLellan, T. Selwood and P. J. Thornalley, J. Biol. Chem., 1994, 269, 32299–32305 CAS
.
- J. M. Acimovic, B. D. Stanimirovic and Lj. M. Mandic, J. Serb. Chem. Soc., 2009, 74, 867–883 CrossRef CAS
.
- S. W. Vetter and V. S. K. Indurthi, Clin. Chim. Acta, 2011, 412, 2105–2116 CrossRef CAS PubMed
.
- K. Nakajou, H. Watanabe, U. Kragh-Hansen, T. Maruyama and M. Otagiri, Biochim. Biophys. Acta, 2003, 1623, 88–97 CrossRef CAS PubMed
.
- K. Mera, K. Takeo, M. Izumi, T. Maruyama, R. Nagai and M. Otagiri, J. Pharm. Sci., 2010, 99, 1614–1625 CrossRef CAS PubMed
.
- J. Anguizola, R. Matsuda, O. S. Barnaby, K. S. Hoy, C. Wa, E. DeBolt, M. Koke and D. S. Hage, Clin. Chim. Acta, 2013, 425, 64–76 CrossRef CAS PubMed
.
- American Diabetes Association, Diabetes Care, 2008, 31, S55–S60 CrossRef PubMed
.
- N. Ahmed, Diabetes Res. Clin. Pract., 2005, 67, 3–21 CrossRef CAS PubMed
.
- S. A. Phillips and P. J. Thornalley, Eur. J. Biochem., 1993, 212, 101–105 CrossRef CAS PubMed
.
- A. Lapolla, R. Flamini, A. Dalla Vedova, A. Senesi, R. Reitano, D. Fedele, E. Basso, R. Seraglia and P. Traldi, Clin. Chem. Lab. Med., 2003, 41, 1166–1173 CrossRef CAS PubMed
.
- P. J. Thornalley, Ann. N. Y. Acad. Sci., 2005, 1043, 111–117 CrossRef CAS PubMed
.
- P. J. Thornalley, Biochem. Soc. Trans., 2003, 31, 1372–1377 CrossRef CAS
.
- P. Faure, R. Tamisier, J. P. Baguet, A. Favier, S. Halimi, P. Levy and J.-L. Pepin, Eur. Respir. J., 2008, 31, 1046–1053 CrossRef CAS PubMed
.
- P. Matafome, C. Sena and R. Seica, Endocrine, 2013, 43, 472–484 CrossRef CAS PubMed
.
- S. S. Mohanty, V. B. Pinnelli, R. Murgod and D. S. Raghavendra, Asian J. Pharm. Clin. Res., 2013, 6, 188–190 Search PubMed
.
- M. D. Argirova and B. J. Ortwerth, Arch. Biochem. Biophys., 2003, 420, 176–184 CrossRef CAS PubMed
.
- A. Guerin-Dubourg, A. Catan, E. Bourdon and P. Rondeau, Diabetes Metab., 2012, 38, 171–178 CAS
.
- I. D. Pavićević, V. B. Jovanović, M. M. Takić, A. Z. Penezić, J. M. Aćimović and Lj. M. Mandić, Chem.-Biol. Interact., 2014, 224, 42–50 CrossRef PubMed
.
- V. B. Jovanovic, A. Z. Penezic Romanjuk, I. D. Pavicevic, J. M. Acimovic and Lj. M. Mandić, Anal. Biochem., 2013, 439, 17–22 CrossRef CAS PubMed
.
- G. Bulaj, T. Kortemme and D. P. Goldenberg, Biochemistry, 1998, 37, 8965–8972 CrossRef CAS PubMed
.
- Y. A. Gryzunov, A. Arroyo, J.-L. Vigne, Q. Zhao, V. A. Tyurin, C. A. Hubel, R. E. Gandley, Y. A. Vladimirov, R. N. Taylor and V. E. Kagan, Arch. Biochem. Biophys., 2003, 413, 53–66 CrossRef CAS
.
- G. R. Kingsley, J. Biol. Chem., 1939, 131, 197–200 CAS
.
- Y. Zhang and D. E. Wilcox, JBIC, J. Biol. Inorg. Chem., 2002, 7, 327–337 CrossRef CAS PubMed
.
- J. Christodoulou, P. J. Sadler and A. Tucker, Eur. J. Biochem., 1994, 225, 363–368 CrossRef CAS PubMed
.
- L. Banci, I. Bertini, S. Ciofi-Baffoni, T. Kozyreva, K. Zovo and P. Palumaa, Nature, 2010, 465, 645–648 CrossRef CAS
.
- J. M. Aćimović, B. D. Stanimirović, N. Todorović, V. B. Jovanović and Lj. M. Mandić, Chem.-Biol. Interact., 2010, 188, 21–30 CrossRef PubMed
.
- P. J. Thornalley, Drug Metab. Drug Interact., 2008, 23, 125–150 CAS
.
- S. D. Sharma, B. N. Pandey, K. P. Mishra and S. Sivakami, J. Biochem., Mol. Biol. Biophys., 2002, 6(4), 233–242 CAS
.
- H. Zoellner, J. Y. Hou, T. Hochgrebe, A. Poljak, M. W. Duncan, J. Golding, T. Henderson and G. Lynch, Biochem. Biophys. Res. Commun., 2001, 284, 83–89 CrossRef CAS PubMed
.
- A. Sarkar, S. Dash, B. K. Barik, M. S. Muttigi, V. Kedage, J. K. Shetty and M. Prakash, Indian J. Clin. Biochem., 2010, 25, 74–76 CrossRef CAS PubMed
.
- J. Xu, Q. Zhou, G. Liu, Y. Tan and L. Cai, Oxid. Med. Cell. Longevity, 2013, 2013, 635214, DOI:10.1155/2013/635214
.
- S. P. Wolff and R. T. Dean, Biochem. J., 1987, 245, 243–250 CrossRef CAS
.
- P. J. Thornalley, A. Langborg and H. S. Minhas, Biochem. J., 1999, 344, 109–116 CrossRef CAS
.
- S. Carballal, B. Alvarez, L. Turell, H. Botti, B. A. Freeman and R. Radi, Amino Acids, 2007, 32, 543–551 CrossRef CAS PubMed
.
- P. Faure, L. Troncy, M. Lecomte, N. Wiernsperger, M. Lagarde, D. Ruggiero and S. Halimi, Diabetes Metab., 2005, 31, 169–177 CAS
.
Footnote |
| † PhD Department of Biochemistry, Faculty of Chemistry, University of Belgrade, P.O. Box 51, Studentski trg 16, 11158 Belgrade, Serbia. |
| This journal is © The Royal Society of Chemistry 2015 |