Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of indole-derived allocolchicine congeners exhibiting pronounced anti-proliferative and apoptosis-inducing properties†
Nikolay S.
Sitnikov
ab,
Alexander V.
Sinzov
a,
Diane
Allegro
c,
Pascale
Barbier
c,
Sebastien
Combes
d,
Liliane Abodo
Onambele
e,
Aram
Prokop
e,
Hans-Günther
Schmalz
b and
Alexey Yu.
Fedorov
*a
aDepartment of Organic Chemistry, Lobachevsky State University of Nizhni Novgorod, Gagarina Av. 23, Nizhni Novgorod 603950, Russia. E-mail: afnn@rambler.ru
bDepartment für Chemie, Universität zu Köln, Greinstr. 4, 50939, Köln, Germany
cAix-Marseille Université, INSERM, CRO2 UMR_S 911, F-13005, Marseille, France
dLaboratory of Integrative Structural and Chemical Biology, Institut Paoli-Calmettes, Aix-Marseille Université, UM 105, F-13009, Marseille, France
eDepartment of Pediatric Hematology/Oncology, Children's Hospital Köln, Amsterdamer Str. 59, 50735 Köln, Germany
First published on 22nd September 2015
Abstract
Based on the natural antimitotic agent allocolchicine as a lead structure, a series of novel indole-based allocolchicine congeners was synthesized and assessed in vitro for their cytostatic properties. Several compounds exhibited potent anti-proliferative and apoptosis-inducing activity towards lymphoma cells along with low unspecific cytotoxicity. The observed activity is supposed to result from the inhibition of microtubule assembly, as indicated by the tubulin polymerisation assay.
Introduction
Colchicine (1),1,2 an alkaloid isolated from plants of the genera Colchiceae, Merendera and Gloriosa, is a long-known natural product exhibiting high levels of cytotoxicity towards proliferating cells. The origin of its biological effect lies in the ability to inhibit polymerisation of tubulin, the main constitutive protein of microtubules.3–7 This effect leads to the disruption of mitotic spindle formation, arrest of the cell cycle in G2/M phase and, eventually, apoptotic cell death. The intriguing biological properties of colchicine as well as its unique structural features8 became a motivation for a number of total syntheses (resulting in one of the most fascinating endeavours in the history of organic synthesis),8 as well as several studies concerning the structure–activity relationship (SAR) of colchicine structural analogs.3,9–11 While high systemic toxicity12,13 (resulting in strong gastrointestinal upset, neuropathy, and bone marrow suppression) has prevented its use in the treatment of cancer, colchicine (1) became a lead structure in the design of novel tubulin polymerisation inhibitors. Along with several classes of structurally related compounds (e.g. combretastatins14–17 and 4-arylcoumarins18), allocolchicine (2)19 and its analogues9–11,20–27 were identified as promising candidates for further development. Recently, our group reported the synthesis and biological evaluation of a series of heterocyclic allocolchicine congeners (for instance 3 and 4, Fig. 1), in which ring C of the parent compound 2 is replaced with an indole28,29 or a benzofuran30 pharmacophore. Allocolchicinoids 3 and 4 showed high levels of proliferation inhibition and apoptosis induction at nanomolar concentrations against different lymphoma cells, although their unspecific cytotoxicity was found to be particularly low.28 Herein, we report the synthesis of pyrrolo-allocolchicinoids of type 5, i.e. the constitutional isomers of 3 and 4, and present the primary results of their biological assessment using a human lymphoma cell line. In addition, the influence of the compounds on tubulin polymerisation was determined in vitro. | ||
| Fig. 1 Structures of colchicine (1), allocolchicine (2), known pyrrolo-allocolchicinoids (3 and 4) and their target constitutional isomers of type 5. | ||
Results and discussion
Synthesis
Multiple strategies to access the tricyclic fused ring system of allocolchicine (2) and its analogues have been developed to date.31–43 For the construction of the carbocyclic skeleton of 5, we followed the strategy depicted in Scheme 1, which relies on intramolecular Friedel–Crafts acylation and Pd-catalysed cross-coupling as the C–C bond forming steps.First, the halogen-selective Suzuki–Miyaura reaction between methyl (iodoaryl)propionate 7 (ref. 30) and indolylboronate 8 generated biaryl 9, which upon basic hydrolysis of the methyl ester yielded acid 6. Treatment of 6 with (1-chloro-2-methylpropenyl)dimethylamine (10, Ghosez reagent)44,45 resulted in the formation of acyl chloride 11 which was used in situ in the intramolecular Friedel–Crafts acylation. Under the previously reported30 cyclisation conditions (ZnCl2, 0.02 M 11 in DCM), tetracycle 12 was formed as a single regioisomer, however, in only 17% yield (as a consequence of the acid-catalysed oligomerisation of the starting material). Application of Et2AlCl or EtAlCl2 as proton-scavenging Lewis acids46 also gave only low yields of 12 due to competing nucleophilic addition of the Al-alkyl reagent to acid chloride 11. However, treatment of 11 with an excess of bulky diisobutylaluminum chloride resulted in efficient seven-membered ring closure and, in addition, in situ reduction of the carbonyl group47,48 (via β-hydride transfer). This way, tetracyclic alcohol rac-13 was obtained in 68% yield over three steps in a one-pot procedure.‡ After cyclization, the bromine in rac-13 was removed via halogen–lithium exchange/protonation to give rac-5a in 96% yield (32% overall from 7) (Scheme 2).
Alcohol rac-5a further served as the substrate for the synthesis of allocolchicinoids with various functionalities at C(7)§ (Scheme 3). Thus, oxidation of rac-5a with N-methylmorpholine-N-oxide in the presence of catalytic Pr4NRuO4 (Ley oxidation)49 gave ketone 5b in 90% yield. Quantitative conversion of rac-5a to the corresponding acetate rac-5c was achieved via transesterification with ethyl acetate. The reaction of rac-5a with Zn(N3)2·2Py under Mitsunobu conditions50 resulted in the formation of azide rac-5d (91% yield) which was subsequently reduced with lithium aluminum hydride to amine rac-5e (93% yield). Finally, acylation of rac-5e with acetic anhydride in pyridine provided acetamide rac-5f in 94% yield.
Biological assessment
The cytostatic activity of the target pyrrolo-allocolchicinoids 5a–f against BJAB (Burkitt-type lymphoma) cells was evaluated using colchicine (1) as a standard (Table 1). All compounds exhibited a clear dose-dependent effect on cell proliferation and apoptosis (see the ESI† for detailed information). As a general tendency, compounds bearing an oxygen-based functionality at C(7) possessed higher cytostatic activity and lower unspecific cytotoxicity compared to the corresponding analogues with a C(7)–N bond. Acetate rac-5c was identified as a particularly potent antimitotic agent, as it caused virtually complete inhibition of cell proliferation at low nanomolar concentrations (Fig. 2), while no necrosis was detected (in a lactate dehydrogenase (LDH) release assay after 1 h) at concentrations of up to 5 μM. It is noteworthy that the novel pyrrolo-allocolchicinoids 5a–f possess biological activity in the same concentration range as the previously reported isomeric series 3 and 4 (Fig. 1). This indicates that the mode of pyrrole ring fusion to the allocolchicine scaffold does not induce a profound influence on the cytostatic properties.| IC50a [μM] | AC50b [μM] | Proliferation inhibition at AC50 [%] | Necrosis at AC50c [%] | |
|---|---|---|---|---|
Each experiment was performed in triplicate; n.d. = not determined.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a IC50: concentration of the compound causing 50% cell growth inhibition after 24 h, as determined by CASY cell counting.b AC50: concentration of the compound causing 50% cell apoptosis after 72 h, as determined by a DNA fragmentation assay.c Necrosis level caused by the compound at AC50 concentration after 1 h, measured by the LDH release assay.d X corresponds to the functional group at C(7) (Fig. 1). a IC50: concentration of the compound causing 50% cell growth inhibition after 24 h, as determined by CASY cell counting.b AC50: concentration of the compound causing 50% cell apoptosis after 72 h, as determined by a DNA fragmentation assay.c Necrosis level caused by the compound at AC50 concentration after 1 h, measured by the LDH release assay.d X corresponds to the functional group at C(7) (Fig. 1). |
||||
| Colchicine (1) | 0.02 | 0.03 | n.d. | n.d. |
| 5a (X = OH)d | 0.001 | 0.001 | 52 | 7 |
| 5b (X = O) | 0.01–0.05 | 0.05 | 98 | 4 |
| 5c (X = OAc) | 0.01–0.05 | 0.05 | 89 | 0 |
| 5d (X = N3) | 50 | >100 | 30 | 1 |
| 5e (X = NH2) | 0.5–1 | 5 | 90 | 29 |
| 5f (X = NHAc) | >100 | >100 | — | — |
 | ||
| Fig. 2 Concentration-dependent inhibition of BJAB lymphoma cell proliferation by 5c as determined by CASY cell counting after 24 h. | ||
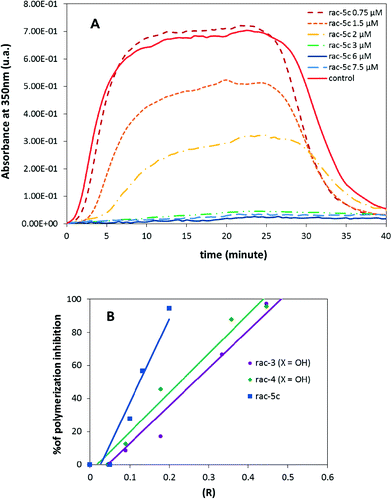
To probe whether the cytostatic activity of the pyrrolo-allocolchicinoids might be a consequence of tubulin binding, acetate rac-5c as well as rac-3 (X = OH) and rac-4 (X = OH) (as the most active of the previously reported compounds) were tested in a fluorescence-based tubulin polymerisation assay (Fig. 3). The depicted turbidimetry curves reflect the effect of all three compounds on the microtubule assembly from purified tubulin. A clear inhibition was noted, as the rate of assembly and the final amount of microtubules were clearly lower in the presence of allocolchicinoids than those in the control experiment. The extent of inhibition increased steadily with the molar ratio of the total ligand to the total tubulin in the solution (R). All three compounds demonstrated a sub-stoichiometric mode of action.51 Half-inhibition of tubulin polymerisation was achieved at a molar ratio (compound/tubulin) of 0.125 for rac-5c, 0.264 for rac-3 (X = OH) and 0.228 for rac-4 (X = OH) (the corresponding value for colchicine (1) is 0.375,52 and that for combretastatin A-4 is 0.09 (own data)). Thus, the high cytostatic activity of pyrrolo-allocolchicinoids 3–5 appears to be a direct consequence of efficient tubulin binding.
Conclusions
A synthetic route to a new structural type of pyrrolo-allocolchicinoids was developed. The cytostatic properties of target compounds 5a–f bearing different functional groups were evaluated employing Burkitt-like lymphoma cells (BJAB). Allocolchicinoids 5a–c exhibited potent anti-proliferative and apoptosis-inducing activity with IC50 and AC50 values in the low nanomolar concentration range along with low unspecific cytotoxicity (according to LDH release measurements). The in vitro tubulin polymerisation assay revealed that compound 5a as well as the previously reported structural isomers rac-3 (X = OH) and rac-4 (X = OH) inhibit the assembly of tubulin into microtubules. This indicates that, similarly to colchicine, the anti-proliferative and pro-apoptotic effects of pyrrolo-allocolchicinoids most probably result from the disruption of the mitotic spindle formation and subsequent cell cycle arrest.Acknowledgements
We gratefully acknowledge the Russian Foundation for Basic Research (project 14-03-91342), the Deutsche Forschungsgemeinschaft (project 857/18-1) and the Ministry of Education and Science of the Russian Federation (project 4.619.2014/K) for financial support. N. S. thanks the Alexander von Humboldt Foundation for a postdoctoral fellowship. A. Prokop gratefully acknowledges support from Dr. Kleist-Stiftung, Berlin.Notes and references
- H.-G. Capraro and A. Brossi, in The Alkaloids: Chemistry and Pharmacology, ed. A. Brossi, Academic Press, Orlando, 1984, vol. 23, p. 1 Search PubMed.
- C. Le Hello, in The Alkaloids: Chemistry and Biology, ed. G. A. Cordell, Academic Press, San Diego, CA, 1999, vol. 53, p. 287 Search PubMed.
- B. Bhattacharyya, D. Panda, S. Gupta and M. Banerjee, Med. Res. Rev., 2008, 28, 155 CrossRef CAS PubMed.
- B. Gigant, A. Cormier, A. Dorleans, R. B. G. Ravelli and M. Knossow, in Topics in Current Chemistry, ed. T. Carlomagno, Springer-Verlag, Berlin, 2009, vol. 286, p. 259 Search PubMed.
- S. Sapra, Y. Bhalla, Nandani, S. Sharma, G. Singh, K. Nepali, A. Budhiraja and K. L. Dhar, Med. Chem. Res., 2013, 22, 531 CrossRef CAS.
- For general reviews on tubulin as a target in anticancer therapy, see: E. Stec-Martyna, M. Ponassi, M. Miele, S. Parodi, L. Felli and C. Rosano, Curr. Cancer Drug Targets, 2012, 12, 658 CrossRef CAS PubMed; C. D. Katsetos and P. Dráber, Curr. Pharm. Des., 2012, 18, 2778 CrossRef PubMed.
- For general reviews on microtubule-binding agents, see: C. Dumontet and M. A. Jordan, Nat. Rev. Drug Discovery, 2010, 9, 790 CrossRef CAS PubMed; R. A. Stanton, K. M. Gernert, J. H. Nettles and R. Aneja, Med. Res. Rev., 2011, 31, 443 CrossRef PubMed.
- T. Graening and H.-G. Schmalz, Angew. Chem., Int. Ed., 2004, 43, 3230 CrossRef CAS PubMed.
- J. Chen, T. Liu, X. W. Dong and Y. Z. Hu, Mini-Rev. Med. Chem., 2009, 9, 1174 CrossRef CAS PubMed.
- Y. Lu, J. J. Chen, M. Xiao, W. Li and D. D. Miller, Pharm. Res., 2012, 29, 2943 CrossRef CAS PubMed.
- G. Sivakumar, Curr. Med. Chem., 2013, 20, 892 CAS.
- C. Putterman, E. Benchetrit, Y. Caraco and M. Levy, Semin. Arthritis Rheum., 1991, 21, 143 CrossRef CAS PubMed.
- Y. Finkelstein, S. E. Aks, J. R. Hutson, D. N. Juurlink, P. Nguyen, G. Dubnov-Raz, U. Pollak, G. Koren and Y. Bentur, Clin. Toxicol., 2010, 48, 407 CrossRef CAS PubMed.
- M. Marrelli, F. Conforti, G. A. Statti, X. Cachet, S. Michel, F. Tillequin and F. Menichini, Curr. Med. Chem., 2011, 18, 3035 CrossRef CAS PubMed.
- Y. Shan, J. Zhang, Z. Liu, M. Wang and Y. Dong, Curr. Med. Chem., 2011, 18, 523 CrossRef CAS PubMed.
- H. Rajak, P. K. Dewangan, V. Patel, D. K. Jain, A. Singh, R. Veerasamy, P. C. Sharma and A. Dixit, Curr. Pharm. Des., 2013, 19, 1923 CrossRef CAS PubMed.
- A. Brancale and R. Silvestri, Med. Res. Rev., 2007, 27, 209 CrossRef CAS PubMed.
- S. Combes, P. Barbier, S. Douillard, A. McLeer-Florin, V. Bourgarel-Rey, J.-T. Pierson, A. Yu. Fedorov, J.-P. Finet, J. Boutonnat and V. Peyrot, J. Med. Chem., 2011, 54, 3153 CrossRef CAS PubMed; O. G. Ganina, E. Daras, V. Bourgarel-Rey, V. Peyrot, A. N. Andresyuk, J.-P. Finet, A. Yu. Fedorov, I. P. Beletskaya and S. Combes, Bioorg. Med. Chem., 2008, 16, 8806 CrossRef PubMed.
- O. Boyé and A. Brossi, in The Alkaloids: Chemistry and Pharmacology, ed. A. Brossi and G. A. Cordell, Academic Press, San Diego, CA, 1992, vol. 41, p. 125 Search PubMed.
- R. Brecht, G. Seitz, D. Guenard and S. Thoret, Bioorg. Med. Chem., 2000, 8, 557 CrossRef CAS PubMed.
- F. Buttner, S. Bergemann, D. Guenard, R. Gust, G. Seitz and S. Thoret, Bioorg. Med. Chem., 2005, 13, 3497 CrossRef PubMed.
- A. Joncour, A. Decor, J.-M. Liu, M.-E. T. H. Dau and O. Baudoin, Chem. – Eur. J., 2007, 13, 5450 CrossRef CAS PubMed.
- N. Nicolaus, J. Zapke, P. Riesterer, J.-M. Neudoerfl, A. Prokop, H. Oschkinat and H.-G. Schmalz, ChemMedChem, 2010, 5, 661 CrossRef CAS PubMed.
- F.-D. Boyer, J. Dubois, S. Thoret, M.-E. T. H. Dau and I. Hanna, Bioorg. Chem., 2010, 38, 149 CrossRef CAS PubMed.
- N. Jain, D. Yada, T. B. Shaik, G. Vasantha, P. S. Reddy, S. V. Kalivendi and B. Sreedhar, ChemMedChem, 2011, 6, 859 CrossRef CAS PubMed.
- N. Nicolaus, J. Reball, N. Sitnikov, J. Velder, A. Termath, A. Y. Fedorov and H.-G. Schmalz, Heterocycles, 2011, 82, 1585 CAS.
- E. Chosson, F. Santoro, C. Rochais, J. Sopkova-de Oliveira Santos, R. Legay, S. Thoret, T. Cresteil, M. S. Sinicropi, T. Besson and P. Dallemagne, Bioorg. Med. Chem., 2012, 20, 2614 CrossRef CAS PubMed.
- N. Sitnikov, J. Velder, L. Abodo, N. Cuvelier, J.-M. Neudorfl, A. Prokop, G. Krause, A. Y. Fedorov and H.-G. Schmalz, Chem. – Eur. J., 2012, 18, 12096 CrossRef CAS PubMed.
- N. S. Sitnikov, A. S. Kokisheva, G. K. Fukin, J.-M. Neudörfl, H. Sutorius, A. Prokop, V. V. Fokin, H.-G. Schmalz and A. Yu. Fedorov, Eur. J. Org. Chem., 2014, 29, 6481 CrossRef.
- Y. V. Voitovich, E. S. Shegravina, N. S. Sitnikov, V. Faerman, V. V. Fokin, H.-G. Schmalz, S. Combes, D. Allegro, P. Barbier, I. P. Beletskaya, E. V. Svirshchevskaya and A. Y. Fedorov, J. Med. Chem., 2015, 58, 692 CrossRef CAS PubMed.
- For a review, see: N. S. Sitnikov and A. Y. Fedorov, Russ. Chem. Rev., 2013, 82, 393 CrossRef.
- J. S. Sawyer and T. L. Macdonald, Tetrahedron Lett., 1988, 29, 4839 CrossRef CAS.
- A. V. Vorogushin, A. V. Predeus, W. D. Wulff and H.-J. Hansen, J. Org. Chem., 2003, 68, 5826 CrossRef CAS PubMed.
- M. Leblanc and K. Fagnou, Org. Lett., 2005, 7, 2849 CrossRef CAS PubMed.
- W. M. Seganish and P. DeShong, Org. Lett., 2006, 8, 3951 CrossRef CAS PubMed.
- G. Besong, K. Jarowicki, P. J. Kocienski, E. Sliwinski and F. T. Boyle, Org. Biomol. Chem., 2006, 4, 2193 CAS.
- S. Djurdjevic and J. R. Green, Org. Lett., 2007, 9, 5505 CrossRef CAS PubMed.
- S. D. Broady, M. D. Golden, J. Leonard, J. C. Muir and M. Maudet, Tetrahedron Lett., 2007, 48, 4627 CrossRef CAS.
- G. Besong, D. Billen, I. Dager, P. Kocienski, E. Sliwinski, L. R. Tai and F. T. Boyle, Tetrahedron, 2008, 64, 4700 CrossRef CAS.
- F.-D. Boyer and I. Hanna, Eur. J. Org. Chem., 2008, 4938 CrossRef CAS.
- N. Nicolaus, S. Strauss, J.-M. Neudoerfl, A. Prokop and H.-G. Schmalz, Org. Lett., 2009, 11, 341 CrossRef CAS PubMed.
- N. Nicolaus and H.-G. Schmalz, Synlett, 2010, 2071 CAS.
- S. Djurdjevic, F. Yang and J. R. Green, J. Org. Chem., 2010, 75, 8241 CrossRef CAS PubMed.
- A. Devos, J. Remion, A. M. Frisque-Hesbain, A. Colens and L. Ghosez, J. Chem. Soc., Chem. Commun., 1979, 1180 RSC.
- B. Haveaux, A. Dekoker, M. Rens, A. R. Sidani, J. Toye and L. Ghosez, Org. Synth., 1979, 59, 26 CrossRef.
- T. Okauchi, M. Itonaga, T. Minami, T. Owa, K. Kitoh and H. Yoshino, Org. Lett., 2000, 2, 1485 CrossRef CAS PubMed.
- G. Giacomelli and L. Lardicci, J. Org. Chem., 1981, 46, 3116 CrossRef CAS.
- J. S. Cha, O. O. Kwon, S. Y. Kwon, J. M. Kim, W. W. Seo and S. W. Chang, Synlett, 1995, 1055 CrossRef CAS.
- S. V. Ley, J. Norman, W. P. Griffith and S. P. Marsden, Synthesis, 1994, 639 CrossRef.
- M. C. Viaud and P. Rollin, Synthesis, 1990, 130 CrossRef CAS.
- B. Perez-Ramirez, J. M. Andreu, M. J. Gorbunoff and S. N. Timasheff, Biochemistry, 1996, 35, 3277 CrossRef CAS PubMed.
- H. Sternlicht and I. Ringel, J. Biol. Chem., 1979, 254, 10540 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures and characterisation data for all new compounds; details of biological experiments. See DOI: 10.1039/c5md00320b |
| ‡ To our best knowledge, this is the first example demonstrating the feasibility of the tandem Friedel–Crafts acylation–carbonyl group reduction using (i-Bu)2AlCl as an activator (Lewis acid) and an in situ reducing agent. |
| § Colchicine numbering (Fig. 1) is used throughout the manuscript. |
| This journal is © The Royal Society of Chemistry 2015 |