Synthesis and biological evaluation of novel 7-O-lipophilic substituted baicalein derivatives as potential anticancer agents†
Abstract
We synthesized derivatives of baicalein, wogonin, and chrysin through alkylation at the 7-O-position of the A ring with lipophilic terphenyl or long chain n-alkyl groups, and studied the in vitro anticancer activity of the derivatives through the growth inhibition MTT assay. We discovered that baicalein and two of its derivatives were good free radical scavengers. Among the 20 synthesized derivatives, 7-O-farnesylbaicalein (5d) and 7-O-dodecylbaicalein (5i) demonstrated stronger growth inhibition against human colon cancer SW480 cells compared with baicalein, with half maximal inhibitory concentration (IC50) values of 1.15 and 1.57 μM, respectively. Furthermore, 5d and 5i dose- and time-dependently inhibited the growth of SW480 cells. Cell cycle distribution analysis showed that 5d and 5i induced SW480 cell arrest at the S phase through an apoptotic mechanism, which was associated with an increase in the generation of reactive oxygen species. In conclusion, the potent anticancer activity of the baicalein derivatives (5d and 5i) suggested that the derivatives are potential anticancer agents for human colon cancer.
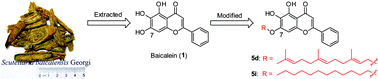

 Please wait while we load your content...
Please wait while we load your content...