An ultrastable porous metal–organic framework luminescent switch towards aromatic compounds†
Fei-Yan
Yi
a,
Ying
Wang
a,
Jian-Ping
Li
a,
Dai
Wu
a,
Ya-Qian
Lan
*b and
Zhong-Ming
Sun
*a
aState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, 5625 Renmin Street, Changchun, Jilin 130022, P. R. China. E-mail: szm@ciac.ac.cn; Web: http://zhongmingsun.weebly.com
bSchool of Chemistry and Materials Science, Nanjing Normal University, Nanjing 210023, P. R. China. E-mail: yqlan@njnu.edu.cn
First published on 27th November 2014
Abstract
In this work, a highly stable MOF luminescent switch {Cd3(L)(bipy)2·4DMA}n (1) has been successfully constructed, which exhibits clear fluorescence enhancement and “turn-off” quenching responses for benzene and nitrobenzene vapors, respectively, with high selectivity and sensitivity, as well as being fully reusable. Remarkably, the porous MOF (1) remains intact in aqueous solution over an extensive pH range of 2–13. This MOF sensor realizes fast detection for benzene vapor with a response time of less than one minute and ∼8-fold fluorescence enhancement. Furthermore, it as a porous multifunctional MOF also shows fully reversible adsorption behaviour for benzene vapor at room temperature. Thus the MOF material will be a promising luminescent sensor and adsorbent material for benzene vapor with important practical applications from environmental and health points of view.
Conceptual insightsFluorescence chemosensors as an expedient detection technique have been widely used for the detection of common volatile organic compounds (VOCs). They have many advantages over other sophisticated electrochemical devices, such as high signal output, simple detection, low expense, and reliability. Metal-organic framework (MOF) materials are promising luminescent sensors as their structures and functions are systematically and predictably designable and readily modulated. The reported MOF sensors have also verified their effectiveness, but most of those explored thus far show only a single “turn-off” or “turn-on” luminescence response for solvent solution. These are not efficient enough for detecting trace solvent vapors. In this paper we successfully synthesized an ultrastable MOF sensor, which realizes excellent ‘on–off’ switch-functions for the probing of benzene and nitrobenzene vapor. We investigate its various parameters to assess the practicality of the sensor, such as selectivity, sensitivity, response rate, repeatability and stability. This MOF-based luminescent switch opens up a new sensor platform with unprecedented practical applications. |
Introduction
Rapid detection and reduction of volatile organic compounds (VOCs) are very important subjects of widespread societal concern related to environmental and health issues.1 The release of VOCs as main sources of atmospheric pollution such as aromatic species (benzene, nitroaromatic explosives, etc.) also poses various threats to homeland security. So it is a critical and significant task to selectively recognize these harmful small molecules, and subsequently to be efficient enough to eliminate them. Particularly, benzene as a colourless and highly volatile liquid can lead to the reduction of leukocytes, and even leukaemia,2 so the determination of indoor benzene vapor is extremely important. Till now, besides sophisticated and expensive electrochemical devices,3 some metal coordination and other reported systems, such as organic–inorganic hybrids, as expedient luminescent sensing methods have been used in the detection of VOCs,4 but it has not been reported that a practical sensory material can effectively detect benzene vapor with high sensitivity and fast response. Metal–organic frameworks (MOFs),5 also known as porous coordination polymers, are attractive for these purposes because (1) their structural and chemical tunability can afford good selectivity through pore sieving functions with different pore sizes or host framework–guest interactions; (2) MOFs with high internal surface areas can concentrate analytes to high density, thereby decreasing detection limits and exhibiting high sensitivity; (3) another striking feature of porous MOFs is their ability to remove polluting guest small molecules as adsorbents. Therefore, MOFs as fluorescent sensing materials are of considerable interest and have been widely explored by researchers.6–8 Furthermore, MOF-based fluorescent sensors exhibit many advantages such as high signal output, simple detection, low cost, and good reliability, which are of great significance for practical use of the sensors. Although there are a number of reports on MOF sensors or guest-dependent luminescence for common small molecular solvents (for example, Li and Chen et.al.9 have reported “turn-off” luminescence sensors for nitrobenzene based on a quenching mechanism), fluorescent sensing of trace solvent vapors is still challenging due to their low vapor pressures at room temperature, so the luminescence detection in the vapor phase is much more difficult than in the liquid form.10 Fluorescence enhancement is more attractive and rare; a MOF-based luminescent switch showing clearly two functions of fluorescence enhancement and quenching effects has not been explored until now.9f So the exploitation of novel and efficient MOF sensory on–off materials for the detection of vaporized analytes is progressing with an extraordinary effort. Herein, we report a porous Cd-MOF as a luminescent switch exhibiting significant fluorescence enhancement for benzene vapor and a turn-off quenching effect for nitrobenzene vapor. This MOF material features very fast response rate, full reusability, and high sensitivity. Notably, it exhibits excellent water stability and chemical stability. Furthermore, it shows fully reversible adsorption behavior for benzene vapor at room temperature. To the best of our knowledge, no available literature thus far has investigated a MOF sensory material showing high selectivity and sensitivity for trace benzene vapor based on significant fluorescence enhancement.9f,11Experimental
Synthesis of {Cd3(L)(bipy)2·4DMA}n (1)
A mixture of Cd(NO3)2·4H2O (0.12 mmol, 37.0 mg), H6L (0.04 mmol, 39.0 mg) and bipy (0.12 mmol, 18.7 mg) in mixed N,N′-dimethylacetamide (DMA, 6 mL)/distilled water (H2O, 1 mL) solvents was sealed in a 20 mL pressure-resistant Teflon-lined stainless steel vessel, and heated in an oven to 100 °C for 3 days, and then slowly cooled to room temperature. The colorless rodlike crystals of 1 were obtained in a yield of 66.1 mg (84% based on CdII), and washed with distilled water (H2O). The purity was confirmed by PXRD (see the ESI†). Anal. Calcd (%) for 1 C88H92Cd3N8O23 (Mr = 1966.9): C, 53.74; H, 4.71; N, 5.70. Found: C, 53.91; H, 4.84; N, 5.66. IR peaks (cm−1) for 1: 3432 (w), 3097 (w), 2942 (w), 2879 (w), 1671 (m), 1604 (s), 1512 (vs), 1415 (m), 1384 (m), 1332 (w), 1296 (s), 1250 (vs), 1162 (m), 1106 (m), 1049 (m), 998 (m), 957 (m), 905 (m), 843 (w), 813 (m), 766 (w), 735 (w), 658 (m).Fluorescence measurements
The luminescent properties of Cd-MOF (1) were examined in the solid state, in organic solvent suspensions and vapors at room temperature. The photoluminescence (PL) excitation and emission spectra were recorded on a Hitachi F-7000 spectrophotometer equipped with a 150 W xenon lamp as the excitation source. Prior to the measurements, some preparatory work was done. The as-synthesized sample of 1 was immersed into anhydrous methanol for 3 days, methanol was refreshed three times during the exchange. Then similar immersion was utilized to treat the sample with dichloromethane to remove methanol molecules. After the removal of dichloromethane by centrifuging, the wet sample was dried under vacuum at 80 °C for 15 h to yield activated 1 (denoted as 1a). Solid samples of activated 1a were ground into powder and used for vapor sensing experiments.1a (30 mg) was placed into a glass tube (5 mL), then put under different solvent vapors for 24 h, including benzene, benzene-d6 (C6D6), toluene, 1,2-dichlorobenzene, 4-chlorotoluene, bromobenzene, nitrobenzene, nitromethane, methanol, ethanol, CH2Cl2, CCl4, 2-propanol, phenylmethanol and H2O. Subsequently the sample tube was taken out and quickly sealed, then the emission spectra were recorded.
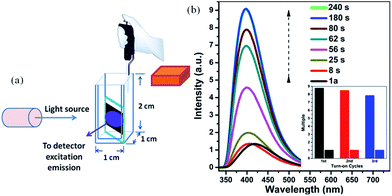
The 1a sample used as in situ time-dependent photoluminescence sensor for solvent vapors was prepared as follows. A ground sample of 1a (100 mg) was pressed into a firm sheet, which was attached to a quartz slide and placed into a cuvette about 1.2 cm from the bottom. The cuvette containing the sensor slide was positioned on the solid sample holder of a Hitachi F-7000 spectrophotometer. The solid state emission spectra of firm sheet sample were recorded as an initial standard, then ∼120 μL benzene (or benzene-d6, nitrobenzene) solution was carefully added into the cuvette. The volume of cuvette used was 2 cm3 with a length and width of 1 cm and a height of 2 cm. So the concentration of the targeted solvent vapor in the cuvette was about 52.6 mg cm−3 (0.67 mmol cm−3).
Results and discussion
The Cd-MOF sensory material was synthesized by mixing a hexacarboxylate ligand H6L (hexa[4-(carboxyphenyl)oxamethyl]-3-oxapentane acid) (Scheme S1†) with Cd(NO3)2·4H2O and coligand bipy (bipy = 2,2′-bipyridine) via a solvothermal method in mixed solvent N,N′-dimethylacetamide (DMA)/distilled water (H2O) at 100 °C for 3 days. It was formulated as {Cd3(L)(bipy)2·4DMA}n (1) based on elemental analysis, single-crystal X-ray diffraction study, thermogravimetric analysis (TGA), and powder XRD analysis. Compared with the previously reported ligands, H6L is flexible and can be restricted to different functional conformations during the formation of MOFs, leading to different fluorescent responses. The constructed flexible framework is very sensitive to external stimuli: it can shrink or expand (breathing effect) for the removal or addition of guest species, making it effective as a fluorescent sensor.Single-crystal X-ray diffraction analysis reveals that 1 crystallizes in a monoclinic C2/c space group (Table S1†).‡ In 1, two Cd(1) situated at the two ends and one Cd(2) at the center form a trinuclear secondary building unit (SBU) (CdII3) (Fig. 1a). In this SBU, Cd(1) is sevenfold-coordinated by two N atoms of one 2,2′-bipy ligand and five carboxylate oxygen atoms from three L6− ligands in a distorted {CdN2O5} pentagonal bipyramid geometry. The Cd(2) ion lies at an inversion centre and thus has 1/2 occupancy, adopting the typical {CdO6} octahedral coordination mode with six carboxylate oxygen atoms of anti–anti coordination conformations from four L6− ligands (Fig. S1†). The bond lengths of Cd–O and Cd–N in the range 2.166(7)–2.587(6) Å and 2.330(9)–2.392(11) Å, respectively, are comparable to reported values.12 Each unique L6− ligand with six carboxylate arms links ten Cd atoms by four μ2-η1,η2 carboxylate groups and two μ2-η1,η1 ones. Each SBU is connected to four adjacent units via four L6− ligands to further form a 2D double layer (Fig. S2†), which stack together to give the overall 3D structure with one-dimensional (1D) open channels having approximates sizes of 16.3 × 11.7 Å2 and 11.5 × 10.9 Å2 along the c-axis and 5.1 × 8.5 Å2 along the b-axis (measured between opposite atoms without taking van der Waals radii of the atoms into account) (Fig. 1b and c). The free DMA guest molecules reside in the channels (Fig. S3†). The overall structure can be simplified into a (4,4)-connected uninodal sql double layer (Fig. S4†) analyzed by the freely available computer program TOPOS.13 The solvent-accessible volume was calculated to be 43.3% by PLATON.14
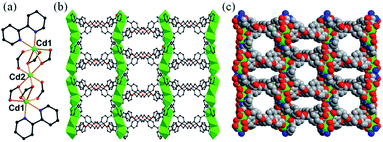 | ||
| Fig. 1 (a) CdII3 SBU. (b) View of the 3D porous framework along the c-axis. (c) The face-filling representation of 1. Color mode: Cd, green; C, grey; N, blue; O, red. | ||
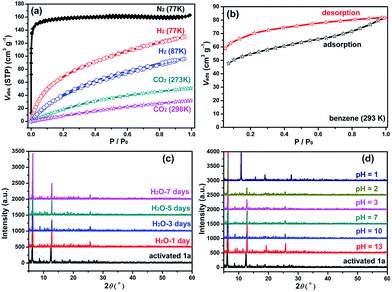
The TGA result of activated 1 (see the ESI and Fig. S5†) indicates 1 may completely release its free solvent molecules to form guest-free 1 (referred to as 1a hereafter). The resulting 1a is thermally stable up to 265 °C. Adsorption and desorption isotherms of N2 (Fig. 2a) for 1a exhibit a single step type I curve with Brunauer–Emmett–Teller (BET) and Langmuir surface areas of 475.0 and 698.6 m2 g−1, respectively, as well as a total pore volume of 0.25 cm3 g−1. The pore size distribution (Fig. S6†) is around 8.6–9.3 Å, matching well with the crystal structure model. The adsorption isotherms of H2 at 77 and 87 K, and of CO2 at 273 and 298 K, were also measured up to 1 atm (Fig. 2a). They show smooth type-I isotherms with uptakes of 129.8 cm3 g−1 at 77 K and 96.0 cm3 g−1 at 87 K for H2, 50.9 cm3 g−1 at 273 K and 32.0 cm3 g−1 at 298 K for CO2. All of the above results demonstrate the robustness and the permanent microporous nature of 1a. More interesting is that 1 can be regenerated by soaking 1a in DMA. Such release and capture for solvent molecules are desirable characteristics, which prompted us to examine its ability as a potential sensory material and adsorbent for solvents. In addition, 1a is insoluble in water and common organic solvents, and it can still remain intact in the air for 3 months and in aqueous solution for 7 days (Fig. 2c). Each ground sample of 1a (30 mg) was immersed into aqueous solutions with different pH values from 1 to 13 for 24 h, then separated by simple centrifugation, washed three times with water (3 mL), then dried at 50 °C for 12 h. Their PXRD results (Fig. 2d) reveal that the characteristic peaks maintain nearly the same positions as those of as-synthesized 1a over a wide pH range from 2 to 13, suggesting that 1a is chemically stable with strong acid and base resistance, which is prerequisite for many important industrial applications. Meanwhile, the PXRD patterns and FTIR spectra also further illustrate its good water/solvent stability after solvent treatment (Fig. S7–S10†). Such high water stability and chemical stability demonstrate that 1a is ultrastable. Adding its thermal stability and porous characteristics lays a firm foundation for the further practical study of this material in chemical sensing applications.
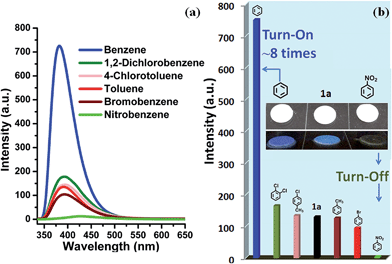
As shown in Fig. S11,†1a exhibits a broad luminescence emission centered at ∼427 nm upon excitation at 324 nm. Compared to those of the neutral ligands H6L (three bands: 382, 401 and 423 nm) and bipy (two shoulder peaks: 392 and 411 nm), the emission band of 1a covers the whole range of H6L and bipy ligands, concomitant with a red shift of ≈16–26 nm, hence it can be assigned to ligand-to-ligand charge transition (LLCT), admixing with metal-to-ligand transition (MLCT) for such Cd2+ coordination complexes.6b,k,l The ground powder of 1a was put under water and organic solvent vapors (such as methanol, ethanol, CH2Cl2, CCl4, 2-propanol, phenylmethanol, benzene, toluene, bromobenzene, 1,2-dichlorobenzene, 4-chlorotoluene, nitrobenzene, 2,4-dinitrotoluene, and nitromethane) for 24 h (Fig. S12†). Notably, 1a shows significantly different luminescence responses (Fig. 3 and S13†) toward the vaporized analytes: an ∼8-fold fluorescence enhancement and a strong ‘turn-off’ fluorescence quenching effect occurred after exposure to benzene and nitro-explosives vapors, respectively. The results of the enhancing or quenching levels are in accordance with the ones in liquid media (Fig. S14†). More dramatically, 1a after exposure to benzene (1a/benzene) exhibits a hypsochromic shift of ∼67 nm (λmax = 381 nm, violet color) compared with 1a/nitro-aromatic explosives (λmax = 448 nm, blue color; Fig. S13c†). Such a luminescence enhancement effect, where the presence of the analyte triggers an increase in the luminescence intensity, is rare; in particular, high selectivity for benzene vapor with a colour shift in the emission wavelength is much rarer and practical. Interestingly, when benzene-d6 (C6D6) was employed as the target vapor, a clearly different fluorescent intensity from 1a/benzene was observed (Fig. S13d†), and an ∼4-times fluorescence enhancement was obtained. To further understand the energy levels of 1a in different solvents environment and host–guest interactions, a theoretical calculation using the Gaussian 09 package was employed based on an idealized non-periodic cluster model constructed according to the experimental data and the computational details are depicted in the ESI.† The calculation results are shown in Fig. 4, and indicate that the electron density of the highest occupied molecular orbital (HOMO) is dominated by p-orbitals of the carboxylate ligand, and the lowest unoccupied molecular orbital (LUMO) is mainly distributed on the N and C atoms in the bipy aromatic ligand. So the energy transfer from the carboxylate ligand to bipy ligand contributes directly to the luminescence of 1a. The corresponding calculated energy gap is ca. 2.75 eV (ca. 450 nm), which is far from the experimental fluorescence data (427 nm). That may be caused by the computational method or the selected ideal structural model of (1) a non-periodic basic trinuclear cluster, and (2) deleting all free solvent molecules, because the crystal data is actually periodic and it is difficult to completely remove all free solvent molecules in its channels although some preparatory work has been done. Such ligand-based emission in MOFs is more desirable, since the bandgaps of these materials can be altered by changing the degree of conjugation in the ligand, affecting the intensity and position of emission.15 In host–guest chemistry, exchanged solvent molecules can diffuse into the channels, leading to the different fluorescent responses by analyte–ligand interactions.
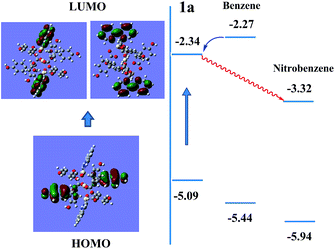 | ||
| Fig. 4 Schematic drawings of the electronic structures of 1a in benzene and nitrobenzene environments. | ||
For 1a/benzene, the calculated energy gap increases from 2.75 eV to 3.17 eV, and the difference (0.42 eV) relative to 1a is very close to the observed blue-shift in the experiment of the luminescence (0.49 eV). Its LUMO is at a high-lying π* antibonding state with higher energy, so energy transfer from benzene to the LUMO of compound 1 occurs and leads to a fluorescence enhancement upon excitation. The significant shifting of emission λmax and fluorescence enhancement could be ascribed to the π⋯π stacking interaction between MOF host and benzene molecules, so that the energy levels of the ground state and excited state were changed (Fig. 4). In contrast, the excited electrons are transferred from the LUMO of 1 to nitrobenzene, thereby leading to a quenching effect for 1a/nitrobenzene. This mechanism is consistent with one previously proposed by other groups and us.9f,16 The UV-Vis adsorption spectra (Fig. S15†) of 1, ligands (H6L and bipy) and solvents (benzene and nitrobenzene) further verify and provide deeper understanding of the luminescent response. It is obvious that the adsorption peaks of 1 and bipy ligand at 220–322 nm almost cover the entire range of the absorption of solvents. Upon illumination, there is competition for excitation energy between the absorption of nitrobenzene and 1 because of the presence of the electron-withdrawing –NO2 group, whereas benzene molecules may have a similar excited energy level to 1a, which has been confirmed by experimental results. As shown in Fig. S16,† the fluorescent spectrum of pure benzene solution exhibits a main sharp emission maxima at ca. 328 nm and one very weak sloping band at ∼390 nm under normal 286 nm excitation. When under excitation at 314 nm, which is the excitation condition of 1a, benzene molecules can emit very weakly at λmax = 386 nm. Combined with the UV-Vis absorption data, it is most likely to lead to energy transfer from benzene to 1a or bipy ligands under excitation, which results in the increase of the luminescent intensity.11b
In order to test the sensitivity and response rate of 1a as a vapor sensor, an in situ solid-state luminescent sensor setup was designed and used (Fig. 5). The time-dependent enhancing and quenching efficiency shows that the response rate of 1a for benzene vapor is 56 s, and it exhibits ∼8 fold fluorescence enhancement in 2–3 min (Fig. S17†); for benzene-d6 vapor it is 30 s and reaches the maximum (∼4 times fluorescent intensity) in 2 min (Fig. S18†); and the fluorescence quenching for nitrobenzene reaches a maximum in 1 min with a quenching percentage of more than 90% (Fig. S19†). Such rapid, sensitive response to benzene vapor is still challenging. Repeatability for a sensory material is a very important parameter to assess the sensor practicability. After detection of benzene and nitrobenzene, samples of 1a/benzene and 1a/nitrobenzene can be recovered by simply heating at 80 °C under vacuum for 3 h, which can be evidenced by the PXRD patterns (Fig. S8†), FTIR spectra (Fig. S10†) and TGA curves (Fig. S20†). For 1a/benzene, its PXRD characteristic peaks match well with the ones of as-synthesized 1a, just the relative intensity shows a slight change (Fig. S8c†), which may be attributed to the benzene molecules filling into the MOF channels, giving rise to the high electronic density in this direction. The FTIR spectrum of 1a/benzene exhibits an obviously increasing infrared absorption peak around 676 cm−1 that belongs to the frame vibration absorption of benzene rings (Fig. S10k and l†) in the channels of 1a. The PXRD of 1a/nitrobenzene undergoes a small shift of ∼0.23° toward low angle and shows a few new peaks compared with 1a. So the interlayer distance along the (200) direction increases by 1.1 Å according to Bragg's equation. These changes evidence the structural change of 1a, caused by a large number of nitrobenzene molecules inserting in the channels of 1a as well as the hydrogen bonding and π⋯π interactions between the 1a host and nitrobenzene molecules. The IR spectrum for 1a/nitrobenzene further verifies (1) the presence of nitrobenzene molecules, where –NO2 characteristic νs and νas stretching vibrations (∼1519 cm−1 and ∼1343 cm−1) as labelled can be apparently observed (Fig. S10m and n†); (2) the stretches are associated with the inserted nitrobenzene molecules; (3) the framework of 1a/nitrobenzene keeps constant as 1a. The PXRD and IR results for 1a after heating treatment from 1a/benzene are in accordance with the ones of 1a-as-synthesized. The PXRD pattern for 1a regenerated from 1a/nitrobenzene also returns to be consistent with the initial one of 1a-as-synthesized. The –NO2 characteristic peaks decrease clearly in its IR spectrum. The changes during the adsorption and desorption processes suggest the “breathing effect” occurs in the flexible framework. Then a recovered 1a sample was reused in the next cycle. The results are presented in Fig. 4b and S17–S19.† It was observed that the response rate and sensitivity after three cycles were mainly maintained, and its PXRD patterns after treatment with benzene and nitrobenzene match well with the simulated pattern generated from the result of single-crystal diffraction data and as-synthesized product (Fig. S8†), confirming its good stability and reusability. The foregoing two aspects of rapid response and repeatability are of great significance for practical use of the sensor. One interesting feature can be easily observed during the process of in situ luminescent test: the emission wavelength of 1a/benzene is gradually blue-shifted as time increases. To deeply understand the influence of retention time for 1a in benzene vapor on its luminescence, a series of detailed studies were carried out, where the PL spectra of 1a in excess benzene vapor for different time intervals were recorded and are shown in Fig. S21.† These results show that the blue-shift is very rapid at the beginning and is complete after about 6 h, which is exactly identical with the foregoing results in Fig. 3 and 5.
As depicted in the experimental section, all in situ fluorescent tests were observed based on a concentration of 52.6 mg cm−3 for benzene vapor (∼120 μL). The fluorescent intensity change is similar to that with higher benzene concentration as shown in Fig. 3, where the sample was placed in excess benzene vapor. But it is highly possible that the luminescence enhancement is dependent on the vapor concentration. In order to further clarify the relationship, in situ fluorescent experiment was carried out in benzene vapor of a low concentration (8.8 mg cm−3; Fig. S22†). The observation indicates 1a/benzene still exhibits a clear enhancement effect, but only ∼3-fold luminescence enhancement was observed, which is far less than the ∼8-fold enhancement in the vapor concentration of 52.6 mg cm−3. This study demonstrates not only that the enhancement is significantly dependent on the concentration of benzene vapor, but also suggests that 1a may be a potential adsorbent material for benzene.
At the same time, one more important feature is also found that the firm sheet sample used for in situ solid-state luminescent testing expanded significantly, even breaking into a lot of small pieces after exposure to benzene vapor, yet not after exposure to nitrobenzene (Fig. S23†). This result may be caused by a large amount of adsorption for benzene molecules, so 1a is a potential MOF adsorbent for benzene vapor. As expected, the total weight of the solid sheet for 1a after treatment with benzene vapor increases by about 55–80% (repeating three times) of the original weight. The TGA curve of 1a after immersing in benzene (Fig. S20†) shows that a weight loss of ∼20% (200 mg g−1) from 50 to 250 °C is observed, which is equivalent to approximately 6 molecules per formula unit stably trapped inside the channels. But in fact, the weight loss of benzene adsorbed on the surface began at room temperature, so the amount of benzene molecules trapped by the framework of 1a is much greater than 20%. Furthermore, the sorption isotherm of benzene (Fig. 2b) by 1a has been measured and shows a rapid increase in the low-pressure range (P/P0 < 0.1), and then slowly approaches saturation with the amount adsorbed of 81.6 cm3 g−1 at P/P0 = 0.99, confirming the good adsorption properties of 1a. The desorption curve shows pronounced hysteresis and incomplete desorption, and does not retrace the adsorption isotherm, which may be attributed to the pore expansion in 1a when benzene molecules are adsorbed into the pores. Such a large adsorbed amount of benzene vapor into the channels may be another reason for the luminescence enhancement. But the amount for each of the other solvent vapors is difficult to measure, so the correlation between the adsorbed amounts of vapor molecules and luminescence intensity is still not clear at this moment. Similarly, the sample of 1a after thermal treatment does not affect the next adsorption. In other words, highly luminescent 1a will be a potential adsorbent for the removal of benzene molecules based on its high porosity, robust stability and reusability, which is needed as an environmentally friendly and effective method.
Conclusions
In summary, the porous Cd-MOF material obtained is not only a practical luminescent switch demonstrated experimentally, which represents high selectivity and sensitivity for detecting trace benzene (benzene-d6) and nitrobenzene explosives in the vapor phase through a luminescence enhancement and quenching response, respectively, but also an effective adsorbent for benzene vapor with recyclability. Its response time reaches ∼56 s for benzene vapor. In addition, its remarkable water and chemical stability as well as reusable characteristics are very important to realize practical use. Further research will be centered on making fluorescent MOF films for more straightforward sensing of vapors, which will be easily manipulated as versatile devices.Acknowledgements
This work was supported by NSFC (21171162 and 21201162), SRF for ROCS (State Education Ministry) and Jilin Province Youth Foundation (20130522132JH and 20130522170JH).References
-
(a)
R. E. Hester and R. M. Harrison, Volatile Organic Compounds in the Atmosphere, Royal Society of Chemistry, Cambridge, UK, 1995 Search PubMed
; (b) L. Caprino and G. Tonga, Environ. Health Perspect., 1998, 106, 115–125 CrossRef CAS
; (c) H. Lin, M. Jang and K. S. Suslick, J. Am. Chem. Soc., 2011, 133, 16786–16789 CrossRef CAS PubMed
.
-
(a) R. Snyder, J. Toxicol. Environ. Health, Part A, 2000, 61, 339–346 CrossRef CAS PubMed
; (b) C. M. McHale, L. Zhang and M. T. Smith, Carcinogenesis, 2012, 33, 240–252 CrossRef CAS PubMed
; (c) L. A. Wallace, Annu. Rev. Energy Environ., 2001, 26, 269–301 CrossRef
.
-
(a) T. Salthammer and M. Bahadir, Clean: Soil, Air, Water, 2009, 37, 417–435 CrossRef CAS
; (b) G. A. Eiceman, J. Gardea-Torresdey, F. Dorman, E. Overton, A. Bhushan and H. P. Dharmasena, Anal. Chem., 2006, 78, 3985–3996 CrossRef CAS PubMed
.
-
(a) X. Zhang, B. Li, Z.-H. Chen and Z.-N. Chen, J. Mater. Chem., 2012, 22, 11427–11441 RSC
; (b) M. A. Rawashdeh-Omary, M. D. Rashdan, S. Dharanipathi, O. Elbjeirami, P. Rameshb and H. V. Rasika Dias, Chem. Commun., 2011, 47, 1160–1162 RSC
; (c) J. Pang, E. J.-P. Marcotte, C. Seward, R. S. Brown and S. Wang, Angew. Chem., 2001, 113, 4166–4169 ( Angew. Chem., Int. Ed. , 2001 , 40 , 4042–4045 ) CrossRef
; (d) H. Ma, R. Gao, D. Yan, J. Zhao and M. Wei, J. Mater. Chem. C, 2013, 1, 4128–4137 RSC
; (e) Y. Zhao, H. Lin, M. Chen and D. Yan, Ind. Eng. Chem. Res., 2014, 53, 3140–3147 CrossRef CAS
.
-
(a) G. Férey, Chem. Soc. Rev., 2008, 37, 191–214 RSC
; (b) O. M. Yaghi, Nat. Mater., 2007, 6, 92–93 CrossRef CAS PubMed
; (c) J. An, O. K. Farha, J. T. Hupp, E. Pohl, J. I. Yeh and N. L. Rosi, Nat. Commun., 2012, 3, 1618 Search PubMed
; (d) L. J. Murray, M. Dincă and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294–1314 RSC
; (e) J. Y. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC
; (f) J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869–932 CrossRef CAS PubMed
; (g) J.-P. Zhang, Y.-B. Zhang, J.-B. Lin and X.-M. Chen, Chem. Rev., 2012, 112, 1001–1033 CrossRef CAS PubMed
; (h) H.-L. Zhou, R.-B. Lin, C.-T. He, Y.-B. Zhang, N. Feng, Q. Wang, F. Deng, J.-P. Zhang and X.-M. Chen, Nat. Commun., 2013, 4, 3534 Search PubMed
; (i) H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 974–987 CrossRef CAS PubMed
; (j) N. W. Ockwig, O. Delgado-Friedrichs, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2005, 38, 176–182 CrossRef CAS PubMed
; (k) S. Horike, D. Umeyama and S. Kitagawa, Acc. Chem. Res., 2013, 46, 2376–2384 CrossRef CAS PubMed
.
-
(a) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed
; (b) Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126–1162 CrossRef CAS PubMed
; (c) B. Chen, Y. Yang, F. Zapata, G. Lin, G. Qian and E. B. Lobkovsky, Adv. Mater., 2007, 19, 1693–1696 CrossRef CAS
; (d) B. Chen, L. Wang, F. Zapata, G. Qian and E. B. Lobkovsky, J. Am. Chem. Soc., 2008, 130, 6718–6719 CrossRef CAS PubMed
; (e) Y. Cui, H. Xu, Y. Yue, Z. Guo, J. Yu, Z. Chen, J. Gao, Y. Yang, G. Qian and B. Chen, J. Am. Chem. Soc., 2012, 134, 3979–3982 CrossRef CAS PubMed
; (f) B. Chen, S. Xiang and G. Qian, Acc. Chem. Res., 2010, 43, 1115–1124 CrossRef CAS PubMed
; (g) Y.-Q. Lan, H.-L. Jiang, S.-L. Li and Q. Xu, Adv. Mater., 2011, 23, 5015–5020 CrossRef CAS PubMed
; (h) X.-Z. Song, S.-Y. Song, S.-N. Zhao, Z.-M. Hao, M. Zhu, X. Meng, L.-L. Wu and H.-J. Zhang, Adv. Funct. Mater., 2014, 24, 4034–4041 CrossRef CAS
; (i) Y. Takashima, V. M. Martínez, S. Furukawa, M. Kondo, S. Shimomura, H. Uehara, M. Nakahama, K. Sugimoto and S. Kitagawa, Nat. Commun., 2011, 2, 168–176 CrossRef PubMed
; (j) X.-L. Yang, C. Zou, Y. He, M. Zhao, B. Chen, S. Xiang, M. O'Keeffe and C.-D. Wu, Chem. - Eur. J., 2014, 20, 1447–1452 CrossRef CAS PubMed
; (k) L.-Y. Zhang, J.-P. Zhang, Y.-Y. Lin and X.-M. Chen, Cryst. Growth Des., 2006, 6, 1684–1689 CrossRef CAS
; (l) S.-L. Zheng, J.-M. Yang, X.-L. Yu, X.-M. Chen and W.-T. Wong, Inorg. Chem., 2004, 43, 830 CrossRef CAS PubMed
.
-
(a) P. Wu, J. Wang, C. He, X. Zhang, Y. Wang, T. Liu and C. Duan, Adv. Funct. Mater., 2012, 22, 1698–1703 CrossRef CAS
; (b) X. Zhao, X. Bu, T. Wu, S.-T. Zheng, L. Wang and P. Feng, Nat. Commun., 2013, 4, 2344 Search PubMed
; (c) D. Ma, B. Li, X. Zhou, Q. Zhou, K. Liu, G. Zeng, G. Li, Z. Shi and S. Feng, Chem. Commun., 2013, 49, 8964–8966 RSC
; (d) Q.-K. Liu, J.-P. Ma and Y.-B. Dong, J. Am. Chem. Soc., 2010, 132, 7005–7017 CrossRef CAS PubMed
; (e) H.-L. Jiang, Y. Tatsu, Z.-H. Lu and Q. Xu, J. Am. Chem. Soc., 2010, 132, 5586–5587 CrossRef CAS PubMed
; (f) G.-L. Liu, Y.-j. Qin, L. Jing, G.-y. Wei and H. Li, Chem. Commun., 2013, 49, 1699–1701 RSC
; (g) T. Wen, D.-X. Zhang, J. Liu, R. Lin and J. Zhang, Chem. Commun., 2013, 49, 5660–5662 RSC
.
-
(a) J. Ferrando-Soria, P. Serra-Crespo, M. de Lange, J. Gascon, F. Kapteijn, M. Julve, J. Cano, F. Lloret, J. Pasán, C. Ruiz-Pérez, Y. Journaux and E. Pardo, J. Am. Chem. Soc., 2012, 134, 15301–15304 CrossRef CAS PubMed
; (b) Y.-N. Gong, L. Jiang, T.-B. Lu, J.-X. Ma, X.-F. Huang, X.-Q. Song and W.-S. Liu, Chem. - Eur. J., 2013, 19, 3590–3595 CrossRef PubMed
; (c) X. Wang, X. Wang, Y. Wang and Z. Guo, Chem. Commun., 2011, 47, 8127–8129 RSC
; (d) Q.-K. Liu, J.-P. Ma and Y.-B. Dong, Chem. - Eur. J., 2009, 15, 10364–10368 CrossRef CAS PubMed
; (e) Z. Chen, Y. Sun, L. Zhang, D. Sun, F. Liu, Q. Meng, R. Wang and D. Sun, Chem. Commun., 2013, 49, 11557–11559 RSC
; (f) H. Li, W. Shi, K. Zhao, Z. Niu, H. Li and P. Cheng, Chem. - Eur. J., 2013, 19, 3358–3365 CrossRef CAS PubMed
; (g) R. Saha, B. Joarder, A. S. Roy, S. M. Islam and S. Kumar, Chem. - Eur. J., 2013, 19, 16607–16614 CrossRef CAS PubMed
; (h) W. J. Rieter, K. M. L. Taylor and W. Lin, J. Am. Chem. Soc., 2007, 129, 9852–9853 CrossRef CAS PubMed
; (i) N. B. Shustova, A. F. Cozzolino, S. Reineke, M. Baldo and M. Dincă, J. Am. Chem. Soc., 2013, 135, 13326–13329 CrossRef CAS PubMed
.
-
(a) Y.-S. Xue, Y. He, L. Zhou, F.-J. Chen, Y. Xu, H.-B. Du, X.-Z. You and B. Chen, J. Mater. Chem. A, 2013, 1, 4525–4530 RSC
; (b) H. Xu, F. Liu, Y. Cui, B. Chen and G. Qian, Chem. Commun., 2011, 47, 3153–3155 RSC
; (c) A. Lan, K. Li, H. Wu, D. H. Olson, T. J. Emge, W. Ki, M. Hong and J. Li, Angew. Chem., Int. Ed., 2009, 48, 2334–2338 CrossRef CAS PubMed
; (d) S. Zhang, L. Han, L. Li, J. Cheng, D. Yuan and J. Luo, Cryst. Growth Des., 2013, 13, 5466–5472 CrossRef CAS
; (e) S. Pramanik, Z. Hu, X. Zhang, C. Zheng, S. Kelly and J. Li, Chem. - Eur. J., 2013, 19, 15964–15971 CrossRef CAS PubMed
; (f) S. Pramanik, C. Zheng, X. Zhang, T. J. Emge and J. Li, J. Am. Chem. Soc., 2011, 133, 4153–4155 CrossRef CAS PubMed
.
-
(a) Y. Li, S. Zhang and D. Song, Angew. Chem., Int. Ed., 2013, 52, 710–713 CrossRef CAS PubMed
; (b) J. Chen, F.-Y. Yi, H. Yu, S. Jiao, G. Pang and Z.-M. Sun, Chem. Commun., 2014, 50, 10506–10509 RSC
.
-
(a) J.-M. Zhou, W. Shi, H.-M. Li, H. Li and P. Cheng, J. Phys. Chem. C, 2014, 118, 416–426 CrossRef CAS
; (b) A. Planchais, S. Devautour-Vinot, S. Giret, F. Salles, P. Trens, A. Fateeva, T. Devic, P. Yot, C. Serre, N. Ramsahye and G. Maurin, J. Phys. Chem. C, 2013, 117, 19393–19401 CAS
.
-
(a) F.-Y. Yi, W. Yang and Z.-M. Sun, J. Mater. Chem., 2012, 22, 23201–23209 RSC
; (b) P. Lama, R. Kumar Das, V. J. Smith and L. J. Barbour, Chem. Commun., 2014, 50, 6464–6467 RSC
.
-
(a) V. A. Blatov, Struct. Chem., 2012, 23, 955–963 CrossRef CAS PubMed
. TOPOS software is available for download at http://www.topos.samsu.ru; (b) V. A. Blatov, TOPOS, a Multipurpose Crystallochemical Analysis with the Program Package, Russia, 2004 Search PubMed
; (c) M. O'Keeffe, Reticular Chemistry Structure Resource, http://rcsr.anu.edu.au/; (d) V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576–3586 CrossRef CAS
.
-
(a) A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 194–201 CrossRef
; (b) A. L. Spek, PLATON99, a Multipurpose Crystallographic Tool, Utrecht University, Utrecht, The Netherlands, 1999 Search PubMed
.
-
(a) C. A. Bauer, T. V. Timofeeva, T. B. Settersten, B. D. Patterson, V. H. Liu, B. A. Simmons and M. D. Allendorf, J. Am. Chem. Soc., 2007, 129, 7136–7144 CrossRef CAS PubMed
; (b) X.-L. Qi, R.-B. Lin, Q. Chen, J.-B. Lin, J.-P. Zhang and X.-M. Chen, Chem. Sci., 2011, 2, 2214–2218 RSC
; (c) D. Yan, G. O. Lloyd, A. Delori, W. Jones and X. Duan, ChemPlusChem, 2012, 77, 1112–1118 CrossRef CAS
; (d) D. Yan, Y. Tang, H. Lin and D. Wang, Sci. Rep., 2014, 4, 4337–4344 Search PubMed
; (e) Y. Tang, W. He, Y. Lu, J. Fielden, X. Xiang and D. Yan, J. Phys. Chem. C, 2014, 118, 25365–25373 CrossRef CAS
; (f) D. Yan and D. G. Evans, Mater. Horiz., 2014, 1, 46–57 RSC
.
-
(a) S. W. Thomas, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339–1386 CrossRef CAS PubMed
; (b) M. Zhang, G. Feng, Z. Song, Y.-P. Zhou, H.-Y. Chao, D. Yuan, T. T. Y. Tan, Z. Guo, Z. Hu, B. Z. Tang, B. Liu and D. Zhao, J. Am. Chem. Soc., 2014, 136, 7241–7244 CrossRef CAS PubMed
; (c) S. Dang, X. Min, W. Yang, F.-Y. Yi, H. You and Z.-M. Sun, Chem. - Eur. J., 2013, 19, 17172–17179 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and methods, crystal data, structural information, PXRD, FTIR, TGA, fluorescence measurements, UV-Vis and additional figures. CCDC 1017377. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4mh00210e |
‡ Crystal data for 1: C88H92Cd3N8O23, M = 1966.90, monoclinic, space group C2/c, a = 31.136(9) Å, b = 20.048(6) Å, c = 15.849(4) Å, V = 9390(4) Å3, Z = 4, μ = 0.746 mm−1, Dc = 1.391 Mg m−3, F(000) = 4016, 8371 unique (Rint = 0.0792), R1 = 0.0841, wR2 = 0.2592 (I > 2σ(I)), GOF = 1.011. Max./min. residual electron density 1.653 and −2.053 e Å−3. A total of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 data were measured in the range 1.23 < θ < 25.17°. 440 data were measured in the range 1.23 < θ < 25.17°. |
| This journal is © The Royal Society of Chemistry 2015 |