Highly efficient integration of the viral portal proteins from different types of phages into planar bilayers for the black lipid membrane analysis†
Abstract
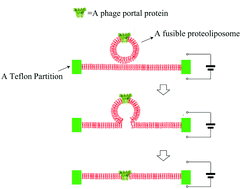
The planar lipid bilayer technology is a technique that yields incredibly useful structural function information about a single channel protein. It is also currently actively utilized as a powerful platform using biological protein nanopores for the development of single-molecule nanopore sensing technology, as well as ultrafast DNA sequencing technology. The portal protein, GP10, from the bacteriophage Φ29 was the first phage portal protein shown to be successfully inserted into planar bilayer membranes, thereby it may inspire more researchers to apply the techniques to portal proteins from the other bacteriophages. However, the technology is far from perfect since the insertion of the channel proteins into planar bilayer membranes is not only technically difficult but also time-consuming. For the fusion of phage portal proteins, vesicles are typically needed to be reconstituted with the portal proteins to form proteoliposomes. However, most of the phage portal proteins have low solubility, and may self-aggregate during the preparation of the proteoliposomes. Furthermore, the fusion of the formed proteoliposomes is sporadic, unpredictable and varied from person to person. Due to the lack of experimental consistency between labs, the results from different methodologies reported for generating fusible proteoliposomes are highly variable. In this research, we propose a new method for the preparation of the fusible proteoliposomes containing portal proteins from bacteriophages, to circumvent the problems aforementioned. Compared to the conventional methods, this method was able to avoid the protein aggregation issues during the vesicle preparation by eliminating the need for detergents and the subsequent time-consuming step for detergent removal. The proteoliposomes prepared by the method were shown to be more efficiently and rapidly inserted into planar bilayer membranes bathed in different conducting buffer solutions including those with nonelectrolytes such as glycerol and PEG. In addition, the method of forming proteoliposomes has significantly extended the shelf life of the proteoliposomes. To further explore its potentials, we have successfully applied the method to the insertion of a mutant portal protein, GP20, from T4 bacteriophage, a hydrophobic portal protein that has not been explored using the planar lipid bilayer membrane technique. The results suggest that this method could be used to prepare proteoliposomes formed by hydrophobic portal proteins from other bacteriophages.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...