Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and cytostatic activity of 7-arylsulfanyl-7-deazapurine bases and ribonucleosides†
Martin
Klečka
ab,
Lenka Poštová
Slavětínská
b,
Eva
Tloušťová
b,
Petr
Džubák
c,
Marián
Hajdúch
c and
Michal
Hocek
*ab
aInstitute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Gilead Sciences & IOCB Research Center, Flemingovo nam. 2, CZ-16610 Prague 6, Czech Republic. E-mail: hocek@uochb.cas.cz; Tel: +420 220183324
bDepartment of Organic Chemistry, Faculty of Science, Charles University in Prague, Hlavova 8, CZ-12843 Prague 2, Czech Republic
cInstitute of Molecular and Translational Medicine, Faculty of Medicine and Dentistry, Palacky University and University Hospital in Olomouc, Hněvotínská 5, CZ-775 15 Olomouc, Czech Republic
First published on 2nd December 2014
Abstract
A series of 7-phenylsulfanyl- or 7-(2-thienyl)sulfanyl-7-deazapurine bases bearing diverse substituents at position 6 was prepared through C–H sulfenylation of 6-chloro-7-deazapurine followed by cross-coupling or nucleophilic substitutions. The corresponding ribonucleosides (as thia-analogues of known nucleoside cytostatics) were prepared by glycosylation of 6-chloro-7-arylsulfanyl-7-deazapurines followed by the same transformations at position 6. The 7-thienylsulfanyl-7-deazapurine bases 2b–2h exerted micromolar cytostatic activities, whereas the nucleosides did not show significant biological effects.
1. Introduction
Several base-modified purine nucleosides are important antitumor agents.1 Also diverse substituted purine and deazapurine bases exert cytostatic effects, typically through inhibition of kinases and other ATP- or GTP-dependent enzymes.2 Recently, we have discovered new types of nucleoside cytostatics: 6-hetaryl-7-deazapurine,3 7-hetaryl-7-deazaadenine4 and 6-substituted 7-hetaryl-7-deazapurine5ribonucleosides. They all showed cytostatic effects at nanomolar concentrations and their mechanism of action is not yet fully understood. They are inhibitors of adenosine kinases,6,7 but they are substrates at the same time and are phosphorylated to nucleoside triphosphates which then interfere with RNA synthesis or are incorporated to DNA and RNA. In all three series, the most active were derivatives bearing thiophene or furan (Chart 1).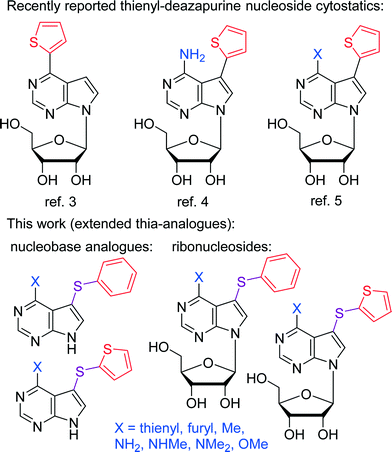 | ||
| Chart 1 Previously reported nucleoside cytostatics and the design of thia-analogues under study. | ||
C–H activation reactions are increasingly popular methods in organic synthesis8 and were also applied in purines and deazapurines. In addition to relatively common and useful C–H arylations of purines reported by us9 and by others,10 we have recently reported C–H borylation11 and C–H sulfenylation12 of 7-deazapurines. The latter method gave access to 7-arylsulfanyl-7-deazapurine bases,12 which can be considered extended thia-analogues of 7-aryl-7-deazapurines that are components of the abovementioned nucleoside cytostatics.4,5 Therefore, we decided to prepare a series of 7-phenylsulfanyl- and 7-(2-thienyl)sulfanyl-7-deazapurine bases and ribonucleosides for screening of their anticancer activity. These extended analogues should show whether the direct conjugation of the (het)aryl group at position 7 is needed for the cytostatic activity of this class of 7-deazapurine nucleosides,4,5 and in principle, they can also be metabolized to other sulfur-containing nucleosides.
2. Results and discussion
2.1. Chemistry
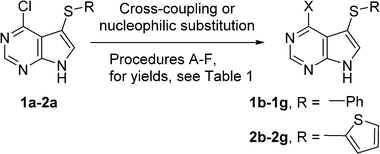
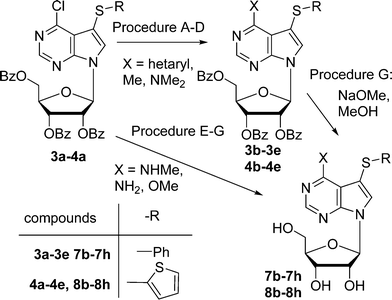
The proposed synthetic approach to our target 7-arylsulfanyl-7-deazapurines was based on recently developed direct C–H sulfenylation12 of 6-chloro-7-deazapurine (correct IUPAC name: 4-chloro-7H-pyrrolo[2,3-d]pyrimidine) catalysed by CuI and 4,4-di-tert-butyl bipyridine (dtbpy) under oxygenatmosphere. This modified procedure (oxygenatmosphere and dtbpy) gave better results than previously published methods developed for related heterocycles.13 By the reaction with diphenyldisulfide and bis(2-thienyl)disulfide, two modified 7-(het)arylsulfanyl-7-deazapurines 1a and 2a were synthesized in excellent yield (90% or 95%) (Scheme 1). After one-pot silylation by N,O-bis(trimethylsilyl)acetamide (BSA) of 1a followed by glycosylation using commercially available 1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose, in analogy to the modified Vorbrüggen procedure,14 the desired protected 7-phenylsulfanyl-7-deazapurine ribonucleoside intermediate 3a was obtained in good yield of 49% (Scheme 1). In case of 7-thienylsulfanyl-7-deazapurine 2a, the silylation was not completed under standard conditions and therefore 2 equiv. of BSA were used to fully dissolve the starting material, even though the yield of the following glycosylation to 4a was only 30%, which was still sufficient to make multigram amounts of this key intermediate.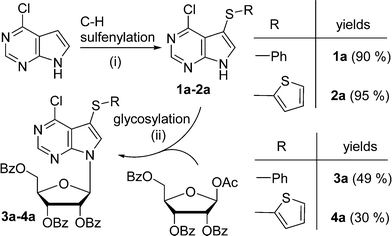 | ||
| Scheme 1 Reagents and conditions: i) RS-SR (1 equiv.), CuI (10%), dtbpy (20%), O2, DMF, 110 °C, 18 h. ii) 1. BSA (1 or 2 equiv.), MeCN, 15 min, rt, 2. TMSOTf (2 equiv.), sugar (1 equiv.), 80 °C, 6 h. | ||
In order to synthesize a series of target 6-substituted 7-deazapurine nucleobase analogues, 6-chlorodeazapurine intermediates 1a and 2a were modified at position 6. The first goal was to introduce thiophene and furan substituents (previously reported3 in cytostaticnucleosides). Since attempted Suzuki–Miyaura cross-coupling reactions with the corresponding thienyl- or furylboronic acids gave very low conversions (<10%), we further focused on the Stille coupling. Thus the Stille reactions of 1a or 2a with thienyl- or furyl(tributyl)stannanes under standard conditions in the presence of PdCl2(PPh3)2 in DMF proceeded smoothly to give desired 6-hetaryl derivatives 1b–1c and 2b–2c in good yields (57–87%) (Scheme 2, Table 1, entries 1, 2, 7, and 8). A methyl group was introduced through Pd-catalysed cross-coupling of 1a or 2a with Me3Al to give 1d and 2d in good yields (entries 3 and 9). Finally, dimethylamino, methylamino and amino groups were introduced through aromatic nucleophilic substitution of 6-chloro-derivative 1a or 2a with amines or ammonia to give 1e–1f and 2e–2f in good yields (58–85%, entries 4–6, 10–12).
| Entry | Procedure | Reagent | X– | R– | Product (yield %) |
|---|---|---|---|---|---|
| 1 | A | 2-ThienylSnBu3 | 2-Thienyl- | Ph– | 1b (80%) |
| 2 | B | 2-FurylSnBu3 | 2-Furyl- | Ph– | 1c (87%) |
| 3 | C | Me3Al | Me– | Ph– | 1d (73%) |
| 4 | D | Me2NH | Me2N– | Ph– | 1e (84%) |
| 5 | E | MeNH2 | MeNH– | Ph– | 1f (83%) |
| 6 | F | NH3 | NH2– | Ph– | 1g (85%) |
| 7 | A | 2-ThienylSnBu3 | 2-Thienyl- | 2-Thienyl- | 2b (57%) |
| 8 | B | 2-FurylSnBu3 | 2-Furyl- | 2-Thienyl- | 2c (72%) |
| 9 | C | Me3Al | Me– | 2-Thienyl- | 2d (66%) |
| 10 | D | Me2NH | Me2N– | 2-Thienyl- | 2e (63%) |
| 11 | E | MeNH2 | MeNH– | 2-Thienyl- | 2f (58%) |
| 12 | F | NH3 | NH2– | 2-Thienyl- | 2g (85%) |
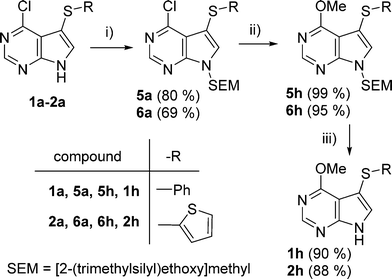
On the other hand, direct methoxylation of 1a–2a by reaction with NaOMe in MeOH was not successful. Therefore, we first protected the NH at position 9 by a SEM group and then the methoxylation of 5a or 6a by MeONa proceeded quantitatively to give intermediates 5h and 6h. Final cleavage of the SEM groups by TFA afforded the desired 6-methoxy-7-deazapurines 1h and 2h in high yields (Scheme 3).
The target nucleoside analogues were prepared by analogous modifications of 6-chloro-7-(het)aryl-7-deazapurine nucleoside intermediates 3a and 4a (Scheme 4, Table 2). The Stille coupling reactions with thienyl- or furylstannanes gave the corresponding benzoylated 6-hetaryl-7-deazapurine nucleosides3b and 3c and 4b and 4c, whereas the coupling with trimethylaluminum afforded 6-methyl derivatives 3d and 4d. The reactions with trimethylamine furnished 6-(dimethylamino)-7-deazapurine nucleosides3e and 4e. Final Zemplén deprotection using sodium methoxide in methanol furnished free 6,7-disubstituted nucleosides7b–7e and 8b–8e in 59–87% yields (Scheme 4, Table 2). Nucleophilic substitutions of protected nucleoside intermediate 3a or 4a with methylamine, ammonia or NaOMe proceeded with concomitant de-benzoylation to give directly unprotected 6-methylamino-, 6-amino or 6-methoxy-7-(het)arylsulfanyl-7-deazapurine ribonucleosides7f–7h and 8f–8h in good yields.
| Procedure | Reagent | X– | R– | Product (yield %) | Deprotection product (yield %) |
|---|---|---|---|---|---|
| A | ThienylSnBu3 | 2-Thienyl- | Ph– | 3b (72%) | 7b (75%) |
| B | FurylSnBu3 | 2-Furyl- | Ph– | 3c (92%) | 7c (78%) |
| C | Me3Al | Me– | Ph– | 3d (55%) | 7d (87%) |
| D | Me2NH | Me2N– | Ph– | 3e (88%) | 7e (87%) |
| E | MeNH2 | MeNH– | Ph– | — | 7f (90%) |
| F | NH3 | NH2– | Ph– | — | 7g (86%) |
| G | NaOMe | MeO– | Ph– | — | 7h (75%) |
| A | ThienylSnBu3 | 2-Thienyl- | 2-Thienyl- | 4b (78%) | 8b (59%) |
| B | FurylSnBu3 | 2-Furyl- | 2-Thienyl- | 4c (41%) | 8c (57%) |
| C | Me3Al | Me– | 2-Thienyl- | 4d (67%) | 8d (64%) |
| D | Me2NH | Me2N– | 2-Thienyl- | 4e (88%) | 8e (65%) |
| E | MeNH2 | MeNH– | 2-Thienyl- | — | 8f (75%) |
| F | NH3 | NH2– | 2-Thienyl- | — | 8g (70%) |
| G | NaOMe | MeO– | 2-Thienyl- | — | 8h (77%) |
2.2. Biological activity profiling
The in vitro cytotoxic/cytostatic activities of all final nucleobases 1b–1h and 2b–2h, as well as nucleosides7b–7h and 8b–8h, were initially evaluated against seven cell lines derived from human solid tumors including lung (A549 cells) and colon (HCT116 and HCT116p53−/−) carcinomas, as well as leukemia cell lines (CCRF-CEM, CEM-DNR, K562 and K562-TAX) and, for comparison, non-malignant BJ and MRC-5 fibroblasts. Concentrations inhibiting the cell growth by 50% (IC50) were determined using a quantitative metabolic staining with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)15 following a 3-day treatment. In addition, the anti-proliferative effect was tested against a human hepatocarcinoma Hep G2, human T-lymphoblastic promyelocytic leukemia HL-60 and cervical carcinoma HeLa S3 growing in liquid suspension. Cell viability was determined following a 3-day incubation using 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay.16Selected results are summarized in Table 3 (for complete data including standard deviations, see Table S1 in the ESI†). Surprisingly, most of the nucleosides, 7 and 8, were entirely inactive in these assays with the exception of 6-amino-7-deazapurine nucleosides7g and 8g showing moderate cytotoxic activities at >20 μM concentrations. Also none of the 7-phenylsulfanyl-7-deazapurine bases 1b–1h exerted any significant cytostatic activity. On the other hand, all the 7-(2-thienyl)sulfanyl-7-deazapurine bases bearing diverse substituents at position 6 showed significant cytostatic effects at micromolar concentrations. The most active were 6-hetaryl- (2b and 2c) and 6-methylamino and -dimethylamino (2e and 2f) derivatives having IC50 values in the low micromolar range. Compounds 2e and 2f were non-toxic to BJ and MRC-5 fibroblasts showing a promising therapeutic index.
| IC50 (μM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A549 | CCRF–CEM | CEM–DNR | HCT116 | HCT116p53– | K562 | K562-TAX | HepG2 | HL60 | HeLa S3 | BJ | MRC-5 | |
| 2b | 16.19 | 10.55 | 17.67 | 13.03 | 5.06 | 5.14 | 21.664 | >25 | 21.1 | >25 | 23.38 | 54.48 |
| 2c | 11.43 | 7.73 | 20.83 | 6.75 | 19.53 | 4.26 | 18.90 | >25 | 7.63 | 8.49 | 22.06 | 32.87 |
| 2d | >50 | >50 | >50 | 38.12 | 29.10 | 13.99 | >50 | >25 | >25 | >25 | >150 | 135.50 |
| 2e | 19.80 | 14.63 | 35.25 | 11.01 | 27.54 | 3.83 | 22.14 | >25 | >25 | >25 | 144.56 | >150 |
| 2f | 28.58 | 14.72 | 26.15 | 18.98 | 45.30 | 4.95 | 21.00 | >25 | 13.5 | 17.6 | 132.24 | 148.21 |
| 2g | 22.82 | 16.68 | 20.34 | 22.79 | >50 | 17.88 | 17.92 | >25 | 13.9 | 17.9 | >150 | 135.71 |
| 2h | 21.47 | 18.23 | >50 | 17.15 | >50 | >50 | 43.95 | >25 | >25 | 23.9 | 122.60 | 148.13 |
| 7g | 22.91 | 33.96 | >50 | 20.80 | 22.41 | 23.09 | 29.62 | >25 | >25 | >25 | 67.88 | 67.70 |
| 8g | 43.76 | 64.66 | >100 | 36.72 | 23.18 | 23.43 | 55.77 | >25 | >25 | >25 | 93.59 | 138.24 |
Since the nucleosides7 and 8 were inactive with the exception of moderately active adenosine analogues 7g and 8g (thia-analogues of cytostatic 7-aryl-7-deazaadenosines4), it can be concluded that replacement of the (het)aryl group at position 7 by the extended (het)arylsulfanyl group is not tolerated by the biological target(s) of the previously developed nucleoside cytostatics.3–5 Further studies will be necessary to explain the significant cytostatic effect of the 7-(thienylsulfanyl)-7-deazapurine bases which is apparently caused by a different mechanism (presumably by kinase inhibition).
In addition, all compounds were also tested on antiviral activity (HCV 1B and 2A replicon and RSV), antimicrobial activity (panel of gram-positive and gram-negative bacteria) and antifungal activity (several strains of Candida species) but did not show any significant activity in these assays.
3. Conclusions
In conclusion, we have developed a facile methodology for the synthesis of a series of 7-(het)arylsulfanyl-7-deazapurine bases and nucleosides bearing diverse substituents at position 6. It was based on Cu-catalysed C–H sulfenylation of 6-chloro-7-deazapurine followed by glycosidation and/or cross-coupling or nucleophilic substitutions. While the ribonucleoside analogues were almost entirely inactive, most of the 7-(thienylsulfanyl)-7-deazapurine bases showed significant cytostatic activities.Acknowledgements
This work was supported by the institutional support of the Charles University and Academy of Sciences of the Czech Republic (RVO: 61388963), by the Czech Science Foundation (P207/12/0205), Ministry of School and Education of the Czech Republic (LO1304) and by Gilead Sciences, Inc. The authors thank Prof. Milan Kolar and Dr. Kateřina Bogdanová (Palacky University) for antimicrobial screening and Dr. Gina Bahador and Dr. Joy Feng (Gilead Sciences, Inc.) for anti-HCV testing.Notes and references
- Reviews: (a) W. B. Parker, J. A. Secrist III and W. R. Waud, Curr. Opin. Invest. Drugs, 2004, 5, 592–596 CAS; (b) C. M. Galmarini, F. Popowycz and B. Joseph, Curr. Med. Chem., 2008, 15, 1072–1082 CrossRef CAS; (c) W. B. Parker, Chem. Rev., 2009, 109, 2880–2893 CrossRef CAS PubMed.
- Reviews: (a) M. Legraverend and D. S. Grierson, Bioorg. Med. Chem., 2006, 14, 3987–4006 CrossRef CAS PubMed; (b) S. Tumkevicius and J. Dodonova, Chem. Heterocycl. Compd., 2012, 48, 258–279 CrossRef CAS.
- P. Nauš, R. Pohl, I. Votruba, P. Džubák, M. Hajdúch, R. Ameral, G. Birkuš, T. Wang, A. S. Ray, R. Mackman, T. Cihlar and M. Hocek, J. Med. Chem., 2010, 53, 460–470 CrossRef PubMed.
- A. Bourderioux, P. Nauš, P. Perlíková, R. Pohl, I. Pichová, I. Votruba, P. Džubák, P. Konečný, M. Hajdúch, K. M. Stray, T. Wang, A. S. Ray, J. Y. Feng, G. Birkus, T. Cihlar and M. Hocek, J. Med. Chem., 2011, 54, 5498–5507 CrossRef CAS PubMed.
- P. Nauš, O. Caletková, P. Konečný, P. Džubák, K. Bogdanová, M. Kolář, J. Vrbková, L. Slavětínská, E. Tloušťová, P. Perlíková, M. Hajdúch and M. Hocek, J. Med. Chem., 2014, 57, 1097–1110 CrossRef PubMed.
- P. Perlíková, P. Konečný, P. Nauš, J. Snášel, I. Votruba, P. Džubák, I. Pichová, M. Hajduch and M. Hocek, Med. Chem. Commun., 2013, 4, 1497–1500 RSC.
- J. Snášel, P. Nauš, J. Dostál, A. Hnízda, J. Fanfrlík, J. Brynda, A. Bourderioux, M. Dušek, H. Dvořáková, J. Stolaříková, H. Zábranská, R. Pohl, P. Konečný, P. Džubák, I. Votruba, M. Hajdúch, P. Řezáčová, V. Veverka, M. Hocek and I. Pichová, J. Med. Chem., 2014, 57, 8268–8279 CrossRef PubMed.
- C-H Activation, Topics in Current Chemistry, ed. J.-Q. Yu and Z. Shi, Springer, 2010, vol. 292, pp. 1–380 (whole book) Search PubMed.
- (a) I. Čerňa, R. Pohl, B. Klepetářová and M. Hocek, Org. Lett., 2006, 8, 5389–5392 CrossRef PubMed; (b) I. Čerňa, R. Pohl and M. Hocek, Chem. Commun., 2007, 4729–4730 RSC; (c) I. Čerňa, R. Pohl, B. Klepetářová and M. Hocek, J. Org. Chem., 2008, 73, 9048–9054 CrossRef PubMed; (d) I. Čerňa, R. Pohl, B. Klepetářová and M. Hocek, J. Org. Chem., 2010, 75, 2302–2308 CrossRef PubMed.
- (a) T. E. Storr, A. G. Firth, K. Wilson, K. Darley, C. G. Baumann and I. J. S. Fairlamb, Tetrahedron, 2008, 64, 6125–6137 CrossRef CAS PubMed; (b) T. E. Storr, C. G. Baumann, R. J. Thatcher, S. De Ornellas, A. C. Whitwood and I. J. S. Fairlamb, J. Org. Chem., 2009, 74, 5810–5821 CrossRef CAS PubMed; (c) S. Sahnoun, S. Messaoudi, J.-D. Brion and M. Alami, Org. Biomol. Chem., 2009, 7, 4271–4278 RSC; (d) B. Vaňková, V. Krchňák, M. Soural and J. Hlaváč, ACS Comb. Sci., 2011, 13, 496–500 CrossRef PubMed.
- M. Klečka, R. Pohl, B. Klepetářová and M. Hocek, Org. Biomol. Chem., 2009, 7, 866–868 Search PubMed.
- M. Klečka, R. Pohl, J. Čejka and M. Hocek, Org. Biomol. Chem., 2013, 11, 5189–5193 Search PubMed.
- C–H sulfenylation: (a) Z. Li, J. Hong and X. Z. Li, Tetrahedron, 2011, 67, 3690–3697 CrossRef CAS PubMed; (b) G. L. Regina, V. Gatti, V. Famiglini, F. Piscitelli and R. Silvestri, ACS Comb. Sci., 2012, 14, 258–262 CrossRef PubMed; (c) L. H. Zou, J. Reball, J. Mottweiler and C. Bolm, Chem. Commun., 2012, 48, 11307–11309 RSC; (d) S. Ranjit, R. Lee, D. Heryadi, C. Shen, J. Wu, P. Zhang, K.-W. Huang and X. Liu, J. Org. Chem., 2011, 76, 8999–9007 CrossRef CAS PubMed; (e) Ch. Dai, Z. Xu, F. Huang, Z. Yu and Y.-F. Gao, J. Org. Chem., 2012, 77, 4414–4419 CrossRef CAS PubMed; (f) X.-L. Fang, R.-Y. Tang, P. Zhong and J.-H. Li, Synthesis, 2009, 24, 4183–4189 Search PubMed.
- X. Ming and F. Seela, Tetrahedron, 2007, 63, 9850–9861 CrossRef PubMed.
- (a) F. Denizot and R. Lang, J. Immunol. Methods, 1986, 89, 271–277 CrossRef CAS; (b) M. Hajduch, V. Mihal, V. Minarik, E. Faber, M. Safarova, E. Weigl and P. Antalek, Cytotechnology, 1996, 19, 243–245 CrossRef CAS.
- D. A. Scudiero, R. H. Shoemaker, K. D. Paull, A. Monks, S. Tierney, T. H. Nofziger, M. J. Currens, D. Seniff and M. R. Boyd, Cancer Res., 1988, 48, 4827–4833 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed table with all cytostatic activity data, experimental part and characterization data for all new compounds. See DOI: 10.1039/c4md00492b |
| This journal is © The Royal Society of Chemistry 2015 |