DOI:
10.1039/C4MD00526K
(Concise Article)
Med. Chem. Commun., 2015,
6, 879-886
Membrane-interacting properties of the functionalised fatty acid moiety of muraymycin antibiotics†
Received
18th November 2014
, Accepted 6th March 2015
First published on
6th March 2015
Abstract
Functional insights into bioactive natural products with medicinal potential are often hindered by their structural complexity. We herein report a simplified model system to investigate the functional significance of a structural motif of biologically potent muraymycin antibiotics of the A-series. These compounds have a highly unusual ω-guanidinylated fatty acid moiety, which has been proposed to mediate membrane penetration, thus enabling the interaction of A-series muraymycins with their intracellular target MraY. Our assay was based on a synthetic conjugate of this fatty acid structure with a negatively charged fluorophore lacking membrane permeability. Using this conjugate, immobilised giant unilamellar lipid vesicles and confocal laser scanning fluorescence microscopy, we demonstrated that the attachment of the ω-N-hydroxy-guanidinyl fatty acid unit led to an enhanced uptake of the fluorophore into the vesicles. This represents the first experimental evidence of this unusual structural motif's functional relevance for the parent natural product, which may support the future design of novel muraymycin analogues.
Introduction
Bacterial strains with resistances towards established antibiotics continue to emerge. However, only very few new antibacterial agents have been developed in recent years.1,2 It is therefore highly desirable to identify novel antimicrobial compounds displaying new or yet unexploited modes of action. Starting with the development of the penicillins in the 1940s, natural products and their analogues have served as clinically useful antibiotic agents. Thus, one may assume that naturally occurring antibiotics will probably play a key role in overcoming the current lack of new antibacterials for clinical use. This will include the identification of such compounds, their total synthesis for structure–activity relationship (SAR) studies, and also their functional elucidation. Due to the structural complexity of many natural products though, their functional analysis is often not trivial and requires time-consuming multi-step syntheses of suitable molecular probes.
Nucleoside
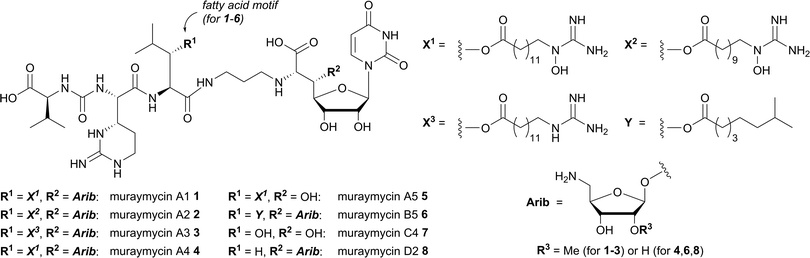
antibiotics represent a class of natural products targeting cell wall biosynthesis.3,4 Antibacterially active nucleosideantibiotics interfere with peptidoglycanassemblyvia inhibition of the bacterial membrane protein MraY, a key enzyme in the intracellular part of peptidoglycan formation.5–9 As the active site of MraY is located at the cytosolic side of the bacterial membrane, MraY inhibitors need to be able to penetrate the bacterial cell wall and the plasma membrane. Streptomyces-produced muraymycins (e.g. muraymycins A1 1 to A5 5, B5 6, C4 7 and D2 8, Fig. 1) represent one subclass of nucleosideantibiotics. They show promising activity against Staphylococcus aureus and Enterococcus strains via inhibition of MraY.10
Some SAR data have already been reported for muraymycins and their analogues.11–15 However, a structural feature of muraymycins which has only found limited attention so far is the presence of ω-guanidinylated fatty acid moieties in the biologically most potent muraymycins of the A-series. These fatty acid units are attached to the muraymycin backbone via esterification of a 3-hydroxy-L-leucine motif. Muraymycins of the B-series carry shorter and unfunctionalised fatty acids, while the fatty acid unit is missing in muraymycins of the C- and D-series. As a general rule, the A-series muraymycins show the best antibacterial activities, followed by the B-series congeners, while C- and D-series muraymycins are pronouncedly less active.10 These SAR findings might indicate (i) that the presence of the fatty acid moiety mediates membrane penetration of the otherwise polar muraymycin scaffold14 (and therefore cellular uptake of the acylated congeners) and (ii) that ω-guanidinylated fatty acids, despite the polarity of the ω-functionality, provide an even more pronounced membrane-penetrating effect.
The most obvious strategy to experimentally study this hypothesis would be to synthesise fluorescently labelled analogues of O-acylated muraymycins and to test them for membrane interaction and permeability e.g. with lipid vesicles. However, an attached fluorophore would potentially alter the molecular properties of muraymycins to a significant degree. Furthermore, the total synthesis of muraymycin-derived fluorescent probes would be time-consuming. We therefore decided to design a pronouncedly simplified model system to assay the fatty acid moiety of muraymycin A1 1 (residue X1, Fig. 1) for its ability to mediate membrane interaction and penetration. The goal was to study potential accumulation at the membrane interface and a possible increase of membrane permeability.
Results and discussion
Experimental design
The concept of the employed assay is depicted in Fig. 2. The fluorescent dye AlexaFluor 488 (AF488) was chosen as it exhibits large photostability. It was envisioned to convert a commercially available COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSazide-labelled derivative 9 of AF488 with the propargyl ester 10 of the fatty acid moiety of muraymycin A1 1 (subsequently named “lipid side chain”, LSC, Fig. 2B). Using the copper-catalysed version of the Huisgen alkyne–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSazidecycloaddition (“click”-chemistry),16–18 this should furnish AF488 LSC conjugate 11. It can then be tested if the presence of the LSC unit makes the AF488 moiety membrane-permeable, i.e. if fluorescence can be detected within lipid vesicles upon treatment with 11 (Fig. 2A). In order to ensure that the linker unit, i.e. the 1,4-substituted COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTStriazole, does not influence the low membrane permeability of the fluorescent dye, acetyl derivative 12 (“AF488 acetate”) lacking the full-length LSC motif was envisioned to serve as a reference (Fig. 2B).
 |
| | Fig. 2 A: Simplified assay system to test the influence of the fatty acid moiety of muraymycin A1 1 (LSC) on membrane-penetrating properties of an attached structure lacking membrane permeability (schematic representation). B: AlexaFluor 488 derivatives 11 and 12 conceived for the assay. | |
Synthesis of fluorescent probes
For the synthesis of AlexaFluor 488 LSC conjugate 11, propargyl ester 10 had to be prepared first (Scheme 1). Starting from erucic acid 13, epoxidation and alkaline epoxide opening gave 13,14-dihydroxy behenic acid 14 as a mixture of stereoisomers in 94% yield. Esterification with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSpropargyl alcohol by COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTScarbodiimide activation then furnished propargylic ester15 in 52% yield. Subsequent periodate cleavage afforded aldehyde16, i.e. an ω-functionalized tridecanoic acid propargyl ester, in 85% yield. Aldehyde16 was then employed in a sequence of oximeformation (product 17, 92% yield) and reduction to give N-alkyl hydroxylamine 18 (66% yield for the reduction step). As part of our synthetic studies on muraymycins and their analogues,19–26 we have systematically investigated the preparation of N-alkyl-N-hydroxy-guanidines by guanidinylation27 of N-alkyl COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTShydroxylamine precursors.28 A protecting group-free method using guanidinylation reagent 19 turned out to be the best method for this transformation. Consequently, treatment of 18 with 19 gave the desired N-alkyl-N-hydroxy-guanidine 10 with high conversion, but due to difficult purification of the amphiphilic product, in a moderate yield of 47% (Scheme 1).
 |
| | Scheme 1
Synthesis of AlexaFluor 488 derivatives 11 and 12. | |
Unexpectedly, the subsequent copper-catalysed “click” reaction towards conjugate 11 turned out to be non-trivial. First attempts under standard conditions only gave low conversions, which we speculate to be a consequence of the amphiphilic nature of alkyne10. Following a thorough variation of the reaction conditions, the reaction could be significantly improved by reductive in situ-generation of the copper(I) catalyst, application of the solvent system COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMF/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSmethanol and addition of the detergentTriton X-100. However, due to the high costs for COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSazide-labelled AF488 derivative 9, the reaction could only be performed on the sub-mg scale, thus making an unambiguous determination of the yield difficult. Product 11 could be obtained after HPLCpurification, and the identity of the product could be confirmed by mass spectrometry as the amount was insufficient for NMR spectroscopy. AF488 acetate 12 was prepared in an analogous manner using propargyl acetate 20 as the alkyne component for the “click” reaction (Scheme 1), and both target compounds 11 and 12 were thus available for the aforementioned fluorescence-based assay.
Membrane partitioning assay
The influence of AlexaFluor 488 LSC conjugate 11 on lipid membranes was investigated by means of fluorescence microscopy based on giant unilamellar vesicles (GUVs). GUVs composed of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSPOPC), fixed to a silicon substrate viaCOMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSbiotin–avidin interaction, were incubated with conjugate 11 and confocal laser scanning microscopy images were taken. The fluorescence image depicted in Fig. 3 clearly shows that conjugate 11 accumulates in the lipid membrane (also see Fig. 4). Part of the GUVs is also filled with the conjugate 11. As a control, the same experiment was performed with AF488 acetate 12 lacking the fatty acid moiety. In this case, no enrichment of the dye in the lipid membrane was observed. To quantitatively analyse the number of GUVs which were filled with the fluorescent conjugate, we defined a GUV as dye-filled if F/F0 > 0.5, with F being the intensity of the GUV interior and F0 being the background intensity. 24% of the POPC GUVs were filled with AF488 LSC conjugate 11 after 40 min and only 6% after incubation with AF488 acetate 12 (with the entire distribution for F/F0 > 0.5 being taken into account, Fig. 5A). It should be noted that values F/F0 > 1.0 could be ruled out as this would have required an active transport process (equilibrium is reached for F/F0 = 1.0). Active transport processes need external sources of energy (e.g. an electrochemical gradient or light coupling) though, and these were absent in the employed assay.
To investigate the influence of the lipidmixture on the dye distribution, we used a POPC/POPE (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine, 7 : 3) mixture to form GUVs better resembling the composition of bacterial membranes. Also for COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSPOPC/POPE membranes, an increased fluorescence intensity in the membrane interface as a result of accumulation of AF488 LSC conjugate 11 was found, leading to an almost four times larger fluorescence intensity at the membrane compared to the bulk solution (Fig. 4). 14% of the GUVs were filled with AF488 LSC conjugate 11, but only 4% in case of AF488 acetate 12 (Fig. 5B). Distribution patterns were similar to those found for the POPC GUVs (vide supra, also see Fig. 5A).
Based on the simplified model system, we were thus able to provide evidence that the fatty acid moiety of A-series muraymycins probably contributes to the accumulation of these compounds at a lipid membrane. An increased number of GUVs were filled with the lipidated fluorescent dye, which might be a result of the accumulation of the compound at the membrane interface, which can disturb the integrity of the bilayer. From these results, we conclude that muraymycin membrane partitioning and cell entry is probably facilitated by the ω-guanidinylated lipophilic side chain.
The role of guanidine moieties in membrane penetration and thus in cellular uptake processes has been widely investigated and discussed.29Arginine-rich cell-penetrating peptides have been extensively studied,30–33 and guanidinylated cationic lipids were reported to be effective transfection agents.34 Guanidinylated dendrimers were found to display pronounced interactions with liposomal phospholipid membranes,35 and conjugates of aminoglycoside antibiotics with guanidinylated cationic lipids showed antibacterial activity.36 However, such oligo-guanidinylated systems are probably prone to cooperative or multivalency effects, and it is therefore quite remarkable that a single ω-guanidinylated fatty acid motif (as in A-series muraymycins or in AlexaFluor 488 LSC conjugate 11) can enable membrane interaction and penetration as reported herein. It should be taken into account though that the N-hydroxylated guanidine moiety of A-series muraymycins and conjugate 11 is likely to have reduced basicity in comparison to non-hydroxylated guanidines.
Conclusions
In summary, we have developed an efficient and facile method to study the interaction of a naturally occurring structural motif with lipid membranes. Using this assay, we were able to demonstrate that the ω-functionalised fatty acid moiety found in some muraymycin nucleosideantibiotics of the A-series can mediate membrane accumulation and even membrane penetration of a fluorescent dye. This represents the first experimental evidence of this unusual structural motif's functional relevance for the parent natural product. While there is no close structural relation of the employed fluorophore and the natural product scaffold, the relative ease of our method will allow to test a significant number of fatty acid motifs for their potential to mediate membrane accumulation and penetration. This will be important to elucidate the relevance of the ω-substituent as well as of the length of the lipophilic moiety, i.e. the supposed interplay of polar ω-functionalisation and lipophilicity. Based on the results reported herein, we will therefore synthesise and investigate varying fatty acid units found in the muraymycin family as well as non-natural congeners with respect to different chain lengths and functional groups in the ω-position (e.g. amino-substituted and unfunctionalised analogues). Selected fatty acid structures will then be used for the synthesis of muraymycin analogues with potentially improved cellular uptake and thus also with enhanced biological activities. Overall, it is anticipated that these results will enable the design of muraymycin analogues with optimised biological profiles. This strategy might also have the potential to be employed within SAR studies on other lipidated natural products,37–41 and it might generally stimulate the functional analysis of medicinally relevant natural products based on simplified, chemically more tractable model systems.
Experimental
General methods.
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSAzide-labelled AlexaFluor 488 was obtained from Life Technologies. All other chemicals were purchased from standard suppliers. SiO2-supported NaIO4 was prepared according to an established procedure.42,43 Reactions involving oxygen and/or moisture sensitive reagents were carried out under an atmosphere of argon using anhydrous solvents. Anhydrous solvents were obtained in the following manner: COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2 was dried over COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCaH2 and distilled, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSpyridine was dried over COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCaH2 and distilled, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMF was dried over activated molecular sieves (4 Å) and degassed. All other solvents were of technical quality and distilled prior to their use, and deionised COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater was used throughout. Column chromatography was carried out on silica gel 60 (0.040–0.063 mm, 230–400 mesh ASTM, VWR) under flash conditions. TLC was performed on aluminium plates precoated with silica gel 60 F254 (VWR). Visualisation of the spots was carried out using UV light (254 nm) and/or staining under heating (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKMnO4 staining solution: 1 g COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKMnO4, 6 g COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 and 1.5 mL 1.25 M COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNaOHsolution, all dissolved in 100 mL COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH2O). Preparative HPLC was performed on a VWR-Hitachi system equipped with an L-2300 pump, an L-2200 autosampler, an L-2300 column oven (25 °C), an L-2455 Diode Array Detector (DAD) and a LiChroCart™ column (4 × 125 mm) containing reversed phase silica gel Purospher™ RP18e (5 μm) purchased from VWR. 300 MHz-1H and 75 MHz-, 76 MHz-, and 126 MHz-13C NMR spectra were recorded on Varian MERCURY 300, UNITY 300 and INOVA 500 spectrometers. All 13C NMR spectra are 1H-decoupled. All spectra were recorded at room temperature except of samples in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMSO-d6 (standard 35 °C) and where indicated otherwise and were referenced internally to solvent reference frequencies wherever possible. Chemical shifts (δ) are quoted in ppm, and coupling constants (J) are reported in Hz. Assignment of signals was carried out using 1H,1H-COSY, HSQC and HMBC spectra obtained on the spectrometers mentioned above. Low resolution ESI mass spectrometry was performed on a Varian MAT 311 A spectrometer operating in positive or negative ionisation mode. High resolution (HR) ESI mass spectrometry was carried out on a Bruker microTOF spectrometer or a Bruker 7T FTICR APEX IV spectrometer. Melting points (mp) were measured on a Büchi instrument and are not corrected. Infrared spectroscopy (IR) was performed on a Jasco FT/IR-4100 spectrometer equipped with an integrated ATR unit (GladiATR™, PIKE Technologies). Wavenumbers (ν) are quoted in cm−1. UV spectroscopy was carried out on a Jasco V-630 spectrometer.
Propargyl 13-(N-hydroxyguanidino)-tridecanoate
10
.
1H-Pyrazole-1-carboxamidine hydrochloride
19 (72 mg, 0.49 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNEt3 (0.14 mL, 0.10 g, 1.00 mmol) were added to a solution of N-alkyl hydroxylamine 18 (100 mg, 0.353 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMF (2 mL) and the reaction mixture was stirred at rt for 15 h. The solvent was evaporated under reduced pressure and the resultant residue was dissolved in sat. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNH4Clsolution (5 mL). The aqueous solution was extracted with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSEt2O (3 × 10 mL) and the combined organics were dried over COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa2SO4 and evaporated under reduced pressure. The resultant crude product was purified by column chromatography (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMeOH, 9 : 1 → 1 : 1) to give 10 (53 mg, 47%) as a colourless solid; mp 77 °C; TLCRf 0.28 (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMeOH, 4 : 1); IR (ATR) νmax/cm−1 2920, 2848, 1728, 1626, 1468, 1166 and 1105; δH (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMSO-d6) 1.09–1.36 (16 H, m, 4-H2-11-H2), 1.41–1.65 (4 H, m, 3-H2, 12-H2), 2.30 (2 H, t, J 7.3, 2-H2), 3.50 (1 H, d, J 2.5, 3′-H), 3.54 (2 H, t, J 7.0, 13-H2), 4.66 (2 H, d, J 2.5, 1′-H2) and 7.61 (4 H, br s, NH, OH); δC (76 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMSO-d6) 24.3, 25.7, 25.7, 28.2, 28.5, 28.7, 28.8, 28.9, 33.1, 50.7, 51.4, 77.4, 78.4, 157.7 and 172.1; m/z (ESI+) 326.2 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH+); m/z (HR-ESI+) 326.2438 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH+, C17H32N3O3 requires 326.2438).
AlexaFluor 488 lipid side chain conjugate 11.
A solution of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSazide-labelled AlexaFluor 488 9 (50 mM in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMF, 2.0 μL, 0.10 μmol 9), a solution of lipid side chain propargyl ester 10 (50 mM in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMeOH, 8.0 μL, 0.40 μmol 10), a solution of sodium L-ascorbate (20 mM in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater, 4.0 μL, 80 nmol sodium L-ascorbate), a solution of CuSO4 (10 mM in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater, 4.0 μL, 40 nmol CuSO4), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMeOH (6 μL) and Triton X-100 (10% in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater, 48 μL) were mixed and stirred in a ThermoMixer (50 °C, 200 rpm) for 15 h. The reaction mixture was then directly purified by preparative HPLC (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMeCN–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater (+0.01% COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTFA), 15 : 85 → 50 : 50, 1.0 mL min−1). Product-containing fractions were pooled, and the solvent was removed with an Eppendorf Concentrator 5301 to give 11 (estimated from UV-Vis data: 9.0 nmol) as a red solid; preparative HPLCtR 13.3 min; m/z (ESI+) 984.3 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH+) and 1006.3 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa+); m/z (HR-ESI+) 984.3594 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH+, C44H58N9O13S2 requires 984.3590).
AlexaFluor 488 acetyl conjugate 12.
A solution of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSazide-labelled AlexaFluor 488 9 (5 mM in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMF, 20 μL, 0.10 μmol 9), a solution of propargyl acetate 20 (1 M in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMeOH, 14 μL, 14 μmol 20), a solution of sodium L-ascorbate (20 mM in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater, 10 μL, 0.20 μmol sodium L-ascorbate), a solution of CuSO4 (10 mM in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater, 10 μL, 0.10 μmol CuSO4) and Triton X-100 (10% in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater, 48 μL) were mixed and stirred in a ThermoMixer (10 °C, 200 rpm) for 17 h. The reaction mixture was then directly purified by preparative HPLC (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMeCN–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater (+0.01% COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTFA), 15 : 85 → 50 : 50, 1.0 mL min−1). Product-containing fractions were pooled, and the solvent was removed with an Eppendorf Concentrator 5301 to give 12 (estimated from UV-Vis data: 32 nmol) as a red solid; preparative HPLCtR 6.8 min; m/z (ESI−) 755.1 (M − COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH+); m/z (HR-ESI−) 755.1446 (M − COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH+, C32H31N6O12S2 requires 755.1447).
13,14-Dihydroxybehenic acid 14.
A solution of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH2O2 (30% in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater, 15.0 mL, 16.7 g, 147 mmol COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH2O2) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSformic acid (50 mL) was added dropwise to a suspension of erucic acid 13 (5.00 g, 14.8 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSformic acid (50 mL) and stirred at rt for 23 h. Excess COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH2O2 was destroyed with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa2SO3 (negative iodinestarch test) and the solvent was evaporated under reduced pressure. The resultant residue was dissolved in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKOHsolution (1 M, 100 mL) and heated under reflux for 4 h. The reaction mixture was then acidified with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSHCl (2 M, 100 mL) and the aqueous layer was extracted with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSEtOAc (3 × 100 mL). The combined organics were dried over COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa2SO4 and the solvent was evaporated under reduced pressure to give 14 (5.17 g, 94%) as a colourless solid; mp 110 °C; IR (ATR) νmax/cm−1 3332, 3254, 2912, 2846, 1703, 1467, 720 and 656; δH (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMSO-d6) 0.68 (3 H, t, J 6.7, 22-H3), 1.15–1.33 (28 H, m, 4-H2-11-H2, 16-H2-21-H2), 1.34–1.43 (4 H, m, 12-H2, 15-H2), 1.44–1.57 (2 H, m, 3-H2), 2.18 (2 H, t, J 7.3, 2-H2), 3.15–3.25 (2 H, m, 13-H, 14-H) and 4.06 (2 H, br s, OH); δC (75 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMSO-d6) 13.8, 22.0, 28.5, 28.6, 28.7, 28.8, 28.9, 28.9, 29.0, 29.0, 29.2, 31.2, 24.4, 25.5, 32.3, 33.6, 73.0 and 174.3; m/z (ESI+) 373.3 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH+) and 395.4 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa+); m/z (HR-ESI+) 373.3311 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH+, C22H45O4 requires 373.3312).
Propargyl 13,14-dihydroxybehenoate
15
.
To a solution of carboxylic acid14 (1.71 g, 4.56 mmol), propargylic alcohol (5.30 mL, 91.7 mmol) and 4-(dimethylamino)-pyridine (DMAP, 59 mg, 0.48 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2 (100 mL), dicyclohexyl carbodiimide (DCC, 1.22 g, 5.91 mmol) was added. The reaction mixture was stirred at rt for 20 h and then diluted with sat. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNH4Clsolution (90 mL). The aqueous layer was extracted with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2 (2 × 90 mL). The combined organics were washed with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater (1 × 90 mL), dried over COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa2SO4 and evaporated under reduced pressure. The resultant crude product was purified by column chromatography (petroleumether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSEtOAc, 3 : 1) to give 15 (980 mg, 52%) as a colourless solid; mp 64 °C; TLCRf 0.36 (petroleumether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSEtOAc, 2 : 1); IR (ATR) νmax/cm−1 3300, 2913, 2846, 1740, 1467, 1170, 721 and 640; δH (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMSO-d6) 0.84 (3 H, t, J 6.6, 22-H3), 1.12–1.46 (32 H, m, 4-H2-12-H2, 15-H2-21-H2), 1.47–1.63 (2 H, m, 3-H2), 2.28 (2 H, t, J 7.4, 2-H2), 3.13 (1 H, t, J 2.4, 3′-H), 3.14–3.27 (2 H, m, 13-H, 14-H), 3.89 (2 H, d, J 4.0, OH) and 4.61 (2 H, d, J 2.4, 1′-H2); δC (76 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMSO-d6) 13.6, 22.0, 25.3, 28.3, 28.5, 28.6, 28.7, 28.9, 28.9, 28.9, 29.0, 29.1, 31.2, 32.6, 24.2, 33.1, 51.1, 73.1, 77.8, 78.5 and 171.7; m/z (ESI+) 433.4 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa+); m/z (HR-ESI+) 433.3288 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa+, C25H46NaO4 requires 433.3288).
Propargyl 13-oxotridecanoate 16.
To a solution of diol15 (857 mg, 2.09 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2 (20 mL), SiO2-supported NaIO4 (ref. 42 and 43) (0.610 mmol g−1, 5.14 g, 3.14 mmol NaIO4) was added. The resultant suspension was stirred at rt for 1 h and then filtered. The filtrate was evaporated under reduced pressure. The resultant crude product was purified by column chromatography (petroleumether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSEtOAc, 6 : 1) to give 16 (473 mg, 85%) as a colourless oil; TLCRf 0.29 (petroleumether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSEtOAc, 6 : 1); IR (ATR) νmax/cm−1 2924, 2853, 1738, 1724, 1157, 1106, 1025, 997 and 666; δH (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMSO-d6) 1.15–1.60 (18 H, m, 3-H2-11-H2), 2.32 (2 H, t, J 7.3, 2-H2), 2.40 (2 H, td, J 7.2, J 1.6, 12-H2), 3.47 (1 H, t, J 2.2, 3′-H), 4.67 (2 H, d, J 2.2, 1′-H2) and 9.66 (1 H, t, J 1.6, 13-H); δC (76 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMSO-d6) 24.2, 28.2, 28.4, 28.5, 28.5, 28.6, 28.7, 28.7, 28.8, 33.1, 42.9, 51.3, 77.2, 78.4, 172.0 and 203.3; m/z (ESI+) 289.2 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa+); m/z (HR-ESI+) 289.1778 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa+, C16H26NaO3 requires 289.1774).
Propargyl 13-hydroxyiminotridecanoate
17
.
A solution of aldehyde16 (359 mg, 1.35 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTShydroxylamine hydrochloride (470 mg, 6.76 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSEtOH (5 mL) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSpyridine (5 mL) was stirred in the presence of molecular sieve (3 Å) at rt for 46 h. The reaction mixture was filtered through a short pad of celite and the solvent of the filtrate was evaporated under reduced pressure. The resultant crude product was purified by column chromatography (petroleumether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSEtOAc, 4 : 1) to give 17 (349 mg, 92%) as a mixture of E/Z-isomers as a colourless solid; TLCRf 0.22 (petroleumether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSEtOAc, 4 : 1); δH (300 MHz, C6D6) 1.05–1.31 (16 H, m, 4-H2-11-H2), 1.43–1.61 (2 H, m, 3-H2), 2.01 (1 H, t, J 2.4, 3′-H), 2.06 (2 H, t, J 7.4, 2-H2), 2.31 (2 H, td, J 7.1, J 5.4, 12-H2), 4.44 (2 H, d, J 2.4, 1′-H2), 6.51 (1 H, t, J 5.4, 13-H) and 8.94 (1 H, br s, OH); δC (126 MHz, C6D6) 25.2, 25.4, 26.4, 29.4, 29.7, 29.7, 29.8, 29.9, 29.9, 30.0, 34.1, 51.6, 74.8, 78.4, 152.2 and 172.2; m/z (ESI+) 304.2 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa+); m/z (HR-ESI+) 304.1884 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa+, C16H27NNaO3 requires 304.1883).
Propargyl 13-hydroxyaminotridecanoate
18
.
NaBH3CN (1.18 g, 18.8 mmol) and freshly prepared COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSHCl in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMeOH (1 M) were added alternately and portionwise to a solution of oxime17 (500 mg, 1.78 mmol) and methyl orange (small amount, indicator) in i-PrOH (25 mL) until the reaction mixture retained a pink color. The reaction mixture was stirred at rt for 24 h and then neutralised with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNEt3 and evaporated under reduced pressure. The resultant residue was dissolved in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSEtOAc (50 mL), washed with sat. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNaHCO3 (2 × 50 mL) and brine (1 × 50 mL), dried over COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa2SO4 and evaporated under reduced pressure. The resultant crude product was purified by column chromatography (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSEtOAc) to give 18 (334 mg, 66%) as a colourless solid; mp 62 °C; TLCRf 0.35 (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSEtOAc); IR (ATR) νmax/cm−1 3299, 2916, 2848, 1740, 1464, 1390, 1276, 1225, 1199 and 1175; δH (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3) 1.18–1.38 (16 H, m, 4-H2-11-H2), 1.46–1.69 (4 H, m, 3-H2, 12-H2), 2.34 (2 H, t, J 7.5, 2-H2), 2.46 (1 H, t, J 2.5, 3′-H), 2.92 (2 H, t, J 7.3, 13-H2), 4.66 (2 H, d, J 2.5, 1′-H2) and 5.83 (2 H, br s, NH, OH); δC (126 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3) 24.8, 26.7, 27.1, 29.1, 29.1, 29.2, 29.4, 29.5, 29.5, 29.6, 34.0, 51.7, 53.6, 74.7, 77.8 and 172.8; m/z (ESI+) 284.2 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH+) and 306.2 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa+); m/z (HR-ESI+) 284.2223 (M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH+, C16H30NO3 requires 284.2220).
Membrane partitioning assay
Materials.
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSPOPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[biotinyl(polyethylene glycol)-2000] (DSPE-PEG2000-biotin) were purchased from Avanti Polar Lipids. Sulforhodamine 101 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanol-L-amine-triethyl ammonium salt (Texas Red DHPE) was obtained from Sigma-Aldrich.
Confocal laser scanning microscopy
.
Fluorescence images were taken with a confocal laser scanning microscope (LSM 710, Carl Zeiss, Jena, Germany) equipped with a water immersion objective W Plan-Apochromat 63×/1.0 n.a. (Zeiss). AlexaFluor 488 and Texas Red were excited at λex = 488 nm and 563 nm, respectively. Emission was detected at λem = 495–538 nm and 633–690 nm, respectively.
Acknowledgements
The authors thank the Deutsche Forschungsgemeinschaft (DFG, SFB 803 “Functionality controlled by organization in and between membranes”) and the Fonds der Chemischen Industrie (FCI, Sachkostenzuschuss) for financial support.
Notes and references
- G. Taubes, Science, 2008, 321, 356–361 CrossRef CAS PubMed.
- M. A. Cooper and D. Shlaes, Nature, 2011, 472, 32 CrossRef CAS PubMed.
- K.-I. Kimura and T. D. H. Bugg, Nat. Prod. Rep., 2003, 20, 252–273 RSC.
- M. Winn, R. J. M. Goss, K.-I. Kimura and T. D. H. Bugg, Nat. Prod. Rep., 2010, 27, 279–304 RSC.
- C. Dini, Curr. Top. Med. Chem., 2005, 5, 1221–1236 CrossRef CAS.
- T. D. H. Bugg, A. J. Lloyd and D. I. Roper, Infect. Disord.: Drug Targets, 2006, 6, 85–106 CrossRef CAS.
- A. J. Lloyd, P. E. Brandish, A. M. Gilbey and T. D. H. Bugg, J. Bacteriol., 2004, 186, 1747–1757 CrossRef CAS.
- M. T. Rodolis, A. Mihalyi, A. O'Reilly, J. Slikas, D. I. Roper, R. E. W. Hancock and T. D. H. Bugg, ChemBioChem, 2014, 15, 1300–1308 CrossRef CAS PubMed.
- M. T. Rodolis, A. Mihalyi, C. Ducho, K. Eitel, B. Gust, R. J. M. Goss and T. D. H. Bugg, Chem. Commun., 2014, 50, 13023–13025 RSC.
- L. A. McDonald, L. R. Barbieri, G. T. Carter, E. Lenoy, J. Lotvin, P. J. Petersen, M. M. Siegel, G. Singh and R. T. Williamson, J. Am. Chem. Soc., 2002, 124, 10260–10261 CrossRef CAS PubMed.
- Y.-I. Lin, Z. Li, G. D. Francisco, L. A. McDonald, R. A. Davis, G. Singh, Y. Yang and T. S. Mansour, Bioorg. Med. Chem. Lett., 2002, 12, 2341–2344 CrossRef CAS.
- A. Yamashita, E. Norton, P. J. Petersen, B. A. Rasmussen, G. Singh, Y. Yang, T. S. Mansour and D. M. Ho, Bioorg. Med. Chem. Lett., 2003, 13, 3345–3350 CrossRef CAS.
- T. Tanino, S. Ichikawa, B. Al-Dabbagh, A. Bouhss, H. Oyama and A. Matsuda, ACS Med. Chem. Lett., 2010, 1, 258–262 CrossRef CAS PubMed.
- T. Tanino, B. Al-Dabbagh, D. Mengin-Lecreulx, A. Bouhss, H. Oyama, S. Ichikawa and A. Matsuda, J. Med. Chem., 2011, 54, 8421–8439 CrossRef CAS PubMed.
- Y. Takeoka, T. Tanino, M. Sekiguchi, S. Yonezawa, M. Sakagami, F. Takahashi, H. Togame, Y. Tanaka, H. Takemoto, S. Ichikawa and A. Matsuda, ACS Med. Chem. Lett., 2014, 5, 556–560 CrossRef CAS PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., 2001, 113, 2056–2075 (
Angew. Chem., Int. Ed.
, 2001
, 40
, 2004–2021
) CrossRef.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., 2002, 114, 2708–2711 (
Angew. Chem., Int. Ed.
, 2002
, 41
, 2596–2599
) CrossRef.
- C. W. Tornoe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef CAS PubMed.
- A. P. Spork, S. Koppermann and C. Ducho, Synlett, 2009, 2503–2507 CAS.
- A. P. Spork, S. Koppermann, B. Dittrich, R. Herbst-Irmer and C. Ducho, Tetrahedron: Asymmetry, 2010, 21, 763–766 CrossRef CAS PubMed.
- A. P. Spork and C. Ducho, Org. Biomol. Chem., 2010, 8, 2323–2326 CAS.
- A. P. Spork, D. Wiegmann, M. Granitzka, D. Stalke and C. Ducho, J. Org. Chem., 2011, 76, 10083–10098 CrossRef CAS PubMed.
- M. Büschleb, M. Granitzka, D. Stalke and C. Ducho, Amino Acids, 2012, 43, 2313–2328 CrossRef PubMed.
- A. P. Spork and C. Ducho, Synlett, 2013, 24, 343–346 CrossRef CAS PubMed.
- O. Ries, M. Büschleb, M. Granitzka, D. Stalke and C. Ducho, Beilstein J. Org. Chem., 2014, 10, 1135–1142 CrossRef PubMed.
- A. P. Spork, M. Büschleb, O. Ries, D. Wiegmann, S. Boettcher, A. Mihalyi, T. D. H. Bugg and C. Ducho, Chem. – Eur. J., 2014, 20, 15292–15297 CrossRef CAS PubMed.
- J. H. Jones, J. Pept. Sci., 2002, 8, 285–287 CrossRef CAS PubMed.
- O. Ries, A. Ochmann and C. Ducho, Synthesis, 2011, 2357–2368 CAS.
- E. Wexselblatt, J. D. Esko and Y. Tor, J. Org. Chem., 2014, 79, 6766–6774 CrossRef CAS PubMed.
- E. Geihe Stanzl, B. M. Trantow, J. R. Vargas and P. A. Wender, Acc. Chem. Res., 2013, 46, 2944–2954 CrossRef PubMed.
- C. Bechara and S. Sagan, FEBS Lett., 2013, 587, 1693–1702 CrossRef CAS PubMed.
- D. M. Copolovici, K. Langel, E. Eriste and Ü. Langel, ACS Nano, 2014, 8, 1972–1994 CrossRef CAS PubMed.
- R. Brock, Bioconjugate Chem., 2014, 25, 863–868 CrossRef CAS PubMed.
- J. Sen and A. Chaudhuri, J. Med. Chem., 2005, 48, 812–820 CrossRef CAS PubMed.
- I. Tsogas, D. Tsiourvas, G. Nounesis and C. M. Paleos, Langmuir, 2006, 22, 11322–11328 CrossRef CAS PubMed.
- S. Bera, G. G. Zhanel and F. Schweizer, J. Antimicrob. Chemother., 2010, 65, 1224–1227 CrossRef CAS PubMed.
-
H. Hashizume and Y. Nishimura, in Natural Products Chemistry, ed. Atta-ur-Rahman, Elsevier, Amsterdam, 2008, vol. 35, pp. 693–751 (and references therein) Search PubMed.
- T. Noguchi, Y. Yasuda, T. Niida and T. Shomura, Nippon Shokubutsu Byori Gakkaiho, 1968, 34, 323–327 CAS.
- A. Takatsuki, K. Arima and G. Tamura, J. Antibiot., 1971, 24, 215–223 CrossRef CAS.
- K. Dobashi, H. Naganawa, Y. Takahashi, T. Takita and T. Takeuchi, J. Antibiot., 1988, 41, 1533–1541 CrossRef CAS.
- A. Nemoto, Y. Hoshino, K. Yazawa, A. Ando, Y. Mikami, H. Komaki, Y. Tanaka and U. Gräfe, J. Antibiot., 2002, 55, 593–597 CrossRef CAS.
- Y.-L. Zhong and S. Chang, J. Org. Chem., 1997, 62, 2622–2624 CrossRef CAS.
- N. K. Dunlap, J. Drake, A. Ward, T. L. J. Salyard and L. Martin, J. Org. Chem., 2008, 73, 2928–2930 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article