An insight into antagonist binding and induced conformational dynamics of class B GPCR corticotropin-releasing factor receptor 1†
Abstract
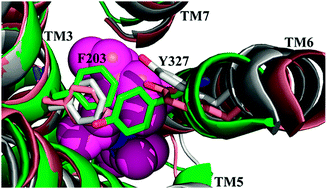
The human corticotropin-releasing factor receptor type 1 (CRF1R) is a class B G-protein-coupled receptor (GPCR), which mediates the response to stress and has been considered as a drug target for depression and anxiety. Based on the CRF1R-antagonist crystal structure, we study the binding mechanism of two distinct antagonists, CP-376395 and MTIP, and the dynamics behaviors of CRF1R induced by an antagonist binding. Key residues interacting with both antagonists and residues specifically binding to one of them are identified. Both antagonists interact with Asn283, Phe203, Met206, Leu280, Tyr316, Leu323, Leu287, Phe284, Val279, Leu319, Phe207, Gly210 and Phe362. CP-376395 specifically binds to Glu209 and Phe160, while MTIP specifically binds to Leu320, Leu213, Ile290, Phe162 and Val313. The total binding free energy of MTIP is lower than that of CP-376395; this is consistent with the experimental observation that MTIP shows higher binding affinity than CP-376395. The conformational dynamic behaviors of antagonist bound holo-CRF1R were found to be different from those of apo-CRF1R in three aspects: (i) the “ionic lock” between side chains of Arg151 in TM2 and Glu209 in TM3 was broken in apo-CRF1R, but was formed in holo-CRF1Rs; (ii) Phe203 in TM3 and Tyr327 in TM6 were in close proximity to each other in apo-CRF1R, while they were far apart resulting from the shift of TM6 in holo-CRF1Rs; and (iii) the “rotamer toggle switch”, Tyr327/Leu323/Phe284, adopted different rotameric conformations in apo-CRF1R and holo-CRF1Rs. We hope that our results could be helpful in further development of the drug design of CRF1R.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...