Formation of hybrid core–shell microgels induced by autonomous unidirectional migration of nanoparticles†
Jianying
Wang‡
ab,
Kai
Song‡
bc,
Lei
Wang
b,
Yijing
Liu
b,
Ben
Liu
b,
Jintao
Zhu
*a,
Xiaolin
Xie
a and
Zhihong
Nie
*b
aKey Laboratory for Large-Format Battery Materials and System of the Ministry of Education, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan, 430074, China. E-mail: jtzhu@mail.hust.edu.cn
bDepartment of Chemistry and Biochemistry, University of Maryland, College Park, Maryland 20742, USA. E-mail: znie@umd.edu
cSchool of Life Science, Changchun Normal University, Changchun 130032, China
First published on 6th November 2015
Abstract
This Communication describes a facile strategy for the fabrication of inorganic nanoparticle (NP)-loaded hybrid core–shell microgels. The formation of core–shell microgels constitutes a novel mechanism in which the ionic-crosslinking of charged polymers (e.g., alginate) drives the unidirectional migration of NPs towards the centre of droplets. This versatile strategy allows the encapsulation of inorganic NPs with different sizes, shapes and surface properties in the core of the microgels in a single step.
Conceptual insightsHybrid materials combine multiple disparate components (e.g., organic/inorganic) at molecular or nanoscale level into one system. The hybridization endows these materials with desired properties that are otherwise often not available from each constituent. As a result, hybrid materials can find applications in diverse fields. Great efforts have been devoted to the development of new strategy for fabricating hybrid materials such as inorganic nanoparticle/hydrogel particles. The ability to manipulate the distribution of nanoparticles within hydrogels offers new opportunities to control the property of hybrid hydrogels. Particularly, the use of autonomous migration of nanoparticles for fabrication is conceptually novel and may advance the application of hybrid hydrogels. |
Hybridization of microgels with inorganic nanoparticles (NPs) can endow the system with new or advanced functionalities that are often not attainable by any of the individual components.1–11 For instance, the integration of Au NPs in thermoresponsive microgels makes them responsive to light due to the photothermal effect of metal NPs (that is, the conversion of absorbed light to heat).12 As a result of combined characteristic of organic and inorganic components, these hybrid microgels have been used as “smart” vehicles for the light triggered delivery of therapeutic agents.6 When magnetic NPs are introduced, the hybrid microgels can be used as “smart” stabilizers for emulsions and the separation and stability of the emulsions can be manipulated by external magnetic field.1 The optical, magnetic or catalytic properties of hybrid microgels are strongly dependent on the nature and spatial arrangement of NPs within the microgels. Among hybrid microgels with different morphologies, core–shell microgels are particularly attractive for applications in photonics, catalysis, sensing, and biomedicine, due to their unique characteristics, such as responsiveness to stimuli, superior biocompatibility, and tailorable physical property.13–19
Currently, hybrid core–shell microgels are usually fabricated by (i) localized deposition of NPs within templates of core–shell emulsions or organic particles20 and (ii) coating as-synthesized inorganic NPs with polymers.21 The former method involves the synthesis of organic core–shell particles, and the subsequent synthesis of inorganic NPs within the hydrogel reactor.22–24 The latter approach relies on the coating of inorganic NPs with a hydrogel layer by various polymerization techniques (e.g., atom transfer radical polymerization).25–27 These conventional approaches mostly utilize existing surfaces or interfaces between different materials to guide the formation of core–shell structures. However, they either require relatively sophisticated surface modification of NPs or can only be applied to limited types of inorganic NPs.
Herein, we report an unconventional strategy for the fabrication of hybrid core–shell microgels encapsulated with inorganic NPs within cores. This novel method utilizes the unexpected unidirectional migration of inorganic NPs radially towards the centre of an aqueous droplet of hydrogel precursors during the ionic cross-linking of charged polymers (e.g., sodium alginate) by metal ions (e.g., Ca2+) (Fig. 1). The shell thickness and core size of the hybrid particles can be controlled by tuning the concentration of NPs ([NP]) or Ca2+ ions ([Ca2+]). This facile approach does not require laborious surface modification of NPs, as in most conventional methods. This method is applicable to the fabrication of hybrid core–shell microgels from different combination of hydrogels (e.g., alginate, carrageenan, and carboxymethyl cellulose) and inorganic NPs, such as Au NPs, Au nanorods (NRs), silica NPs, Fe3O4 magnetic NPs, CdSe quantum dots (QDs) (see Fig. S1 in ESI†). The use of NPs with different surfaces, sizes, shapes, and compositions dictates the unique features and resulting applications of such hybrid core–shell microgels.
Results and discussion
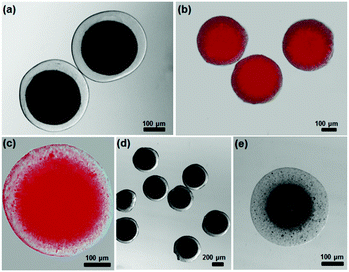
We used a microfluidic technique to generate monodispersed water-in-oil emulsion droplets (see experimental details in ESI†).28–31 An aqueous solution containing sodium alginate, inorganic NPs (e.g., Au NRs), and poly(vinyl alcohol) (PVA) surfactant was used as the droplet phase, while undecanol was used as the continuous phase. When the two liquids were forced through a narrow orifice, monodispersed droplets were generated in the downstream channel (Fig. S2, ESI†). The droplets were collected in a solution of CaI2 (∼0.1–2 wt%) in undecanol. The Ca2+ ions in undecanol slowly diffused into alginate droplets and gradually crosslinked sodium alginate.32 The crosslinking of polymer chains propagated radially from the interface towards the centre of the droplets. It is surprising that the ionic crosslinking of alginate triggered the unidirectional migration of NPs to form a core with concentrated NPs (Fig. 1b). This phenomenon differs from our intuition that gelation traps the NPs locally within the crosslinked polymer networks to produce microgels with uniform distribution of NPs. After the complete solidification, the microgels shrunk by ∼10–50% in diameter, depending on the crosslinking density of the biopolymer.Fig. 2a and b shows representative hybrid core–shell alginate microgels with Au NRs (∼95 nm in length and ∼30 nm in diameter) as cores. The presence of Au NRs in the centre area of microgels provides high contrast between the core and shell phases. The shell thickness of the core–shell microgels can be tailored by controlling the concentration of NRs. Using the same method, spherical Au NPs can be loaded in the core of microgels. The accumulation of NPs in the core was confirmed by imaging the cross-section of a fractured hydrogel particle using scanning electron microscope (SEM) (Fig. 2c). A sharp interface between the core and shell phase can be clearly observed: the majority of NPs are presented in the core, while almost no NPs can be found in the shell region. This approach allows us to readily prepare hybrid core–shell hydrogel particles with controlled shapes (e.g., cylinder-like and hexagonal prism-like microgels) by confining the droplets within a shape-defined geometry (Fig. 2d and e) (see experimental details in ESI†).
The overall diameter of the hybrid core–shell particles can be tuned in the range of 10–900 μm by varying the size of the alginate droplets (Fig. S3a and b, ESI†). The core size of the hybrid microgels can be controlled by varying [Ca2+] or [NP]. When [Ca2+] was increased from 0.1 to 2.0 wt% and other parameters were kept as constant, the ratio of core size to overall size of hybrid microgels (DCore/DEntire) first decreased from ∼0.75 to 0.6 and then increased from ∼0.6 to 0.8 (Fig. S3c, ESI†). This can be explained by the delicate interplay of the driving force for NP migration arising from ionic crosslinking and the friction to slow down the migration of NPs due to the crosslinked polymer networks. On one hand, the fast ionic crosslinking occurred at high [Ca2+] provides strong forces to accelerate the unidirectional movement of NPs.33 On the other hand, the formation of dense polymer networks resulting from fast crosslinking hinders the migration of NPs. Similar trend was also observed when [NP] was increased. The DCore/DEntire first decreased from ∼0.9 to 0.75 and then increased from ∼0.75 to 0.8, when [NP] was increased from 0.001 to 2 mg mL−1 (Fig. 2f).
This general approach is applicable to the generation of core–shell microgels from NPs with different sizes, shapes, compositions, and surface properties (Fig. 3). Firstly, we demonstrated that NPs with size ranging from ∼3 to 350 nm can be encapsulated in the core of microgels in a single step (see images of NPs in Fig. S1, ESI†). They include such as QDs (∼3–5 nm), Fe3O4 NPs (∼10–15 nm), Au NPs (∼30 nm), CaCO3 NPs (∼30–40 nm), and silica NPs (∼350 nm) (Fig. 3a–e). Secondly, NPs with different shapes (e.g., Au NRs and spherical NPs) can be encapsulated into the core of the microgels (Fig. 2a and 3a). Thirdly, it is interesting that the encapsulation of NPs is not sensitive to the surface property of NPs. We demonstrate that all types of NPs can be integrated into alginate microgels as cores (Fig. 2a and 3), regardless of whether they are positively charged (e.g., Au NRs, CaCO3 NPs, or Fe3O4 NPs), negatively charged (e.g., Au NPs, QDs), or neutral (e.g., silica NPs) (see zeta potential measurements in Table S1 in ESI†). Moreover, other negatively charged biopolymers (e.g., I-carrageenan and sodium carboxymethylcellulose) can also be used for generating core–shell structures (Fig. S4, ESI†). The ability to integrate various NPs into core–shell microgels offers us a simple tool to tailor the multi-functionality of the particles (Fig. S5, ESI†). The hybrid microgels exhibit good stability in some organic solvents (e.g., ethanol) or acidic aqueous solution (e.g., pH = 1) for weeks. However, when they are placed in an aqueous salt solution (NaCl at 100 mM), the hybrid microgels slowly dissolved after about 24 h, due to the dissociation of ionic bonding between polymers at high ionic strength.34
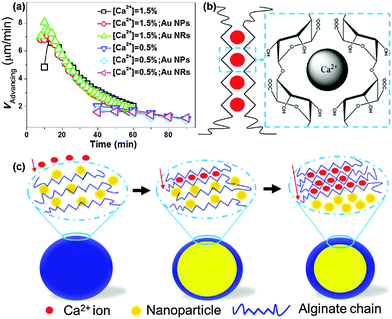
To understand the formation mechanism of hybrid microgels with core–shell structures, we studied the kinetics of the shell-forming process (Fig. S6, ESI†). The propagating front of the gel was carefully measured as a function of time (t). Fig. 4a summarizes the advancing rate of the gel front (vAdvancing) for three cases: (i) pure biopolymer system in the absence of NPs; (ii) system with the presence of positively charged Au NRs; and (iii) system with the presence of negatively charged Au NPs. We observed that the propagating front of crosslinked gels was consistent with the back of the migration line of NP swarms, when NPs were used. Moreover, our control experiment showed that slow UV-initiated polymerization of droplets containing N-isopropyl acrylamide, N,N′-methylene-bis-acrylamide and initiator (Irgacure 2959) did not cause the unidirectional migration of NPs to produce hybrid core–shell microgels (Fig. S7 and S8, ESI†). Together these results suggest that the ionic crosslinking of biopolymers drives the unidirectional movement of NPs towards the centre of the droplet to form cores.
Numerically, vAdvancing can be derived based on the diffusion and reaction stoichiometry.35 The flux of alginate (Ja) and flux of Ca2+ (Jc) consumed by the reaction zone in the vicinity of the gel front are given by:
 | (1) |
| Ja = vAdvancing·Ao | (2) |
| Jc = Nc·Ja | (3) |
 | (4) |
 | (5) |
First of all, the vAdvancing exponentially decays as a function of reaction time from eqn (5) due to the reduced diffusion rate of Ca2+ through crosslinked alginate gels, which is in good agreement with experimental measurements (Fig. 4a). Secondly, both numerical and experimental results indicate that the vAdvancing increased with the increase in the initial concentration of Ca2+ ions (Co) (Fig. 4a). More importantly, we observed that the presence of NPs, and the size and surface property of NPs do not affect the vAdvancing. The vAdvancing of positively or negatively charged NPs are almost consistent with that of pure biopolymer system at various concentrations of Ca2+ ions. This result ruled out the possible diffusiophoresis mechanism in which a charged colloidal particle undergoes diffusiophoretic migration under the electric field arising from the flux of charged ions.36 We are aware that the possible absorption of negatively charged sodium alginate on both positively charged and neutral NPs may alternate the charges on NPs. It is noted that the overall charge of droplets are negative, regardless of the charges carried by NPs (Tables S2 and S3, ESI†).
In the light of current “egg-box” model – coordination between a single Ca2+ cation and four sugar units from two neighboring polymer chains (Fig. 4b) – for ionic crosslinking of hydrogels,37–40 we propose a possible mechanism for the formation of core–shell hydrogel structures through the unidirectional migration of NPs (Fig. 4c). Upon the gradual diffusion of Ca2+ ions into the droplets, alginate chains first form “egg-box” structures and further produce nanofibrils with a diameter of ∼10–15 nm. The long axis of the nanofibrils is perpendicular to the diffusion direction of Ca2+ flux. The formation of fibrils and their orientation has been previously confirmed in different ionic-crosslinked hydrogel systems (e.g., alginate, carrageenan, carboxymethylcellulose, and chitosan) by small-angle X-ray scattering.41 The “zipping” of polymer chains to form “egg-box” structures and nanofibrils pushes NPs to migrate along the direction of Ca2+ flux, that is, the centre of droplets. However, the exact interactions underlying this process are still not clear, and further investigation is needed in future work.
Conclusions
In summary, we have demonstrated an unconventional approach for the preparation of hybrid core–shell hydrogel particles with different shapes and morphologies. The unidirectional NP immigration induced by ionic-crosslinking of polymers distinguishes our technique from any existing approaches. This method we developed shows at least three advantages over conventional techniques: (i) it is simple and robust, and does not require any sophisticated surface modification of NPs; (ii) it can be applied to the fabrication of hybrid core–shell microgels from different combinations of inorganic NPs and ionic polymers; and (iii) it offers excellent control over the overall size, shell thickness, shape, and composition of hybrid microgels. The unique capability offered by this work will potentially advance the application of hybrid core–shell microgels in diverse areas, such as photonics, sensing, biomedicine, catalysts, and micro-motor (Fig. S9, ESI†).Acknowledgements
Z. N. acknowledges the support of NSF Career Award (DMR-1255377) and startup funds from University of Maryland. K. S. acknowledges the funding provided by NSFC (11204021). J. Z. gratefully acknowledges funding provided by MOST (973 program, 2012CB821500), NSFC (51525302, 51173056 and 91127046). We also acknowledge the support of Maryland NanoCenter and its NispLab. The NispLab is supported in part by the NSF as a MRSEC Shared Experimental Facilities.Notes and references
- M. Das, H. Zhang and E. Kumacheva, Annu. Rev. Mater. Res., 2006, 36, 117 CrossRef CAS.
- L. A. Lyon and A. Fernandez-Nieves, Annu. Rev. Phys. Chem., 2012, 63, 25 CrossRef CAS PubMed.
- M. Karg and T. Hellweg, Curr. Opin. Colloid Interface Sci., 2009, 14, 438 CrossRef CAS.
- M. Karg, Colloid Polym. Sci., 2012, 290, 673 CAS.
- J. Pérez-Juste, I. Pastoriza-Santos and L. M. Liz-Marzán, J. Mater. Chem. A, 2013, 1, 20 Search PubMed.
- M. Das, L. Mordoukhovski and E. Kumacheva, Adv. Mater., 2008, 20, 2371 CrossRef CAS.
- Z. X. Guo, M. Zhang, L. B. Zhao, S. S. Guo and X. Z. Zhao, Biomicrofluidics, 2011, 5, 026502 CrossRef PubMed.
- C. H. Yeh, Q. Zhao, S. J. Lee and Y. C. Lin, Sens. Actuators, A, 2009, 151, 231 CrossRef CAS.
- J. M. Köhler, A. März, J. Popp, A. Knauer, I. Kraus, J. Faerber and C. Serra, Anal. Chem., 2013, 85, 313 CrossRef PubMed.
- J. W. Kim, A. S. Utada, A. Fernández-Nieves, Z. B. Hu and D. A. Weitz, Angew. Chem., Int. Ed., 2007, 46, 1819 CrossRef CAS PubMed.
- Y. J. Zhao, H. C. Shum, H. S. Chen, L. L. A. Adams, Z. Z. Gu and D. A. Weitz, J. Am. Chem. Soc., 2011, 133, 8790 CrossRef CAS PubMed.
- B. M. Budhlall, M. Marquez and O. D. Velev, Langmuir, 2008, 24, 11959–11966 CrossRef CAS PubMed.
- W. T. Wu, T. Zhou, A. Berliner, P. Banerjee and S. Q. Zhou, Chem. Mater., 2010, 22, 1966 CrossRef CAS.
- D. Suzuki and H. Kawaguchi, Langmuir, 2006, 22, 3818 CrossRef CAS PubMed.
- Y. Lu, S. Proch, M. Schrinner, M. Drechsler, R. Kempeb and M. Ballauff, J. Mater. Chem., 2009, 19, 3955–3961 RSC.
- S. Shi, L. Zhang, T. Wang, Q. Wang, Y. Gao and N. Wang, Soft Matter, 2013, 9, 10966 RSC.
- R. A. Álvarez-Puebla, R. Contreras-Cáceres, I. Pastoriza-Santos, J. Pérez-Juste and L. M. Liz-Marzán, Angew. Chem., Int. Ed., 2008, 48, 138 CrossRef PubMed.
- W. T. Wu and S. Q. Zhou, Nano Rev., 2010, 1, 5730 Search PubMed.
- A. K. Gaharwar, N. A. Peppas and A. Khademhosseini, Biotechnol. Bioeng., 2014, 111, 441 CrossRef CAS PubMed.
- J. Zhang, S. Xu and E. Kumacheva, J. Am. Chem. Soc., 2004, 126, 7908 CrossRef CAS PubMed.
- R. Contreras-Cáceres, A. Sánchez-Iglesias, M. Karg, I. Pastoriza-Santos, J. Pérez-Juste, J. Pacifico, T. Hellweg, A. Fernández-Barbero and L. M. Liz-Marzán, Adv. Mater., 2008, 20, 1666 CrossRef.
- D. Suzuki and H. Kawaguchi, Colloid Polym. Sci., 2006, 284, 1443 CAS.
- B. Kim, H. Lee, J. Kim and S. H. Kim, Chem. Commun., 2013, 49, 1865 RSC.
- W. C. Jeong, S. H. Kim and S. M. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 826 CAS.
- M. Karg and T. Hellweg, J. Mater. Chem., 2009, 19, 8714 RSC.
- D. J. Kim, S. M. Kang, B. Kong, W. J. Kim, H. J. Paik, H. Choi and I. S. Choi, Macromol. Chem. Phys., 2005, 206, 1941 CrossRef CAS.
- M. Karg, S. Jaber, T. Hellweg and P. Mulvaney, Langmuir, 2011, 27, 820 CrossRef CAS PubMed.
- J. T. Zhu and R. C. Hayward, Angew. Chem., Int. Ed., 2008, 47, 2113 CrossRef CAS PubMed.
- H. Zhang, E. Tumarkin, R. Peerani, Z. H. Nie, R. M. A. Sullan, G. C. Walker and E. Kumacheva, J. Am. Chem. Soc., 2006, 128, 12205 CrossRef CAS PubMed.
- E. Tumarkin and E. Kumacheva, Chem. Soc. Rev., 2009, 8, 2161 RSC.
- H. Zhang, E. Tumarkin, R. M. A. Sullan, G. C. Walker and E. Kumacheva, Macromol. Rapid Commun., 2007, 28, 527 CrossRef CAS.
- J. Y. Sun, X. Zhao, W. R. K. Illeperuma, O. Chaudhuri, K. H. Oh, D. J. Mooney, J. J. Vlassak and Z. G. Suo, Nature, 2012, 489, 133 CrossRef CAS PubMed.
- M. Golmohamadi and K. J. Wilkinson, Carbohydr. Polym., 2013, 94, 82 CrossRef CAS PubMed.
- S. K. Bajpai and S. Sharma, React. Funct. Polym., 2004, 59, 129 CrossRef CAS.
- T. Braschler, A. Valero, L. Colella, K. Pataky, J. Brugger and P. Renaud, Anal. Chem., 2011, 83, 2234 CrossRef CAS PubMed.
- B. Abécassis, C. Cottin-Bizonne, C. Ybert, A. Ajdari and L. Bocquet, Nat. Mater., 2008, 7, 785 CrossRef PubMed.
- G. T. Grant, E. R. Morris, D. A. Rees, P. J. C. Smith and D. Thom, FEBS Lett., 1973, 32, 195 CrossRef CAS.
- E. R. Morris, D. A. Rees, D. Thom and J. Boyd, Carbohydr. Res., 1978, 66, 145 CrossRef CAS.
- L. B. Li, Y. P. Fang, R. Vreeker and I. Appelqvist, Biomacromolecules, 2007, 8, 464 CrossRef CAS PubMed.
- P. Sikorski, F. Mo, G. Skjåk-Bræk and B. T. Stokke, Biomacromolecules, 2007, 8, 2098 CrossRef CAS PubMed.
- Y. Maki, K. Ito, N. Hosoya, C. Yoneyama, K. Furusawa, T. Yamamoto, T. Dobashi, Y. Sugimoto and K. Wakabayashi, Biomacromolecules, 2011, 12, 2145 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5mh00024f |
| ‡ These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |