Open Access Article
Open Access ArticleGlycodendropeptides stimulate dendritic cell maturation and T cell proliferation: a potential influenza A virus immunotherapy†
Ainhoa
Mascaraque
a,
Wioleta
Kowalczyk
b,
Tahia
Fernández
c,
Francisca
Palomares
c,
Cristobalina
Mayorga
*c,
David
Andreu
*b and
Javier
Rojo
*a
aGlycosystems Laboratory, Instituto de Investigaciones Químicas (IIQ), CSIC – Universidad de Sevilla, Américo Vespucio 49, 41092 Seville, Spain. E-mail: javier.rojo@iiq.csic.es; Fax: +34 954460165
bDepartment of Experimental and Health Sciences, Universitat Pompeu Fabra, 08003 Barcelona, Spain. E-mail: david.andreu@upf.edu; Fax: +34 933160901
cLaboratory of Research, UGC de Alergología, IBIMA, Hospital Regional Universitario de Málaga, UMA, 29009 Málaga, Spain. E-mail: mayorga.lina@gmail.com; Fax: +34 951290203
First published on 7th July 2015
Abstract
Mannosylation facilitates uptake and internalization of immunogenic peptides by antigen-processing cells expressing mannose receptors at their surface, such as DC-SIGN, a lectin that plays a key role in the immune response against different pathogens. This internalization, processing and subsequent MHC presentation may result in a strong T cell stimulation. Here, we hypothesized that combining mannose glycodendrons with multivalent presentation of peptide epitopes in a likewise dendron format would yield hybrid constructs, named glycodendropeptides (GDPs), with the capacity to enhance peptide immunogenicity, hence providing a novel and versatile platform for applications in immunotherapy. Thus, GDPs of different valencies displaying the NP366–374 epitope, a conserved sequence from the influenza A virus nucleoprotein (NP), have been built by two click chemistry-based methodologies and assessed as potential flu vaccine candidates. Preliminary evaluation of the ability of these constructs to stimulate dendritic cell maturation and lymphocyte proliferation was promising, showing the highest-functionalized NP366–374 GDPs as inducing the strongest immunostimulatory effect.
Introduction
The biological effect of peptide or protein mannosylation,1 which induces a receptor dependent internalization process in dendritic cells (DCs) and ensues strong T cell stimulation, constitutes an interesting tool for developing novel strategies in the vaccine field.2Compared to the entire protein immunogens, peptides show clear advantages such as stringent chemical definition, fast and efficient large scale production, or simple (cold chain-free) transport and storage.3 These advantages, however, can be offset by the relatively poor immunogenicity of peptides, a limitation often overcome by multiple presentations of the peptide antigen, such as the dendrimer systems pioneered by Tam named multiple antigen peptides (MAP).4
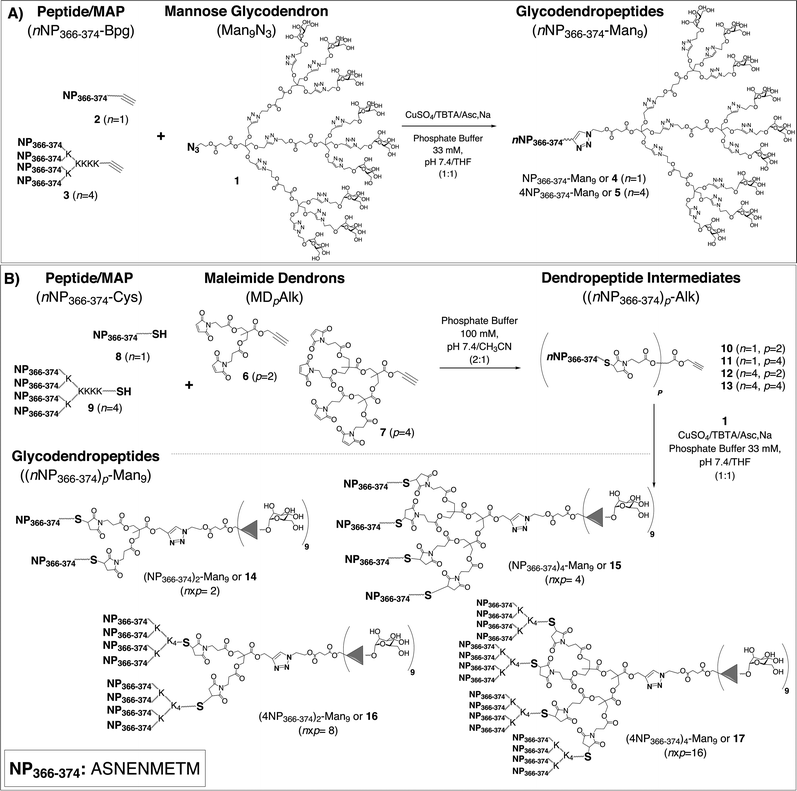
Our group has recently described hybrid constructs named glycodendropeptides (GDPs), where the multivalency of peptide epitopes in a MAP format combined with the improved uptake facilitated by mannosylation may lead to enhanced immunogenicity.5 Specifically, mannosylation would induce MAP internalization into DCs through interactions with the DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin) receptor,6 a lectin expressed at the surface of immature DCs (imDCs). This receptor is involved in the internalization of highly mannosylated antigens to be processed and presented to T cells to elicit an immune response.7 Recently, we have shown that a dendron functionalized with nine copies of mannose (Man) has the ability to interact, in a sugar dependent manner, with DC-SIGN and become internalized into DCs.8 In this context, the nonavalent glycodendron 1 (Scheme 1) was selected as the carbohydrate moiety of the GDPs used in the present study, although other Man valencies could be easily examined as well.
Two different synthetic approaches (methods A and B, Scheme 1)5 provide efficient and convergent routes to chemically well-defined GDPs that combine, in a single platform, multiple carbohydrate and peptide epitopes. The modularity and versatility of these routes allow a variety of biological applications where both the carbohydrate and the peptide, as well as their respective valencies, can be selected at will.
Influenza A is one of the most important respiratory pathogens worldwide, with vaccination being the widely accepted way to control the disease.9 However, in contrast to other pathogens that require vaccination only a few times in a lifetime, influenza vaccines have to be administered yearly. This is due to the remarkable mutation rates – and ensuing antigenic variability – of the two main envelope proteins, hemagglutinin (HA) and neuraminidase (NA), that up to now constitute the cornerstone of practically all influenza vaccines. It has been argued, however, that viral proteins with much lower yearly mutation rates could serve as better immunogens in a future universal flu vaccine.10 One such highly conserved protein is the nucleoprotein (NP) that encapsidates the viral RNA; in particular, the nine-residue epitope NP366–374 has been proposed as a potential candidate immunogen for a universal influenza A vaccine.10,11
With this background, we decided to incorporate the NP366–374 epitope (ASNENMETM) into novel GDPs that might serve as universal flu vaccine candidates. To this end, GDPs 4–5 and 14–17 were synthesized by the previously mentioned methods A and B (Scheme 1).5 Both approaches rely on two varieties of click chemistry reactions, namely the Huisgen's dipolar CuI-catalyzed azide–alkyne cycloaddition (CuAAC)12 and the thiol–ene addition of thiols to maleimides.13 The GDP systems thus generated, providing multiple presentations of a conserved and relevant flu epitope, as well as of Man residues with expected DC internalization capability, constitute novel and versatile platforms with enhanced immunogenic profiles that might eventually find use as synthetic influenza A vaccines.
Results and discussion
The preparation of GDPs by the methods in Scheme 1 required various building blocks (the glycodendron moiety, two maleimide scaffolds and the peptidic components – the peptides and MAPs) that were prepared as previously described.5 Briefly, the nonavalent Man glycodendron 1 was conveniently constructed by a convergent process that used pentaerythritol as the core and 1,3-dipolar cycloadditions for extension; maleimide dendrons 6 and 7 were derived from bis-2,2′-hydroxymethyl propionic acid (bis-MPA),5 and the single and multiple peptide modules (2, 3, 8, and 9) were made by Fmoc solid phase synthesis (SPPS).4,14 All peptides and MAPs were C-terminal carboxamides of either a bishomopropargylglycine (Bpg) residue (2–3), providing the alkyne functionality required for CuAAC (Scheme 1A), or a Cys residue (8–9) supplying the thiol group for thiol–ene ligation (Scheme 1B). In the MAP syntheses, spacers reported to enhance the flexibility of the construct and the quality of the final product were incorporated at all branching points.15 For MAP 3, the spacer was 6-aminohexanoic acid (Ahx), whereas 8-amino-3,6-dioxaoctanoic acid (O2Oc) was selected for 9. All peptide components were obtained in homogeneous form (>95% HPLC), with the MS data consistent with the theoretical values (Table S1, ESI†).To access GDPs by method A (Scheme 1), peptide 2 or MAP 3 was directly conjugated with glycodendron 1 by a single CuAAC reaction. The best results were obtained with 1 equiv. of CuSO4·5H2O, 2 equiv. of TBTA (Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine) and 4 equiv. of sodium ascorbate at room temperature in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) THF/phosphate buffer (33 mM, pH 7.4). The reproducibility and reliability of the conditions enabled the preparation of the final GDPs 4 and 5 (with 9
1 (v/v) THF/phosphate buffer (33 mM, pH 7.4). The reproducibility and reliability of the conditions enabled the preparation of the final GDPs 4 and 5 (with 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 9
1 and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mannose-to-peptide ratios, respectively), with almost quantitative conversions from starting materials to final products and in a monodisperse way, as determined by both analytical RP-HPLC and MS (Table S1, ESI†). The relatively small synthesis scale translated into only moderate yields of HPLC-purified products, typically in the 35–50% range.
4 mannose-to-peptide ratios, respectively), with almost quantitative conversions from starting materials to final products and in a monodisperse way, as determined by both analytical RP-HPLC and MS (Table S1, ESI†). The relatively small synthesis scale translated into only moderate yields of HPLC-purified products, typically in the 35–50% range.
On the other hand, method B (Scheme 1) required two synthetic steps to reach the target products. In this approach, maleimide dendrons 6 and 7 served both to multiply peptide valency and, by way of the alkyne group at their focal positions, to enable conjugation with glycodendron moiety 1. In the first step, peptide 8 or MAP 9, either one featuring a C-terminal Cys, was chemoselectively ligated with di-(6) or tetravalent (7) dendrons by thiol–ene addition in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v) CH3CN/phosphate buffer (100 mM, pH 7.4). The resulting dendropeptide intermediates 10–13 were obtained with almost quantitative conversions and in moderate yields after RP-HPLC purification. This first step allowed access to a dendropeptide (13) with up to 16 peptide copies. Although globally its synthesis involved long reaction times and moderate conversion rates, the end-product was again shown to be clearly monodisperse by analytical RP-HPLC and MS (Table S1, ESI†).
2 (v/v) CH3CN/phosphate buffer (100 mM, pH 7.4). The resulting dendropeptide intermediates 10–13 were obtained with almost quantitative conversions and in moderate yields after RP-HPLC purification. This first step allowed access to a dendropeptide (13) with up to 16 peptide copies. Although globally its synthesis involved long reaction times and moderate conversion rates, the end-product was again shown to be clearly monodisperse by analytical RP-HPLC and MS (Table S1, ESI†).
Once dendropeptide intermediates (10–13) were available, the corresponding GDPs (14–17) were readily obtained by CuAAC-mediated conjugation with glycodendron 1 under the same conditions set up for method A (Scheme 1). By means of this strategy, a GDP with a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 Man/peptide ratio (17) was obtained which, to the best of our knowledge, is the largest multivalent and mannosylated peptide system thus far reported, again obtained as a monodisperse entity as proven by both HPLC and MS (Table S1, ESI†).
16 Man/peptide ratio (17) was obtained which, to the best of our knowledge, is the largest multivalent and mannosylated peptide system thus far reported, again obtained as a monodisperse entity as proven by both HPLC and MS (Table S1, ESI†).
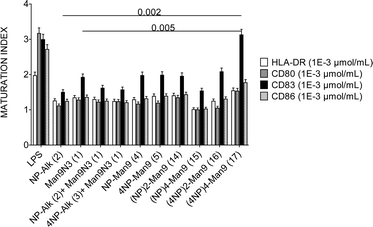
With all the above compounds in hand, we evaluated their effect on specialized cells of the immune system, such as the DCs and lymphocytes. To this end, DCs were incubated with GDPs at different concentrations and the expression of maturation and costimulatory markers (HLA-DR, CD80, CD83 and CD86) was assessed. The results obtained in these experiments indicated that, at a given GDP concentration of 1 μM, the expression patterns of HLA-DR, CD80 or CD86 markers were maintained almost constant regardless of the multivalency of the NP366–374 moiety. In contrast, an increase in CD83 expression was observed with the (4NP366–374)4–Man9 GDP (17), with the highest peptide multiplicity (16 copies), one log above the responses observed for any other compound (p < 0.005 for all) (Fig. 1).
Such an increase in (4NP366–374)4–Man9-induced CD83 expression in DCs may have important implications from an immunological point of view, since different studies have demonstrated that CD83, as the characteristic marker of full DC maturation,16 hence involved in T cell-mediated immune response,17 is essential for DC biology. The effect observed with compound 17 might overcome the down-modulation in CD83 expression produced upon infection by several viruses, which reduces the ability of infected DCs to stimulate T cells.18
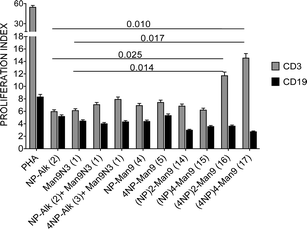
The proliferative responses of diverse lymphocyte subpopulations after incubation with GDPs at different concentrations were studied by analysing CD3, CD4, CD8 and CD19 expressions (Fig. 2 and 3).
In the presence of NP366–374-displaying compounds at a fixed 1 μM concentration, an increase in T lymphocyte (CD3) proliferative response with peptide multiplicity is observed, with highest significant values found for (4NP366–374)2–Man9 (16) and (4NP366–374)4–Man9 (17). Conversely, B lymphocyte (CD19) proliferation decreases with multivalency, although these differences are not significant (Fig. 2).
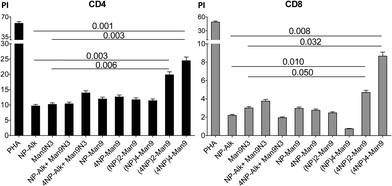
It is worth noting at this point that, during some infection processes, the immunological responses induced by viral agents lead to T cell proliferation, and specifically to CD8 T cells. In the present case, our data show a significant increase (p < 0.05) in the proliferative response of both CD4 and CD8 subsets of T lymphocytes (Fig. 3). As observed with CD83 expression, the highest proliferation index (PI) was observed for the compound with the highest peptide multiplicity, namely (4NP366–374)4–Man9 (17). This could be explained by the fact that, as demonstrated by others, CD83 has an important effect in regulating activation of functional T cells.19
Although a much higher PI was observed for CD4 T cells after incubation with (4NP366–374)4–Man9 (17) (Fig. 3), the proliferative response seems to be more specific for CD8 T cells, since relatively higher increases were found for this compound when compared to the effect induced by either peptide or glycodendron alone. These findings agree with those from other authors who have shown that CD83 expression directly correlates with the ability to induce specific cytotoxic T lymphocyte proliferation.18a Taken together, our results from maturation analysis and proliferative response lead us to conclude that (4NP366–374)4–Man9 (17) could be a good vaccine candidate, given its effectiveness in inducing complete DC maturation (CD83 increase), hence a subsequent increase in specific cytotoxic T lymphocyte proliferation. Further studies to characterize the immunological response of these T cell subpopulations are being carried out.
Experimental section
Materials and methods
Fmoc-protected amino acids were from Iris Biotech (Marktredwitz, Germany). Fmoc-Rink amide ChemMatrix resin, 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) and N-hydroxybenzotriazole (HOBt) were from Matrix Innovation (Montreal, Canada). All other reagents were of the highest quality commercially available from Sigma-Aldrich (Madrid, Spain). All solvents, including HPLC-grade CH3CN, and peptide synthesis-grade DMF, CH2Cl2, DIEA and TFA were from Carlo Erba-SDS (Sabadell, Spain). Non-peptide compounds were purified by flash chromatography using medium or fine silica gel or by Sephadex LH20 or G25 (GE Healthcare, Barcelona, Spain) gel filtration. Thin layer chromatography (TLC) was carried out with pre-coated F254 silica gel plates (Merck, Darmstadt, Germany). Flash chromatography was carried out with silica gel 60 (230–400 mesh) (Macherey-Nagel, Düren, Germany). TLC reaction monitoring was performed using potassium permanganate, phosphomolybdic acid, 10% sulfuric acid in methanol or anisaldehyde as development reagents. Analytical HPLC was carried out on 4.6 × 50 mm, 3 μm C18 or C8 columns (Phenomenex, Torrance, CA) with a LC-2010A system (Shimadzu, Kyoto, Japan). Solvents A and B were 0.045% and 0.036% (v/v) TFA in H2O and CH3CN, respectively. Elution was performed with linear gradients of solvent B into A over 15 min, at a 1 mL min−1 flow rate, with UV detection at 220 nm. Semi-preparative HPLC was done with a 10 × 250 mm, 10 μm, C18 column (Phenomenex) with a Shimadzu LC-8A system. Solvents A and B were 0.1% TFA (v/v) in H2O and CH3CN, respectively. Elution was performed with linear gradients of solvent B into A over 30 min, at a 5 mL min−1 flow rate, with UV detection at 220 nm. Fractions of adequate purity (>95%) by analytical HPLC were pooled and lyophilized. Mass spectra were obtained by electrospray ionization with Esquire 6000 (Bruker), LTQ-Orbitrap XL (Thermo Fisher, Waltham, MA), Q-Star Pulsar (Applied Biosystems, Carlsbad, CA) or LC/MSD-TOF (Agilent Technologies) instruments, or by MALDI-TOF using a Voyager DE-STR (Applied Biosystems). Compounds 1, 6 and 7 were synthesized as described before.5 On the other hand, compounds 2–3 and 8–9 were prepared according to the methodology widely used in our group5 (details are included in the ESI†).General synthetic protocol for dendropeptide intermediates
To a solution of 0.5 μmol (1 equiv.) of maleimide dendron (0.24 mg of 6 or 0.5 mg of 7) in CH3CN (400 μL), a solution of 1.5 equiv. per maleimide group of peptide 8 or MAP 9 in 100 mM phosphate buffer, pH 7.4 (800 μL), was added. To monitor the reaction progress, aliquots of the mixture were taken for analytical HPLC and MALDI-TOF MS analysis, and when no further changes in the HPLC profile were observed (reaction times range from a few minutes to 24 h, depending on the steric hindrance), the reaction was stopped by addition of glacial AcOH. The product was then isolated by preparative RP-HPLC. The target compounds (10–13) were obtained in homogeneous form (>95%) by analytical HPLC and were satisfactorily characterized by MS (see Table S1, ESI†).General protocol for glycodendropeptide synthesis
0.26 μmol of glycodendron 1 and 0.13 μmol of peptide 2, MAP 3 or dendropeptide intermediates 10–13 were dissolved in 100 μL of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v) THF/phosphate buffer (100 mM, pH 7.4). In a separate vial, CuSO4·5H2O (0.13 μmol) was dissolved in H2O (15 μL) and mixed with a solution of TBTA (0.26 μmol) in THF (33 μL). Next, this solution was added to the original mixture followed by 52 μL of sodium ascorbate (0.52 μmol) in H2O. After stirring for about 30 min at room temperature (the progression of the reaction was monitored by analytical RP-HPLC), the reaction mixture was lyophilized, then purified by semi-preparative RP-HPLC to give target compounds 4–5 and 14–17 (see Table S1, ESI†).
2 (v/v) THF/phosphate buffer (100 mM, pH 7.4). In a separate vial, CuSO4·5H2O (0.13 μmol) was dissolved in H2O (15 μL) and mixed with a solution of TBTA (0.26 μmol) in THF (33 μL). Next, this solution was added to the original mixture followed by 52 μL of sodium ascorbate (0.52 μmol) in H2O. After stirring for about 30 min at room temperature (the progression of the reaction was monitored by analytical RP-HPLC), the reaction mixture was lyophilized, then purified by semi-preparative RP-HPLC to give target compounds 4–5 and 14–17 (see Table S1, ESI†).
Generation of monocyte-derived DCs
Fresh peripheral blood mononuclear cells (PBMCs) obtained from 40 mL of human blood from 10 healthy donors were used for monocyte purification by means of anti-CD14 microbeads following the manufacturer's protocol (Miltenyi Biotec, Germany). The CD14− fraction was placed in 10% dimethyl sulfoxide and frozen for a later lymphocyte proliferation test. To generate DCs, CD14+ monocyte cells were incubated in complete medium (CM) containing RPMI 1640 medium (Life Technologies, Invitrogen, USA) supplemented with 10% fetal calf serum (FCS; Life Technologies, USA), streptomycin (100 μg ml−1), gentamicin (1.25 U mL−1) as well as recombinant human rhGM-CSF (200 ng mL−1) and rhIL-4 (100 ng mL−1) (both from R&D Systems Inc., USA) for 5 days at 37 °C and 5% CO2. The resulting immature DCs (imDC) were then recovered and used in the experiments.DC maturation
imDCs derived from monocytes were incubated in complete medium at 105 cells per mL in 96-well plates (Nunc AS, Roskilde, Denmark) with different GDP concentrations (10−3, 10−4 and 10−5 mM). LPS at 1 mg mL−1 (Sigma) was used as the positive control and culture media as the negative control. After 72 h of stimulation at 37 °C in 5% CO2, the DCs were harvested and their maturation state was assessed by upregulation of CD80, CD86, and CD83 costimulatory molecules and HLA-DR, using fluorescently labelled monoclonal antibodies (BD Pharmigen, San Diego, California) in a FACSCanto II cytometer (BD Biosciences, Milpitas, California). The data were processed with FlowJo (TreeStar Inc., Switzerland). The results were expressed as the maturation index calculated as the ratio between the percentages of the stimulated DCs compared with the non-stimulated DCs.Lymphocyte transformation tests (LTTs) by CFSE dilution–proliferation
For this assay, imDCs were used as antigen-presenting cells; the CD14 fraction, which included autologous lymphocytes; and GDPs at 10−3, 10−4 and 10−5 mM concentrations. Autologous lymphocytes at concentrations of 0.5 to 1 × 107 mL−1 were labeled with 5,6-carboxyfluorescein diacetate N-succinimidyl ester (CFSE) following the manufacturer's instructions. A total of 100 μL of CFSE-labeled lymphocytes at a concentration of 1.5 × 106 mL−1 was cultured in a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with imDCs at a final volume of 250 μL of complete medium, in 96-well plates in triplicate, with or without a stimulus, for 6 days at 37 °C and 5% CO2. PHA at 10 μg mL−1 (Sigma) was used as the positive proliferative control and culture media as the negative control. The proliferation of different lymphocyte subsets (T cells and B cells) was assessed by flow cytometry, analysing the percentage of CD3, CD4, CD8 and CD19 cells that show a dim expression of CFSE (CFSEdim). The results were expressed as the proliferation index (PI), calculated for each subset as ([% CFSEdim stimulated lymphocytes–lymphocyte–DC]/% CFSEdim unstimulated lymphocytes).
1 ratio with imDCs at a final volume of 250 μL of complete medium, in 96-well plates in triplicate, with or without a stimulus, for 6 days at 37 °C and 5% CO2. PHA at 10 μg mL−1 (Sigma) was used as the positive proliferative control and culture media as the negative control. The proliferation of different lymphocyte subsets (T cells and B cells) was assessed by flow cytometry, analysing the percentage of CD3, CD4, CD8 and CD19 cells that show a dim expression of CFSE (CFSEdim). The results were expressed as the proliferation index (PI), calculated for each subset as ([% CFSEdim stimulated lymphocytes–lymphocyte–DC]/% CFSEdim unstimulated lymphocytes).
Ethics statement
The institutional review board “Ethics Committee of Málaga Hospital” approved the study. These studies were carried out in accordance with the Declaration of Helsinki. Oral and written informed consent for all the diagnostic procedures was obtained from the subjects before being included in the study.Statistical analysis
The maturation and proliferation results after culturing with the different GDPs were compared with nonparametric analysis for related samples (Wilcoxon test). All reported P values represent 2-sided tests, with the level of significance set at the probabilities of p < 0.05.Conclusions
Our studies indicate that viral peptides such as NP366–374 displayed in the novel GDPs are recognized by the immunological system inducing DC maturation and therefore an increase in the T lymphocyte proliferation. We have found that this effect is oriented to a higher proliferation for CD8 T cells, the typical cytotoxic cells in an immunological response against infectious diseases. These responses are directly related to peptide valency within the structure.Moreover, we have found that the activation marker CD83 appears to act as a maturational DC marker directly related to the proliferative response of CD8 T cells. These promising results suggest that this marker could be useful in evaluating candidates suitable to be used as viral vaccines inducing a T cell cytotoxic response. Further studies are needed to better characterize the immunological response induced by these structures.
Acknowledgements
This work was supported by the Instituto de Salud Carlos III (ISCIII) Thematic Networks and Co-operative Research Centres: RIRAAF (RD012/0013/0001 and RD012/0013/0016) and project ISCIII (PI12/02481); by Junta de Andalucía (CTS-7433) and the Nicolas Monardes Program (C-0044-2012 SAS 2013); and by Generalitat de Catalunya (SGR2009-00492) and Ministerio de Economía y Competitividad (MINECO), projects CTQ2011-23410 and SAF2011-24899. This work was co-financed by the European Regional Development Fund (ERDF).Notes and references
- (a) A. J. Engering, M. Cella, D. Fluitsma, M. Brockhaus, E. C. M. Hoefsmit, A. Lanzavecchia and J. Pieters, Eur. J. Immunol., 1997, 27(9), 2417–2425 CrossRef CAS PubMed; (b) M. C. A. A. Tan, A. M. Mommaas, J. W. Drijfhout, R. Jordens, J. J. M. Onderwater, D. Verwoerd, A. A. Mulder, A. N. van der Heiden, D. Scheidegger, L. C. J. M. Oomen, T. H. M. Ottenhoff, A. Tulp, J. J. Neefjes and F. Koning, Eur. J. Immunol., 1997, 27(9), 2426–2435 CrossRef CAS PubMed.
- (a) J. S. Lam, M. K. Mansour, C. A. Specht and S. M. Levitz, J. Immunol., 2005, 175(11), 7496–7503 CrossRef CAS; (b) M. Luong, J. S. Lam, J. Chen and S. M. Levitz, Vaccine, 2007, 25(22), 4340–4344 CrossRef CAS PubMed; (c) C. Grandjean, C. Rommens, H. Gras-Masse and O. Melnyk, Angew. Chem., Int. Ed., 2000, 39(6), 1068–1072 CrossRef CAS; (d) J. Wang, H. Li, G. Zou and L.-X. Wang, Org. Biomol. Chem., 2007, 5(10), 1529–1540 RSC.
- M. J. Purcell AW and J. Rossjohn, Nat. Rev. Drug Discovery, 2007, 6(5), 404–414 CrossRef PubMed.
- J. P. Tam, Proc. Natl. Acad. Sci. U. S. A., 1988, 85(15), 5409–5413 CrossRef CAS.
- W. Kowalczyk, A. Mascaraque, M. Sánchez-Navarro, J. Rojo and D. Andreu, Eur. J. Org. Chem., 2012,(24), 4565–4573 CrossRef CAS PubMed.
- B. M. Curtis, S. Scharnowske and A. J. Watson, Proc. Natl. Acad. Sci. U. S. A., 1992, 89(17), 8356–8360 CrossRef CAS.
- T. B. H. Geijtenbeek, R. Torensma, S. J. van Vliet, G. C. F. van Duijnhoven, G. J. Adema, Y. van Kooyk and C. G. Figdor, Cell, 2000, 100(5), 575–585 CrossRef CAS.
- R. Ribeiro-Viana, J. J. García-Vallejo, D. Collado, E. Pérez-Inestrosa, K. Bloem, Y. van Kooyk and J. Rojo, Biomacromolecules, 2012, 13(10), 3209–3219 CrossRef CAS PubMed.
- M. De Filette, W. Fiers, W. Martens, A. Birkett, A. Ramne, B. Löwenadler, N. Lycke, W. M. Jou and X. Saelens, Vaccine, 2006, 24(44–46), 6597–6601 CrossRef CAS PubMed.
- J. Kaiser, Science, 2006, 312(5772), 380–382 CrossRef CAS PubMed.
- A. R. M. Townsend, J. Rothbard, F. M. Gotch, G. Bahadur, D. Wraith and A. J. McMichael, Cell, 1986, 44(6), 959–968 CrossRef CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40(11), 2004–2021 CrossRef CAS.
- J. M. Langenhan and J. S. Thorson, Curr. Org. Synth., 2005, 2(1), 59–81 CrossRef CAS.
- (a) L. Yi-An, P. Clavijo, M. Galantino, S. Zhi-Yi, L. Wen and J. P. Tam, Mol. Immunol., 1991, 28(6), 623–630 CrossRef; (b) K. Rose, W. Zeng, L. E. Brown and D. C. Jackson, Mol. Immunol., 1995, 32(14–15), 1031–1037 CrossRef CAS.
- (a) W. Kowalczyk, B. G. de la Torre and D. Andreu, Bioconjugate Chem., 2010, 21(1), 102–110 CrossRef CAS PubMed; (b) P. Vepřek and J. Ježek, J. Pept. Sci., 1999, 5(5), 203–220 CrossRef; (c) W. Kowalczyk, M. Monsó, B. G. de la Torre and D. Andreu, J. Pept. Sci., 2011, 17(4), 247–251 CrossRef CAS PubMed.
- M. Lechmann, S. Berchtold, A. Steinkasserer and J. Hauber, Trends Immunol., 2002, 23(6), 273–275 CrossRef CAS.
- A. Prechtel and A. Steinkasserer, Arch. Dermatol. Res., 2007, 299(2), 59–69 CrossRef CAS PubMed.
- (a) G. Arrode, C. Boccaccio, J.-P. Abastado and C. Davrinche, J. Virol., 2002, 76(1), 142–150 CrossRef CAS; (b) M. Kruse, O. Rosorius, F. Krätzer, G. Stelz, C. Kuhnt, G. Schuler, J. Hauber and A. Steinkasserer, J. Virol., 2000, 74(15), 7127–7136 CrossRef CAS.
- (a) Y. Fujimoto, L. Tu, A. S. Miller, C. Bock, M. Fujimoto, C. Doyle, D. A. Steeber and T. F. Tedder, Cell, 2002, 108(6), 755–767 CrossRef CAS; (b) K. Lüthje, S. O. Cramer, S. Ehrlich, A. Veit, C. Steeg, B. Fleischer, A. V. Bonin and M. Breloer, Eur. J. Immunol., 2006, 36(8), 2035–2045 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Mass spectra, HPLC chromatograms and toxicity assays. See DOI: 10.1039/c5md00133a |
| This journal is © The Royal Society of Chemistry 2015 |