DOI:
10.1039/C4MD00325J
(Concise Article)
Med. Chem. Commun., 2015,
6, 94-104
Received 27th July 2014 , Accepted 8th September 2014
First published on 11th September 2014
1. Introduction
Drug discovery from plant-based compounds has since long been a paradigm for the treatment of various diseases and ailments.1,2 In this journey, a large number of plant-based products has taken a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSlead position in the market as active pharmaceutical agents.3,4 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
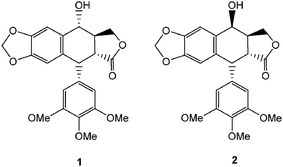
Explore further on Open PHACTSPodophyllotoxin, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1, is a bioactive cyclolignan component of Podophyllum peltatum and Podophyllum hexandrum.5,6 The C-4β epimer, epipodophyllotoxin, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2, has been a focal point for medicinal chemists in the recent past as several of its semi-synthetic derivatives, such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSetoposide and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSteniposide had already taken a place in the market as potential anticancer drugs.7–14 The cytotoxicity of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSpodophyllotoxin, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1, is generally attributed to the inhibition of tubulin polymerization whereas epipodophyllotoxin, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2, and its 4β-congeners viz., COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSetoposide and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSteniposide have been found to target DNA topoisomerase-II.15 Both COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSetoposide and teneposide block the catalytic activity of topoisomerase-II by stabilizing a cleavable enzyme-DNA complex in which the DNA is cleaved and covalently linked to the enzyme which ultimately leads to cell death. Both COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSetoposide and teneposide are active against various cancers such as lymphoma, testicular carcinoma, Kaposi's sarcoma, and small cell lung cancer.7 However, the therapeutic potential of these drugs is often hampered by problems of poor bioavailability and acquired drug resistance.16,17 To address these concerns, libraries of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSpodophyllotoxin, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1, and epipodophyllotoxin, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2, have been synthesized. As per the established structure–activity relationship (SAR),18 the 4β-position of epipodophyllotoxin is the most amenable position for modification. Most of other structural features are very essential for the cytotoxic activity and must be kept intact. Keeping this into consideration, two 4β-analogs GL-331 (ref. 11) and TOP-53 (ref. 19) were reported to exhibit potential anticancer activity.6,20,21 Some of the derivatives such as NK-611 and NPF made it to the clinical stage,22,23 whereas etopophos (a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater-soluble prodrug), is available in the market for the treatment of a variety of malignancies.40 SAR has revealed that bulky substituents at C-4 might be favorable for topoisomerase-II inhibition, and may enhance the cytotoxicity.24 Accordingly, numerous derivatives based on modifications at the C-4 position of epipodophyllotoxin were synthesized to develop better COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSlead molecules. Keeping the SAR into consideration, we, in analogy with some related literature precedents,25,26 hybridized 4β-epipodophyllotxin and chalcones both of which are proven anticancer agents. The reason behind the design lies in the similarity of their modes of action as many recent reports have revealed the topoisomerase-II inhibitory activity of chalcones,27,28 being responsible for their cytotoxicity. Click-chemistry, which enables a modular approach to generate novel pharmacophores, was exploited to conjugate the two moieties. The rationale behind the use of click-chemistry is based on our previous work on steroidal triazoles and 4β-[(4-substituted)-1,2,3-triazol-1-yl] podophyllotoxins, wherein we established that the introduction of a triazole moiety into natural products enhances their anticancer activity.29–32 The epipodophyllotoxin–chalcone hybrids, consisting of structurally different but functionally similar units, provide structurally compact motifs with a possible combined and/or synergistic pharmacological effect. There are chances of competition amongst the ligands when the target is the same. However, as revealed by the cytotoxicity and the docking studies, the binding sites of the two entities are likely to be different leading to better binding interactions of the hybrids with topoisomerase-II. All the designed hybrids were evaluated for their in vitro anticancer activity against a panel of six human cancer cell lines viz., SW-620 (Colon), DU-145 (Prostrate), SKN-SH (CNS), HCT-15 (Colon), Colo-205 (Colon) and HeLa (Cervix). Most of these compounds exhibited promising cytotoxicity with some having IC50 values even better than COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSetoposide. The compounds were also docked against topoisomerase-II and the binding calculations were in good agreement with the observed wet lab results (IC50 values). Compounds COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6d, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6f, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7d and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7f were found to be the most promising in this study especially against SW-620 and SKN-SH cell lines (Table 1).
Table 1 4-(4-Substituted)-1,2,3-triazol-1-yl epipodophyllotoxins differing in substitution at R′′
2. Results and discussion
Design and development of pharmacologically active chemical entities has always been the curiosity of medicinal chemists. Chemists have either used pharmacologically active natural products or relied upon small molecule designer analogs for the development of potent bioactive molecules. However, the exploitation of natural products for the synthesis of lead compounds has been the backbone of medicinal chemistry especially during the recent past.3,4 Various efficient synthetic techniques have been used in various facets of drug discovery enabling a modular approach to generate novel pharmacophores. The present manuscript describes a rational design and synthesis of pharmacologically active compounds wherein chalcone moieties are conjugated with epipodophyllotoxin through the ‘click-chemistry’ approach. The rationality lies in the possible similarity of modes of action of the two structurally different molecules. The hybrid conjugates thus synthesized were screened for their cytotoxic activity against a panel of human cancer cell lines and were found to be very active (Fig. 1). 2.1. Chemistry
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSPodophyllotoxin was converted to C4-β-azido podophyllotoxin, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3, and C4-β-azido-4′-O-demethyl podophyllotoxin, COMPOUND LINKS
Read more about this on ChemSpider
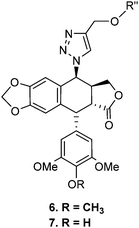
Download mol file of compound4, in accordance with established literature procedures.33,34 As illustrated in Scheme 1, 4β-[(4-substituted)-1,2,3-triazol-1-yl] podophyllotoxin (6a–6j) and 4β-[(4-substituted)-1,2,3-triazol-1-yl]-4′-O-demethyl podophyllotoxin (7a–7j) derivatives were synthesized by the cycloaddition reaction of C4-β-azido podophyllotoxin, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3, and C4-β-azido-4′-O-demethyl podophyllotoxin, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4, with O-aryl-propyne ethers, 5, obtained by reacting COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSchalcone with proporgyl COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSbromide in the presence of potassium carbonate in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSacetone under reflux conditions. 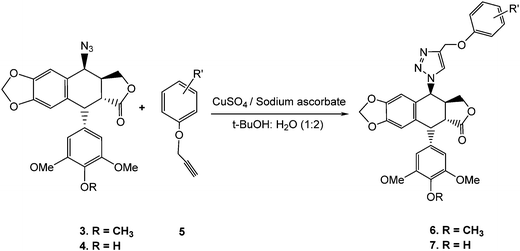 |
| | Scheme 1 Click-chemistry strategy for the synthesis of novel triazolyl epipodophyllotoxin congeners. | |
Azides, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3 and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4 were allowed to react with the O-propargylated phenols/chalcones, 5, as terminal alkynes in the presence of CuSO4·5H2O and sodium ascorbate in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSt-butyl alcohol and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater (1 : 2) at room temperature to selectively generate a focused library of 1,2,3-triazolyl derivatives (6a–j and 7a–j) in excellent yields (Scheme 1).
All the products obtained were characterized by 1H NMR, 13C NMR, IR, ESI-MS and elemental analysis. In 1H NMR, cyclization of azides to triazoles was confirmed by the shift of 4-α H to around δ 6 and the resonance of H-5 in the triazole ring in the aromatic region. The structure was further supported by 13C NMR, which showed all the carbon signals corresponding to triazole derivatives. ESI-MS of all compounds showed M + H or M + Na adduct as the molecular ion.
The human cancer cell lines used for the test were SW-620 (Colon), DU-145 (Prostrate), SKN-SH (CNS), HCT-15 (Colon), Colo-205 (Colon) and HeLa (Cervix). Cellular viability in the presence and absence of experimental agents was determined using the standard sulforhodamine B assay as described in Experimental section. By comparing the cytotoxic potential of compounds with different substitutions, it is evident that 4′-O-demethyl analogs (7a–j) are generally more active than the corresponding methylated analogs (6a–j). Further, the non-chalconated derivatives (6a–6c and 7a–7c) exhibited less cytotoxicity compared to the chalconated hybrids. This may be attributed to the synergistic effect which is expected from the hybridization of functionally similar molecules interacting with the same target, i.e. topoisomerase-II. Based on our previous studies,30 it is more likely that the designed hybrids induced apoptosis due to topoisomerase-II inhibition. The exact mechanism of action towards the inhibition of both topoisomerase-II and topoisomerase-I, is currently under investigation. However, as indicated by the docking calculations, it is evident that the designed hybrids have much better binding affinities than COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSetoposide towards topoisomerase-II. Most of the designed compounds exhibited better in vitro cytotoxicity than COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSetoposide. The increased activity may be attributed either to a better target binding or to the increased solubility leading to better bioavailability. Further, the IC50 values and the docking calculations of non-chalconated derivatives indicate that the presence of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSchalcone affects the binding of epipodophyllotoxin to the binding site of topoisomerase-II which was previously established as the ATPase domain. As the target site of chalcones on topoisomerase-II is not yet known, it is more likely that the binding site is different from that of epipodophyllotoxin. Had it been the same, the two might compete with each other resulting in lower binding-interaction (higher glide score) and higher IC50 values of the hybrids. The IC50 values calculated from the in vitro screening studies further revealed that the compounds COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6d, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6f, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7d and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7f showed significant cytotoxic activity. Compound COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7f, which contained a fluorinated chalcone, showed the highest cytotoxicity, selectively against SW-620 and SKN-SH cell lines. It is pertinent to mention here that the parent epipodophyllotoxin COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSazide did not show any appreciable cytotoxicity on any of the cancer cell lines under scrutiny. 2.3. Docking studies
By considering the ATPase domain of topoisomerase-II (PDB ID : 1ZXM) as the binding site30,35 observed in the co-crystallized structure of 1ZXM, all the molecules were docked as per the procedure given in Experimental section. The ATPase domain was selected for being the main binding site for epipodophyllotoxin analogs, i.e. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSetoposide, as reported earlier.35,36 In agreement with the observed IC50 values, the best glide scores were obtained for the potent ligands COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7d (GS: −6.819) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7f (GS: −7.409) and these were considered for interpreting the interactions. The interactions of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7d and COMPOUND LINKS
Read more about this on ChemSpider
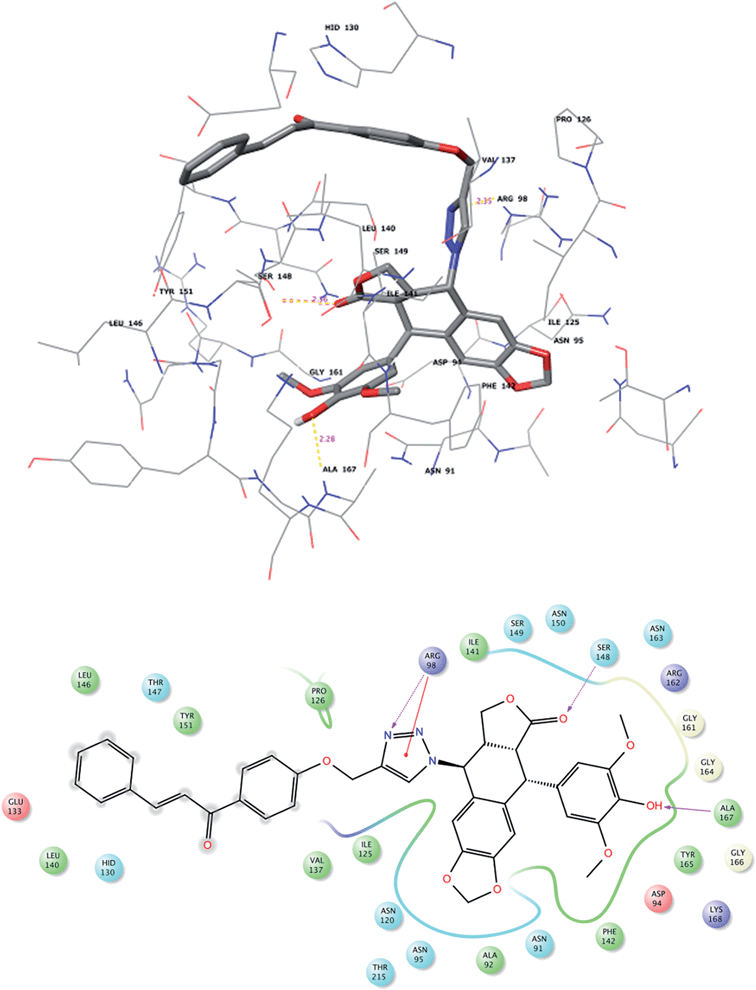
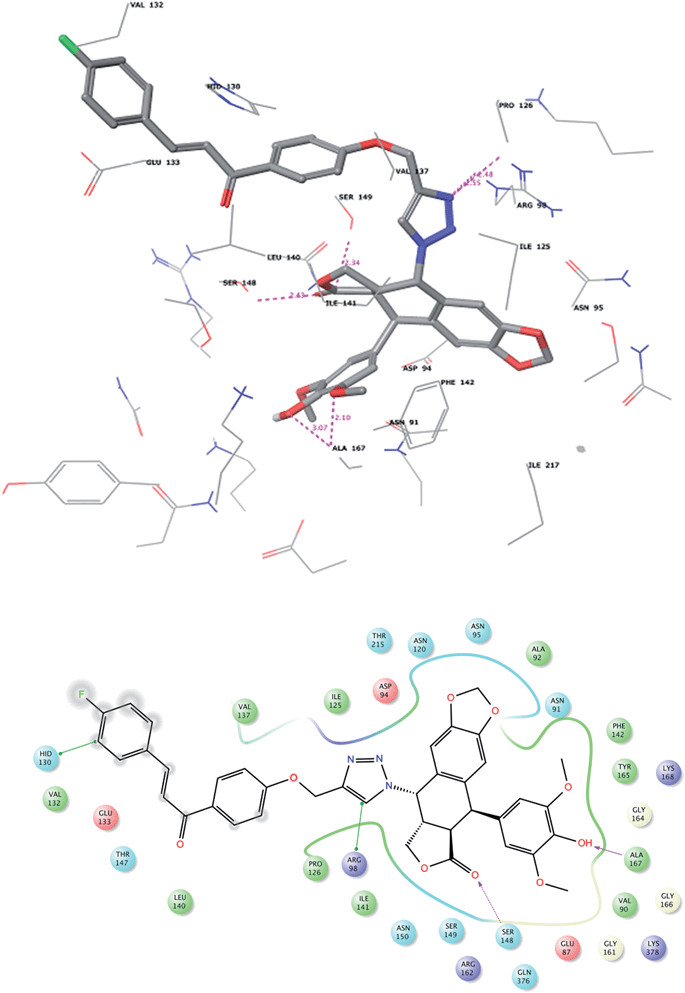
Download mol file of compound7f ligands with the binding domain of the receptor were found to be similar. The ligands maintained a distributed but close hydrophobic contact within the 5 Å hydrophobic pocket of Leu140, Phe142, Ile141, Pro126, Ile125, Val137 and Ala167 of the protein. The chalcone fragment of the ligands is within 4 Å from His130 and Val132. Arg98 maintains a relatively close π–π contact with the triazole group of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7d and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7f (similar contacts were observed with purine of ANP bound to topoisomerase-II of 1ZXM). H-bond strengths with the guanidinium group are 2.35 Å (for COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7d), and 2.48–2.55 Å (for COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7f). Ser148 has a notable H-bond interaction exhibiting a strength of 2.36 Å for COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7d and 2.43 Å for COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7f. Ser149 additionally offers stabilization of the lactone ring of the ligands. The phenolic moiety of the ligand is well stabilized by H-bond interactions of 2.28 Å and 2.10 Å for COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7d and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7f, respectively with Ala167. The ligand interactions of the potent ligands (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7d and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7f) with the receptor are shown in Fig. 2 and 3. 3. Conclusions
In conclusion, a series of 4β-[(4-substituted)-1,2,3-triazol-1-yl] podophyllotoxins were evaluated for the in vitro cell cytoxicity. Evaluation of the anticancer activity of these designed conjugates against a panel of six human cancer cell lines proved their cytotoxic nature. Further, the compounds were docked against topoisomerase-II and the glide scores were in good agreement with the observed IC50 values. As indicated by the docking calculations, it is evident that the designed hybrids have much better binding affinities than COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSetoposide towards topoisomerase-II. Compounds COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6d, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6f, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7d and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7f exhibited significant in vitro cytotoxicity. Among all the compounds evaluated, compound COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7f was found to be the most promising, especially selective against SW-620 and SKN-SH cell lines. The exact mechanism of action of the designed hybrids towards the inhibition of both topoisomerase-II and topoisomerase-I, is currently under investigation. We, further, intend to investigate the target site of chalcones on topoisomerase-II and to study the inhibitory activity of all the active compounds against topoisomerase-II.
4. Experimental
Proton and 13C spectra were recorded using a Bruker DPX400 instrument in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3 as the solvent with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTMS as the internal standard for protons and solvent signals as the internal standard for carbon spectra. The δ scale (ppm) has been used for reporting chemical shift values, and coupling constants are given in Hz. The Buchi MP apparatus D-545 was used for recording the melting points; IR spectra (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr) were recorded using a Bruker Vector 22 instrument. An ESI-esquire 3000 Bruker Daltonics instrument was used for recording mass spectra. The progress of all reactions was monitored by TLC on 2 × 5 cm pre-coated silica gel 60 F254 plates of thickness 0.25 mm (Merck). The chromatograms were visualized under UV 254-366 nm and iodine. 4.1. General procedure for chemical synthesis
Compound 5 (2 mmol) was dissolved in t-BuOH/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater (1 : 2, 12 mL) followed by the addition of CuSO4·5H2O (2.0 mmol) and sodium ascorbate (10.0 mmol). After 10 min, epipodophyllotoxin azide COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3 or COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4 (2 mmol) was charged into the reaction mixture which was then stirred at room temperature for 6 h. After completion, as indicated by TLC, the reaction mixture was diluted with 60 mL of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater and extracted with ethyl acetate (3 × 25 mL). All the extracts were combined and dried over COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNa2SO4 followed by in vacuo evaporation. The crude products obtained after the evaporation were crystallized from COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSdiethyl ether/hexanes to yield the pure products (6a–j and 7a–j). The spectral data of all the products obtained is given as follows. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6a. 9-[4-(4-Acetyl-phenoxymethyl)-[1,2,3]triazol-1-yl]-5-(3,4,5-trimethoxy-phenyl)-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho[2,3-d][1,3]dioxol-6-one. White solid; mp: 135–137 °C; [α]25D − 51 (0.75, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 2.48 (s, 3H), 3.04 (m, 1H), 3.24 (m, 2H), 3.72 (s, 6H), 3.82 (s, 3H), 4.31 (t, J = 7.6, 1H), 4.65 (d, J = 4.8, 1H), 5.15 (s, 2H), 5.92 (d, J = 5.94, 2H), 6.07 (d, J = 3.67, 1H), 6.24 (s, 2H), 6.56 (d, J = 5.68, 2H), 6.96 (d, J =7.21, 2H), 7.37 (s, 1H), 7.84 (d, J = 7.47, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 29.4, 37.0, 41.4, 43.4, 55.9, 58.8, 60.8, 62.4, 67.5, 105.3, 111.3, 111.5, 112.0, 113.8, 113.9, 117.9, 118.0, 127.1, 134.1, 146.7, 150.4, 150.5, 151.5, 152.7, 177.3, 202.2; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3346.3, 1771.6, 1731.2, 1598.5, 1516.3, 1477.1, 1338.0, 1016.6, 756.7 cm−1; ESI-MS: 636.2 (M + Na). Anal. calc. for C33H31N3O9: C, 64.59; H, 5.09; N, 6.85 found: C, 64.54; H, 5.03; N, 6.90%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6b. 5-(3,4,5-Trimethoxy-phenyl)-9-{4-[4-(3-oxo-but-1-enyl)-phenoxymethyl]-[1,2,3]triazol-1-yl}-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho[2,3-d][1,3]-6-one. White solid; mp: 129–131 °C; [α]25D − 23.3 (0.75, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 2.35 (s, 3H), 3.05 (m, 1H), 3.27 (m, 2H), 3.78 (s, 6H), 3.81 (s, 3H), 4.40 (t, J = 7.6, 1H), 4.75 (d, J = 4.8, 1H), 5.20 (s, 2H), 5.48 (s, 1H), 6.02 (d, J = 4.19, 2H), 6.11 (d, J = 4.0, 1H), 6.33 (s, 2H), 6.62 (m, 2H), 7.02 (d, J = 8.72, 2H), 7.28 (m, 2H), 7.53 (d, J = 8.78, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 25.3, 27.4, 29.7, 35.0, 41.7, 43.5, 56.5, 58.8, 60.87, 62.0, 67.4, 82.5, 84.4, 102.0, 107.8, 108.8, 110.6, 115.2, 123.1, 124.4, 125.4, 127.8, 129.6, 130.0, 133.4, 134.4, 142.9, 143.8, 146.6, 148.1, 150.5, 159.9, 172.1, 198.4; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3362.9, 1771.6, 1595.5, 1505.3, 1485.2, 1238.0, 1013.6, 764.8 cm−1; ESIMS: 662.2 (M + Na). Anal. calc. for C35H33N3O9: C, 65.72; H, 5.20; N, 6.57 found: C, 65.80; H, 5.24; N, 6.67%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6c. 5-(3,4,5-Trimethoxy-phenyl)-9-{4-[2-methoxy-4-(3-oxo-but-1-enyl)-phe-noxy-methyl]-[1,2,3]triazol-1-yl}-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho [2,3-d][1,3]dioxol-6-one. White solid; mp: 124–126 °C; [α]25D − 34.6 (0.70, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 2.33 (s, 3H), 3.05 (m, 1H), 3.27 (m, 2H), 3.72 (s, 6H), 3.76 (s, 3H), 3.80 (s, 3H), 4.40 (t, J = 7.6, 1H), 4.79 (d, J = 4.8, 1H), 5.22 (s, 2H), 5.40 (s, 1H), 6.02 (d, J = 3.67, 2H), 6.25 (s, 1H), 6.53 (m, 1H), 6.69 (m, 2H), 7.01 (s, 2H), 7.28–7.48 (m, 2H), 7.53 (d, J = 8.78, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 24.3, 27.4, 29.7, 37.0, 41.6, 43.4, 55.9, 56.5, 58.8, 60.6, 62.0, 67.4, 82.5, 84.4, 102.0, 107.8, 108.9, 110.5, 115.2, 122.7, 123.4, 125.8, 127.8, 129.6, 130.0, 133.4, 134.4, 142.9, 143.8, 146.6, 148.1, 149.5, 159.9, 173.1, 196.8; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3364.6, 1775.2, 1598.2, 1507.1, 1485.2, 1239.2, 1004.6, 755.7 cm−1; ESI-MS: 6792.2 (M + Na). Anal. calc. for C36H35N3O10: C, 64.57; H, 5.27; N, 6.27 found: C, 64.54; H, 5.24; N, 6.34%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6d. 5-(3,4,5-Trimethoxy-phenyl)-9-{4-[4-(3-oxo-3-phenyl-propenyl)-phenoxymethyl]-[1,2,3]triazol-1-yl}-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho[2,3-d] [1.3] dioxol-6-one.. White solid; mp: 145–147 °C; [α]25D − 41 (0.75, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 3.04 (m, 1H), 3.28 (m, 2H), 3.78 (s, 6H), 3.83 (s, 3H), 4.41 (t, J = 7.6, 1H), 4.76 (d, J = 4.6, 1H), 5.26 (s, 2H), 5.47 (s, 1H), 6.01 (d, J = 3.7, 2H), 6.12 (d, J = 4.0, 1H), 6.32 (s, 2H), 6.63 (d, J = 7.7, 2H), 7.05 (d, J = 8.6, 2H),7.28–7.54 (m, 3H), 7.57–7.79 (m, 4H), 8.04 (d, J = 8.78, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 29.6, 37.1, 41.7, 43.5, 56.6, 58.9, 60.8, 62.1, 67.4, 102.1, 107.9, 108.8, 110.6, 114.7, 121.8, 123.3, 124.5, 128.4, 129.0, 129.7, 130.5, 130.9, 131.8, 134.5, 135.0, 144.3, 146.7, 148.2, 149.5, 186.7; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3365.7, 1778.6, 1598.5, 1504.3, 1485.2, 1233.4, 1005.3, 758.3 cm−1; ESI-MS: 724.2 (M + Na). Anal. calc. for C40H35N3O9: C, 68.46; H, 5.03; N, 5.99 found: C, 68.50; H, 5.04; N, 5.93%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6e. 5-(3,4,5-Trimethoxy-phenyl)-9-{4-[4-(3-phenyl-acryloyl)-phenoxy methyl]-[1,2,3]triazol-1-yl}-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho[2,3-d] [1,3]dioxol-6-one. White solid; mp: 137–138 °C; [α]25D − 48.8 (0.50, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 3.06 (m, 1H), 3.25 (m, 2H), 3.79 (s, 6H), 3.82 (s, 3H), 4.39 (t, J = 7.6, 1H), 4.76 (d, J = 4.5, 1H), 5.22 (s, 2H), 5.46 (s, 1H), 6.03 (d, J = 3.65, 2H), 6.14 (d, J = 3.65, 1H), 6.32 (s, 2H), 6.64 (d, J = 8.2, 2H), 7.02 (d, J = 8.6, 2H),7.24–7.32 (m, 2H), 7.34–7.54 (m, 3H), 7.56–7.77 (m, 2H), 8.04 (d, J = 8.57, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 29.5, 37.2, 41.7, 43.5, 56.6, 58.9, 60.8, 62.1, 67.4, 102.1, 107.9, 108.8, 110.6, 114.7, 121.8, 124.3, 124.5, 128.5, 129.0, 129.7, 130.5, 132.9, 131.8, 134.5, 135.0, 144.3, 146.7, 147.8, 150.4, 186.6; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3342.1, 1777.7, 1587.2, 1598.3, 1485.2, 1287.0, 1001.6, 758.5 cm−1; ESIMS: 724.2 (M + Na). Anal. calc. for C40H35N3O9: C, 68.46; H, 5.03; N, 5.99 found: C, 68.51; H, 5.03; N, 5.95%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6f. 9-(4-{4-[3-(4-Fluoro-phenyl)-acryloyl]-phenoxymethyl}-[1,2,3]triazol-1-yl)-5-(3,4,5-trimethoxy-phenyl)-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho [2,3-d] [1,3] dioxol-6-one. White solid; mp: 154–156 °C; [α]25D − 33 (0.75, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 3.05 (m, 1H), 3.26 (m, 2H), 3.79 (s, 6H), 3.82 (s, 3H), 4.42 (t, J = 7.6, 1H), 4.75 (d, J = 4.7, 1H), 5.26 (s, 2H), 5.50 (s, 1H), 6.03 (d, J = 3.88, 2H), 6.12 (d, J = 4.2, 1H), 6.32 (s, 2H), 6.64 (d, J = 7.5, 2H), 7.05–7.24 (m, 5H), 7.66–7.80 (m, 3H), 8.03 (d, J = 8.77, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 35.9, 42.1, 56.9, 59.27, 60.8, 62.3, 66.2, 67.8, 67.7, 102.41, 106.8, 107.8, 109.6, 113.9, 120.7, 122.4, 124.4, 126.4, 128.3, 129.4, 129.8, 131.8, 132.0, 132.5, 133.5, 138.3, 157.6, 160.8, 162.0, 189.6; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3407.1, 1776.6,1598.5, 1505.3, 1485.2, 1238.0, 1012.6, 758.8 cm−1; ESI-MS: 720 (M + H). Anal. calc. for C40H34FN3O9: C, 66.75; H, 4.76; N, 5.84 found: C, 66.79; H, 4.74; N, 5.80%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6g. 9-(4-{4-[3-(3-Fluoro-phenyl)-acryloyl]-phenoxymethyl}-[1,2,3]triazol-1-yl)-5-(3,4,5-trimethoxy-phenyl)-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho [2,3-d] [1,3] dioxol-6-one. White solid; mp: 165–167 °C; [α]25D − 58.3 (0.65, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 3.07 (m, 1H), 3.26 (m, 2H), 3.79 (s, 6H), 3.82 (s, 3H), 4.43 (t, J = 7.6, 1H), 4.76 (d, J = 4.5, 1H), 5.27 (s, 2H), 5.51 (s, 1H), 6.02 (d, J = 4.1, 2H), 6.16 (d, J = 4.1, 1H), 6.33 (s, 2H), 6.65 (d, J = 4.1, 2H), 7.08 (m, 3H), 7.22–7.35 (m, 3H), 7.54–7.73 (m, 2H), 8.06 (d, J = 8.44, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 37.3, 42.1, 43.8, 56.9, 59.3, 60.8, 62.2, 67.8, 102.4, 108.3, 109.1, 110.9, 114.5, 115.0, 117.4, 117.8, 123.3, 123.6, 124.9, 129.9, 130.8, 131.3, 131.8, 133.8, 143.2, 147.1, 148.5, 149.9, 162.3, 173.7, 190.2; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3363.6, 1775.6,1598.5, 1505.3, 1485.2, 1238.0, 1002.6, 753.9 cm−1; ESI-MS: 720.2 (M + H). Anal. calc. for C40H34FN3O9: C, 66.75; H, 4.76; N, 5.84 found: C, 66.78; H, 4.73; N, 5.82%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6h. 9-(4-{4-[3-(2-Fluoro-phenyl)-acryloyl]-phenoxymethyl}-[1,2,3]triazol-1-yl)-5-(3,4,5-trimethoxy-phenyl)-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho [2,3-d] [1,3] dioxol-6-one. White solid; mp: 137––138 °C; [α]25D − 69.4 (0.75, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 3.06 (m, 1H), 3.29 (m, 2H), 3.79 (s, 6H), 3.82 (s, 3H), 4.42 (t, J = 7.5, 1H), 4.76 (d, J = 4.7, 1H), 5.26 (s, 2H), 5.49 (s, 1H), 6.03 (d, J = 3.88, 2H), 6.13 (d, J = 4.2, 1H), 6.33 (s, 2H), 6.64 (d, J = 7.5, 2H), 7.05–7.33 (m, 5H), 7.65–7.83 (m, 3H), 8.04 (d, J = 8.76, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 37.2, 42.1, 56.9, 59.27, 60.8, 66.2, 67.8, 67.7, 102.41, 106.8, 107.8, 109.6, 113.9, 120.7, 122.2, 123.4, 127.4, 128.3, 129.4, 129.8, 130.8, 132.0, 131.8, 133.5, 137.2, 156.6, 160.8, 163.0, 188.3; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3415.3, 1786.5, 1587.4, 1505.1, 1485.2, 1262.0, 1062.6, 738.9 cm−1; ES-IMS: 720.2 (M + H). Anal. calc. for C40H34FN3O9: C, 66.75; H, 4.76; N, 5.84 found: C, 66.77; H, 4.75; N, 5.80%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6i. 5-(3,4,5-Trimethoxy-phenyl)-9-{4-[4-(3-m-tolyl-acryloyl)-phenoxy methyl]-[1,2,3]triazol-1-yl}-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho[2,3-d] [1,3]dioxol-6-one. White solid; mp: 122–123 °C; [α]25D − 42 (0.70, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 2.41 (s, 3H), 3.06 (m, 1H), 3.26 (m, 2H), 3.80 (s, 6H), 3.83 (s, 3H), 4.41 (t, J = 7.6, 1H), 4.75 (d, J = 4.5, 1H), 5.26 (s, 2H), 5.45 (s, 1H), 6.02 (d, J = 3.65, 2H), 6.13 (d, J = 3.65, 1H), 6.33 (s, 2H), 6.64 (d, J = 8.2, 2H), 7.08 (d, J = 8.8, 2H), 7.24–7.29 (m, 4H), 7.57–7.79 (m, 2H), 8.04 (d, J = 8.57, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 18.6, 36.0, 40.7, 42.5, 55.3, 57.9, 60.7, 61.0, 66.4, 101.0, 106.9, 107.8, 109.6, 113.6, 120.5, 122.2, 123.4, 124.8, 127.8, 128.6, 129.8, 130.3, 131.2, 132.5, 133.5, 133.9, 137.6, 143.4, 145.6, 147.1, 148.5, 160.7, 172.1, 186.7; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3425, 2915, 1775, 1747, 1599, 1455, 1240, 1114, 1037, 763 cm−1; ESI-MS: 716.3 (M + H). Anal. calc. for C41H37N3O9: C, 68.80; H, 5.21; N, 5.87 found: C, 68.85; H, 5.14; N, 5.89%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6j. 5-(3,4,5-Trimethoxy-phenyl)-9-(4-{4-[3-(4-methoxy-phenyl)-acryloyl]-phenoxymethyl}-[1,2,3]triazol-1-yl)-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7] naphtho [2,3-d][1,3]dioxol-6-one. White solid; mp: 145–147 °C; [α]25D − 38.5 (0.75, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 3.08 (m, 1H), 3.26 (m, 2H), 3.79 (s, 9H), 3.82 (s, 3H), 4.41 (t, J = 7.6, 1H), 4.76 (d, J = 4.5, 1H), 5.26 (s, 2H), 5.47 (s, 1H), 6.03 (d, J = 3.83, 2H), 6.13 (d, J = 4.1, 1H), 6.33 (s, 2H), 6.64 (d, J = 7.59, 2H), 7.07 (d, J = 7.59, 2H), 7.24–7.41 (m, 4H), 7.66–7.67 (m, 2H), 8.06 (d, J = 8.78, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 36.2, 41.2, 42.5, 55.8, 58.1, 60.7, 61.0, 65.0, 66.4, 70.2, 101.0, 106.8, 106.8, 109.6, 113.9, 120.7, 122.2, 124.4, 127.4, 128.3, 129.4, 129.8, 130.8, 132.0, 132.5, 133.5, 134.0, 143.1, 145.8, 147.1, 148.2, 156.6, 160.8, 172.1, 191.7; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3386.3, 1778.6, 1597.3, 1507.2, 1485.2, 1237.0, 1001.5, 733.3 cm−1; ESI-MS: 732.3 (M + H). Anal. calc. for C41H37N3O10: C, 67.30; H, 5.10; N, 5.74 found: C, 67.35; H, 5.04; N, 5.77%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7a. 9-[4-(4-Acetyl-phenoxymethyl)-[1,2,3]triazol-1-yl]-5-(4-hydroxy-3,5-dimethoxy-phenyl)-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho[2,3-d][1,3]dioxol-6-one. White solid; mp: 143–145 °C; [α]25D −45 (0.75, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 2.49 (s, 3H), 3.05 (m, 1H), 3.26 (m, 2H), 3.74 (s, 6H), 4.33 (t, J = 7.6, 1H), 4.65 (d, J = 4.8, 1H), 5.15 (s, 2H), 5.92 (d, J = 5.94, 2H), 6.06 (d, J = 3.67, 1H), 6.24 (s, 2H), 6.56 (d, J = 5.68, 2H), 6.96 (d, J =7.21, 2H), 7.37 (s, 1H), 7.85 (d, J = 7.47, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 29.5, 37.0, 41.4, 43.4, 55.9, 58.8, 62.2, 67.5, 105.3, 111.3, 111.5, 112.0, 113.8, 113.9, 117.9, 118.0, 127.1, 134.1, 146.7, 150.4, 150.5, 151.5, 152.8, 177.1, 201.2; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3385.3, 1773.6, 1731.2, 1598.5, 1515.3, 1477.2, 1338.0, 1016.6, 759.7 cm−1; ESI-MS: 622 (M + Na). Anal. calc. for C32H29N3O9: C, 64.10; H, 4.88; N, 7.01 found: C, 64.24; H, 5.01; N, 6.92%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7b. 5-(4-Hydroxy-3,5-dimethoxy-phenyl)-9-{4-[4-(3-oxo-but-1-enyl)-phenoxymethyl]-[1,2,3]triazol-1-yl}-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho[2,3-d][1,3]-6-one. White solid; mp: 139–141 °C; [α]25D − 16.3 (0.760, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 2.36 (s, 3H), 3.06 (m, 1H), 3.26 (m, 2H), 3.80 (s, 6H), 4.40 (t, J = 7.6, 1H), 4.75 (d, J = 4.8, 1H), 5.20 (s, 2H), 5.47 (s, 1H), 6.02 (d, J = 4.19, 2H), 6.11 (d, J = 4.0, 1H), 6.33 (s, 2H), 6.62 (m, 2H), 7.00 (d, J = 8.72, 2H), 7.28 (m, 2H), 7.52 (d, J = 8.78, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 24.1, 27.4, 29.7, 37.0, 41.7, 43.5, 56.5, 58.8, 62.0, 67.4, 82.5, 84.4, 102.0, 107.8, 108.8, 110.6, 115.2, 123.1, 124.4, 125.4, 127.8, 129.6, 130.0, 133.4, 134.4, 142.9, 143.8, 146.6, 148.1, 149.5, 159.9, 173.1, 198.4; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3389.9, 1778.6, 1598.5, 1505.3, 1485.2, 1238.0, 1011.6, 774.7 cm−1; ESI-MS: 648 (M + Na). Anal. calc. for C34H31N3O9: C, 65.27; H, 4.99; N, 6.72 found: C, 65.10; H, 4.84; N, 6.79%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7c. 5-(4-Hydroxy-3,5-dimethoxy-phenyl)-9-{4-[2-methoxy-4-(3-oxo-but-1-enyl)-phenoxy-methyl]-[1,2,3]triazol-1-yl}-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho [2,3-d][1,3]dioxol-6-one. White solid; mp: 136–138 °C; [α]25D − 25.6 (0.75, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 2.31 (s, 3H), 3.05 (m, 1H), 3.26 (m, 2H), 3.73 (s, 6H), 3.77 (s, 3H), 4.40 (t, J = 7.6, 1H), 4.79 (d, J = 4.8, 1H), 5.22 (s, 2H), 5.40 (s, 1H), 6.02 (d, J = 3.67, 2H), 6.25 (s, 1H), 6.53 (m, 1H), 6.69 (m, 2H), 7.01 (s, 2H), 7.28–7.48 (m, 2H), 7.52 (d, J = 8.78, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 24.1, 27.4, 29.7, 37.0, 41.6, 43.4, 55.9, 56.5, 58.8, 62.0, 67.4, 82.5, 84.4, 102.0, 107.8, 108.9, 110.5, 115.2, 122.7, 123.4, 125.8, 127.8, 129.6, 130.0, 133.4, 134.4, 142.9, 143.8, 146.6, 148.1, 149.5, 159.9, 173.1, 197.9; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3388.6, 1775.2, 1598.2, 1507.1, 1485.2, 1239.2, 1002.6, 754.7 cm−1; ESI-MS: 678 (M + Na). Anal. calc. for C34H33N3O9: C, 64.12; H, 5.07; N, 6.41 found: C, 64.24; H, 5.16; N, 6.53%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7d. 5-(4-Hydroxy-3,5-dimethoxy-phenyl)-9-{4-[4-(3-oxo-3-phenyl-propenyl)-phenox-ymethyl]-[1,2,3]triazol-1-yl}-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho[2,3-d] [1,3]dioxol-6-one. White solid; mp: 151–153 °C; [α]25D − 37 (0.75, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 3.06 (m, 1H), 3.29 (m, 2H), 3.80 (s, 6H), 4.41 (t, J = 7.6, 1H), 4.76 (d, J = 4.6, 1H), 5.26 (s, 2H), 5.47 (s, 1H), 6.01 (d, J = 3.7, 2H), 6.12 (d, J = 4.0, 1H), 6.33 (s, 2H), 6.64 (d, J = 7.7, 2H), 7.05 (d, J = 8.6, 2H),7.24–7.54 (m, 3H), 7.57–7.79 (m, 4H), 8.05 (d, J = 8.78, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 29.7, 37.1, 41.7, 43.5, 56.6, 58.9, 62.1, 67.4, 102.1, 107.9, 108.8, 110.6, 114.7, 121.8, 123.3, 124.5, 128.4, 129.0, 129.7, 130.5, 130.9, 131.8, 134.5, 135.0, 144.3, 146.7, 148.2, 149.5, 186.7; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3382.7, 1778.6,1598.5, 1505.3, 1485.2, 1233.4, 1005.3, 769.3 cm−1; ESI-MS: 710 (M + Na). Anal. calc. for C39H33N3O9: C, 68.11; H, 4.84; N, 6.11 found: C, 68.20; H, 4.94; N, 6.02%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7e. 5-(4-Hydroxy-3,5-dimethoxy-phenyl)-9-{4-[4-(3-phenyl-acryloyl)-phenoxy methyl]-[1,2,3]triazol-1-yl}-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho[2,3-d] [1,3]dioxol-6-one. White solid; mp: 145–148 °C; [α]25D − 44.4 (0.60, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 3.06 (m, 1H), 3.26 (m, 2H), 3.80 (s, 6H), 4.39 (t, J = 7.6, 1H), 4.75 (d, J = 4.5, 1H), 5.23 (s, 2H), 5.46 (s, 1H), 6.02 (d, J = 3.65, 2H), 6.14 (d, J = 3.65, 1H), 6.33 (s, 2H), 6.64 (d, J = 8.2, 2H), 7.02 (d, J = 8.6, 2H),7.24–7.32 (m, 2H), 7.34–7.54 (m, 3H), 7.57–7.79 (m, 2H), 8.05 (d, J = 8.57, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 29.6, 37.1, 41.7, 43.5, 56.6, 58.9, 62.1, 67.4, 101.1, 107.9, 108.8, 110.6, 114.7, 121.8, 124.3, 124.5, 128.4, 129.0, 129.7, 130.5, 132.9, 131.8, 134.5, 135.0, 144.3, 146.7, 148.2, 150.5, 186.7; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3347.1, 1777.7, 1587.2, 1598.3, 1485.2, 1287.0, 1002.6, 738.6 cm−1; ESI-MS: 710 (M + Na). Anal. calc. for C39H33N3O9: C, 68.11; H, 4.84; N, 6.11 found: C, 68.21; H, 4.72; N, 6.23%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7f. 9-(4-{4-[3-(4-Fluoro-phenyl)-acryloyl]-phenoxymethyl}-[1,2,3]triazol-1-yl)-5-(4-hyd-roxy-3,5-dimethoxy-phenyl)-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho [2,3-d] [1,3] dioxol-6-one. White solid; mp: 162–164 °C; [α]25D − 28 (0.75, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 3.06 (m, 1H), 3.26 (m, 2H), 3.81 (s, 6H), 4.41 (t, J = 7.6, 1H), 4.76 (d, J = 4.7, 1H), 5.26 (s, 2H), 5.50 (s, 1H), 6.02 (d, J = 3.88, 2H), 6.12 (d, J = 4.2, 1H), 6.33 (s, 2H), 6.64 (d, J = 7.5, 2H), 7.05–7.23 (m, 5H), 7.65–7.82 (m, 3H), 8.04 (d, J = 8.77, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 36.4, 42.1, 56.9, 59.27, 60.6, 66.2, 67.8, 67.7, 102.41, 106.8, 107.8, 109.6, 113.9, 120.7, 122.2, 124.4, 126.4, 128.3, 129.4, 129.8, 131.8, 132.0, 132.5, 133.5, 138.3, 157.6, 160.8, 161.0, 189.4; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3409.1, 1778.6,1598.5, 1505.3, 1485.2, 1238.0, 1012.6, 756.8 cm−1; ESI-MS: 706 (M + H). Anal. calc. for C39H32FN3O9: C, 66.38; H, 4.57; N, 5.95 found: C, 66.29; H, 4.64; N, 5.83%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7g. 9-(4-{4-[3-(3-Fluoro-phenyl)-acryloyl]-phenoxymethyl}-[1,2,3]triazol-1-yl)-5-(4-hydro-xy-3,5-dimethoxy-phenyl)-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho [2,3-d] [1,3] dioxol-6-one. White solid; mp: 173–175 °C; [α]25D − 53.3 (0.55, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 3.08 (m, 1H), 3.28 (m, 2H), 3.80 (s, 6H), 4.42 (t, J = 7.6, 1H), 4.76 (d, J = 4.5, 1H), 5.28 (s, 2H), 5.51 (s, 1H), 6.02 (d, J = 4.1, 2H), 6.15 (d, J = 4.1, 1H), 6.33 (s, 2H), 6.64 (d, J = 4.1, 2H), 7.08 (m, 3H), 7.22–7.36 (m, 3H), 7.54–7.72 (m, 2H), 8.05 (d, J = 8.44, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 37.4, 42.1, 43.8, 56.9, 59.3, 62.3, 67.8, 102.4, 108.3, 109.1, 110.9, 114.5, 115.0, 117.4, 117.8, 123.3, 123.6, 124.9, 129.9, 130.8, 131.3, 131.8, 133.8, 143.2, 147.1, 148.5, 149.9, 162.3, 173.7, 191.2; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3378.6, 1778.6,1598.5, 1505.3, 1485.2, 1238.0, 1002.6, 754.9 cm−1; ESI-MS: 706 (M + H). Anal. calc. for C39H32FN3O9: C, 66.38; H, 4.57; N, 5.95 found: C, 66.47; H, 4.68; N, 5.87%. 7h. 9-(4-{4-[3-(2-Fluoro-phenyl)-acryloyl]-phenoxymethyl}-[1,2,3]triazol-1-yl)-5-(4-hydro-xy-3,5-dimethoxy-phenyl)-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho [2,3-d] [1,3] dioxol-6-one. White solid; mp: 142–146 °C; [α]25D − 62.4 (0.75, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 3.06 (m, 1H), 3.29 (m, 2H), 3.80 (s, 6H), 4.41 (t, J = 7.5, 1H), 4.76 (d, J = 4.7, 1H), 5.26 (s, 2H), 5.49 (s, 1H), 6.02 (d, J = 3.88, 2H), 6.13 (d, J = 4.2, 1H), 6.33 (s, 2H), 6.64 (d, J = 7.5, 2H), 7.05–7.33 (m, 5H), 7.65–7.82 (m, 3H), 8.05 (d, J = 8.77, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 37.4, 42.1, 56.9, 59.27, 60.6, 66.2, 67.8, 67.7, 102.41, 106.8, 107.8, 109.6, 113.9, 120.7, 122.2, 123.4, 127.4, 128.3, 129.4, 129.8, 130.8, 132.0, 132.5, 133.5, 137.3, 156.6, 160.8, 163.0, 188.4; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3405.3, 1789.5, 1587.4, 1505.1, 1485.2, 1262.0, 1063.6, 736.9 cm−1; ESI-MS: 706 (M + H). Anal. calc. for C39H32FN3O9: C, 66.38; H, 4.69; N, 5.95 found: C, 66.29; H, 4.60; N, 5.87%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7i. 5-(4-Hydroxy-3,5-dimethoxy-phenyl)-9-{4-[4-(3-m-tolyl-acryloyl)-phenoxy methyl]-[1,2,3]triazol-1-yl}-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7]naphtho[2,3-d] [1,3]dioxol-6-one. White solid; mp: 129–132 °C; [α]25D − 35 (0.70, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 2.40 (s, 3H), 3.06 (m, 1H), 3.28 (m, 2H), 3.80 (s, 6H), 4.41 (t, J = 7.6, 1H), 4.76 (d, J = 4.5, 1H), 5.26 (s, 2H), 5.46 (s, 1H), 6.02 (d, J = 3.65, 2H), 6.15 (d, J = 3.65, 1H), 6.33 (s, 2H), 6.64 (d, J = 8.2, 2H), 7.07 (d, J = 8.8, 2H), 7.24–7.29 (m, 4H), 7.57–7.79 (m, 2H), 8.05 (d, J = 8.57, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 18.3, 36.0, 40.7, 42.5, 55.5, 57.9, 61.0, 66.4, 101.0, 106.9, 107.8, 109.6, 113.6, 120.5, 122.2, 123.4, 124.6, 127.8, 128.6, 129.8, 130.3, 130.9, 132.5, 133.5, 133.9, 137.6, 143.4, 145.6, 147.1, 148.5, 160.7, 172.1, 187.7; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3408, 2925, 1775, 1747, 1599, 1457, 1240, 1114, 1037, 760 cm−1; ESI-MS:702 (M + H). Anal. calc. for C40H35N3O9: C, 68.46; H, 5.03; N, 5.99 found: C, 68.35; H, 5.14; N, 5.87%. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7j. 5-(4-Hydroxy-3,5-dimethoxy-phenyl)-9-(4-{4-[3-(4-methoxy-phenyl)-acryloyl]-phenoxymethyl}-[1,2,3]triazol-1-yl)-5,8,8a,9-tetrahydro-5aH-furo[3′,4′:6,7] naphtho [2,3-d][1,3]dioxol-6-one. White solid; mp: 152–154 °C; [α]25D − 33.2 (0.70, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCHCl3); 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): 3.06 (m, 1H), 3.27 (m, 2H), 3.80 (s, 9H), 4.41 (t, J = 7.6, 1H), 4.76 (d, J = 4.5, 1H), 5.26 (s, 2H), 5.48 (s, 1H), 6.02 (d, J = 3.83, 2H), 6.13 (d, J = 4.1, 1H), 6.33 (s, 2H), 6.64 (d, J = 7.59, 2H), 7.07 (d, J = 7.59, 2H), 7.22–7.42 (m, 4H), 7.64–7.66 (m, 2H), 8.05 (d, J = 8.78, 2H); 13C NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 36.0, 41.2, 42.5, 55.6, 58.1, 61.0, 65.0, 66.4, 70.2, 101.0, 106.8, 107.8, 109.6, 113.9, 120.7, 122.2, 123.4, 127.4, 128.3, 129.4, 129.8, 130.8, 132.0, 132.5, 133.5, 134.0, 143.3, 145.6, 147.1, 148.2, 156.6, 160.8, 172.1, 191.9; IR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSKBr): 3388.3, 1778.6, 1596.4, 1507.3, 1485.2, 1238.0, 1002.6, 732.3 cm−1; ESI-MS: 718 (M + H). Anal. calc. for C40H35N3O10: C, 66.94; H, 4.92; N, 5.85 found: C, 66.79; H, 5.04; N, 5.97%. 4.2. Evaluation of anticancer activity
The anticancer activity of all the derivatives was screened by the inhibition of growth of various cancer cell lines. We have adopted a method recommended by the National Cancer Institute (NCI), which uses the protein-binding dye COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSsulforhodamine B to estimate cell growth. Cellular viability in the presence and absence of experimental agents was determined by using the standard sulforhodamine B assay. The cells were harvested, counted and seeded (104 cells per well in 100 μL medium) in the log phase of their growth in 96-well microtitre plates. Cultures were treated with varying concentrations of test samples (10–100 μM) which were made of 1 : 10 serial dilutions, after 24 h of incubation at 37 °C and 5% COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCO2 to allow cell attachment. Six replicate wells were set up for each experimental condition. The cells were left in contact with the test samples for 48 h under the same conditions. Cold COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTStrichloroacetic acid (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTCA, 50%) was used for fixing the cells which were left at 4 °C for 1 h, followed by washing and air drying. Sulforhodamine-B dye was used to stain all the cells. The adsorbed dye was dissolved in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTris–buffer and plates were gently shaken for 10 min, on a mechanical shaker. The optical density (OD) was recorded using an ELISA reader at 540 nm. Subtraction of mean OD value of the respective blank from the mean OD value of an experimental set was used for calculation of the cellular growth. Percent growth in the presence of a test material was calculated by considering the growth in the absence of any test material as 100%, and in turn percent growth inhibition in the presence of a test material was calculated in terms of IC50 values. The different epipodophyllotoxin derivatives (test material) were dissolved in a mixture of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMSO : COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSWater (1 : 1) and then introduced into the medium containing the cancer cell lines. The cells were allowed for proliferation in the presence of the test material for 48 h. With COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSetoposide as the positive control, cell growth inhibition results are presented in terms of IC50 values (Table 2).
Table 2 IC50 values (μM) of in vitro cytotoxicity of 4β-[(4-substituted)-1,2,3-triazol-1-yl] podophyllotoxin congeners against a panel of human cancer cell linesa
4.3. Molecular docking
Structure building and calculations were performed on a Mac OSX (v10.7.5) using the Schrodinger molecular modeling software (Schrödinger Release 2013-2, Schrödinger, LLC, New York, 2013). The ligands were built using Maestro (v9.5) and minimized. Conformational search application (Macromodel v10.1) was used to generate an extensive set of conformations using advanced option. OPLS-2005 (ref. 37) force field in solvent COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater conditions with default extended cutoff values was used. Optimizations were converged into an energy convergence or continued until the limit of 50 000 iterations was reached. For the conformational search the mixed torsional/low mode sampling method was used with 10 000 steps. The 100 lowest energy conformers were saved and were further used for docking studies (Table 3).
Table 3 Docking scores of various 4β-[(4-substituted)-1,2,3-triazol-1-yl] podophyllotoxin analogs
The crystal structure coordinates of the human topoisomerase-II ATPase-AMP-PNP complex (PDB ID: 1ZXM from the protein data bank, http://www.rcsb.org) were imported for protein preparation using the protein preparation wizard. The protein complex was preprocessed, by replacing the missing amino acids and removing all COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSwater molecules including the ones near the co-crystallized AMP. By using the OPLS-2005 minimization method, a refined structure of the protein was obtained which was further used for grid calculations and docking studies using Glide (v60014).38,39 The generation for the receptor-grid values was computed using OPLS-2005 force fields. The ligands were docked with the obtained conformers using extra precision settings of the docking panel with default settings.
References
- T. Beghyn, R. Deprez-Poulain, N. Willand, B. Folleas and B. Deprez, Chem. Biol. Drug Des., 2008, 72, 3–15 CAS.
- F. E. Koehn and G. T. Carter, Nat. Rev. Drug Discovery, 2005, 4, 206–220 CrossRef CAS PubMed.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2007, 70, 461–477 CrossRef CAS PubMed.
- K. Gransalke, Drug Discovery, 2011, 11, 16–19 Search PubMed.
- M. G. Kelly and J. L. Hartwell, J. Natl. Cancer Inst., 1954, 14, 967–1010 CAS.
- K. H. Lee, Med. Res. Rev., 1999, 19, 569–596 CrossRef CAS.
- L. Bohlin and B. Rosen, Drug Discovery Today, 1996, 1, 343–351 CrossRef CAS.
- K. H. Lee, S. A. Beers, M. Mori, Z. Wang, Y. Kuo, L. Li, S. Liu, Y. Cheng, F. Han and Y. Cheng, J. Med. Chem., 1990, 33, 1364–1368 CrossRef CAS.
- H. Stahelin and A. V. Wartburg, Prog. Drug Res., 1989, 33, 169–266 CAS.
- T. W. Doyle, Etoposide (VP-16) Current Status and New Developments, Academic press, New York, 1984 Search PubMed.
- Z. Q. Wang, Y. H. Kuo, D. Schnur, J. P. Bowen, S. Y. Liu, F. S. Han, Y. C. Cheng and K. H. Lee, J. Med. Chem., 1990, 33, 2660–2666 CrossRef CAS.
- B. F. Issell, Cancer Chemother. Pharmacol., 1982, 7, 73–80 CAS.
- D. R. Budman, Semin. Oncol., 1996, 23, 8–14 CAS.
- F. A. Greco and J. D. Hainsworth, Semin. Oncol., 1996, 23, 45–50 CAS.
- T. L. Macdonald, E. K. Lehenert, J. T. Loper, K. C. Chow and W. E. Ross, in DNA topoisomerase in cancer, ed. M. Potmesil and K. W. Kohn, Oxford University Press, New York, 1991, p. 119 Search PubMed.
- P. I. Clark and M. L. Slevin, Clin. Pharmacokinet., 1987, 12, 223–252 CrossRef CAS PubMed.
- A. Kamal, M. Arifuddin, B. A. Kumar and S. G. Dastidar, US Pat. 0066837, 2007.
- D. Imperio, T. Pirali, U. Galli, F. Pagliai, L. Cafici, P. Luigi, G. Sorba, A. A. Genazzani and G. C. Tron, Bioorg. Med. Chem., 2007, 15, 6748–6757 CrossRef CAS PubMed.
- T. Terada, K. Fujimoto, M. Nomura, J. Yamashita, K. Wierzba, R. Yamazaki, J. Shibata, Y. Sugimoto, Y. Yamada, T. Kobunai, S. Takeda, Y. Minami, K. Yoshida and H. Yamaguchi, J. Med. Chem., 1993, 36, 1689–1699 CrossRef CAS.
- T. Utsugi, J. Shibata, Y. Sugimoto, K. Aoyagi, K. Wierzba, T. Kobunai, T. Terada, T. Ohhara, T. Tsuruo and Y. Yamada, Cancer Res., 1996, 56, 2809–2814 CAS.
- Z. Xiao, Y. D. Xiao, A. Golbraikh, A. Tropsha and K. H. Lee, J. Med. Chem., 2002, 45, 2294–2309 CrossRef CAS PubMed.
- K. Mross, A. Huttmann, K. Herbst, A. R. Hanauske, T. Schilling, C. Manegold, K. Burk and D. K. Hossfeld, Cancer Chemother. Pharmacol., 1996, 38, 217–224 CrossRef CAS.
- Y. Zhang, A. Tropsha, A. T. McPhail and K. H. Lee, J. Med. Chem., 1994, 37, 1460–1464 CrossRef CAS.
- Z. Xiao, K. F. Bastow, J. R. Vance and H. Lee, Bioorg. Med. Chem., 2004, 12, 3339–3344 CAS.
- M. Abdel-Aziz, S. U. Park, G. E.-D. A. A. Abuo-Rahma, M. A. Sayed and Y. Kwon, Eur. J. Med. Chem., 2013, 69, 427–438 CrossRef CAS PubMed.
- X. Zhang, B. Bao, X. Yu, L. Tong, Y. Luo, Q. Huang, M. Su, L. Sheng, J. Li, H. Zhu, B. Yang, X. Zhang, Y. Chen and W. Lu, Bioorg. Med. Chem., 2008, 15, 6981–6995 Search PubMed.
- S. Ohira, K. Takaya, T. Mitsui, M. Kido, K. Kakumoto, K. Hayashi, A. Kuboki, H. Tani, S. Ikeda, M. Linuma, Y. Akao and H. Nozaki, Tetrahedron Lett., 2013, 54, 5052–5055 CrossRef CAS PubMed.
- Y. Na and J. M. Nam, Bioorg. Med. Chem. Lett., 2011, 21, 211–214 CrossRef CAS PubMed.
- A. H. Banday, S. A. Shameem, B. D. Gupta and H. M. S. Kumar, Steroids, 2010, 75, 801–804 CrossRef CAS PubMed.
- D. M. Reddy, J. Srinivas, G. Chashoo, A. K. Saxena and H. M. S. Kumar, Eur. J. Med. Chem., 2011, 46, 1983–1991 CrossRef CAS PubMed.
- P. B. Reddy, S. K. Agrawal, S. Singh, B. A. Bhat, A. K. Saxena, H. M. S. Kumar and G. N. Qazi, Chem. Biodiversity, 2008, 5, 1792–1802 CAS.
- B. A. Bhat, P. B. Reddy, S. K. Agrawal, A. K. Saxena, H. M. S. Kumar and G. N. Qazi, Eur. J. Med. Chem., 2008, 43, 2067–2072 CrossRef CAS PubMed.
- A. Kamal, B. A. Kumar and M. Arifuddin, Tetrahedron Lett., 2003, 44, 8457–8459 CrossRef CAS PubMed.
- A. Kamal, N. Laxman and G. Ramesh, Bioorg. Med. Chem. Lett., 2000, 10, 2059–2062 CrossRef CAS.
- D. Leroy, A. V. Kajava, C. Frei and S. M. Gasser, Biochemistry, 2001, 40, 1624–1634 CrossRef CAS PubMed.
- A. K. Patel, S. Patel and P. K. Naik, Curr. Res. J. Biol. Sci., 2010, 2, 13–23 CAS.
- G. A. Kaminski, R. A. Friesner, J. Tirado-Rives and W. L. Jorgensen, J. Phys. Chem. B, 2001, 105, 6474–6487 CrossRef CAS.
- R. A. Friesner, R. B. Murphy, M. P. Repasky, L. L. Frye, J. R. Greenwood, T. A. Halgren, P. C. Sanschagrin and D. T. Mainz, J. Med. Chem., 2006, 49, 6177–6196 CrossRef CAS PubMed.
- R. A. Friesner, J. L. Banks, R. B. Murphy, T. A. Halgren, J. J. Klicic, D. T. Mainz, M. P. Repasky, E. H. Knoll, D. E. Shaw, M. Shelley, J. K. Perry, P. Francis and P. S. Shenkin, Glide, J. Med. Chem., 2004, 47, 1739–1749 CrossRef CAS PubMed.
- A. Kumar, V. Kumar, A. E. Alegria and S. V. Malhotra, Curr. Med. Chem., 2011, 18, 3853–3870 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4md00325j |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here.