Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dynamic acoustic field activated cell separation (DAFACS)†
G. D.
Skotis‡
a,
D. R. S.
Cumming‡
a,
J. N.
Roberts‡
b,
M. O.
Riehle‡
b and
A. L.
Bernassau‡
*a
aSchool of Engineering, University of Glasgow, Glasgow, G12 8LT, UK. E-mail: Anne.Bernassau@glasgow.ac.uk
bCentre for Cell Engineering, Institute for Molecular, Cell and Systems Biology, CMVLS, University of Glasgow, Glasgow G12 8QQ, UK
First published on 4th December 2014
Abstract
Advances in diagnostics, cell and stem cell technologies drive the development of application-specific tools for cell and particle separation. Acoustic micro-particle separation offers a promising avenue for high-throughput, label-free, high recovery, cell and particle separation and isolation in regenerative medicine. Here, we demonstrate a novel approach utilizing a dynamic acoustic field that is capable of separating an arbitrary size range of cells. We first demonstrate the method for the separation of particles with different diameters between 6 and 45 μm and secondly particles of different densities in a heterogeneous medium. The dynamic acoustic field is then used to separate dorsal root ganglion cells. The shearless, label-free and low damage characteristics make this method of manipulation particularly suited for biological applications. Advantages of using a dynamic acoustic field for the separation of cells include its inherent safety and biocompatibility, the possibility to operate over large distances (centimetres), high purity (ratio of particle population, up to 100%), and high efficiency (ratio of separated particles over total number of particles to separate, up to 100%).
Introduction
Separating and sorting cells and micro-organisms from a heterogeneous mixture is a fundamental step in basic biological,1 chemical and clinical studies,2 enabling regenerative medicine, stem cell research, clinical sample preparation, and improved food safety.3 Biological cell separation needs to enrich a target cell population whilst minimising the presence of unwanted cells or contaminants. At present, the standard systems for cell separation are fluorescently activated cell sorters (FACS) and sedimentation.4 FACS requires fluorescent labelling, making it complex and expensive, whereas sedimentation has a low ratio of recovery leading to loss of material. As a consequence, there is a considerable demand for the discovery of alternative methods. Microfluidic systems,5–7e.g. deterministic sorting,8 inertial microfluidics,9 dielectrophoretic,10 and ultrasonic devices,11–15 present viable routes to superior technologies for cell sorting. Techniques utilising ultrasonics are particularly valuable because of the potential for efficient sorting of relatively large microfluidic volumes in a robust, harmless and scalable technology.Ultrasonic forces are non-invasive and can effectively manipulate cells for applications such as medium exchange,16 sample concentration,17–19 sorting,15,20 enhanced bio-detection and immuno-assays. Achieving cell separation21 utilizing ultrasonic manipulation by frequency sweeping11,22 has been previously demonstrated; however, this method has several critical disadvantages. Firstly, unstable forces are generated which leads to differences in the movement of individual trapped particles of the same size or property.23,24 Secondly, this method exclusively allows small particle displacement, which in turn limits sorting efficiency. Thirdly, frequency sweep sorting inherently lacks flexibility because the frequencies that can be used are limited by the types and dimensions of the transducers. Finally, the dimensional properties of the whole resonator may pose some constraints on the frequency regime.
An alternative to manipulating acoustic frequency is to control the signal phase; it has been demonstrated that shifting the phase of acoustic travelling waves can be used to control the position of micro-particles suspended in an aqueous medium.25 When two opposing transducers are excited, a linear interference pattern of nodes and antinodes is formed in the interstitial media. The micro-particles are trapped at the minima of the potential acoustic energy density.26 Electronically shifting the excitation phase of one of the transducers, with respect to the other, proportionally translates the linear interference pattern in the direction of the added phase delay. The shift of the node position translates to a change in the position of the trapped micro-particles.27,28
The new technique devised utilizes a dynamic acoustic field (DAF) with a time-varying phase delay between two opposing travelling waves that results in the separation of particles or cells over large distances (of the order of centimetres). The method shows a high degree of separation selectivity and throughput, making it suitable for applications such as cell sorting. The forces generated by this method are very stable,23 allowing better spatial separation (improved control and manipulation) of particles and cells to be achieved and maintained compared to results realized using frequency sweeping. This ultimately results in separation of sub-populations in the sample volume with much higher purity.
In this paper, we demonstrate that the flow-less DAF method can be used to separate particles within a sample volume depending on their size or density. We also study the discriminative ability of the method for particles of different sizes or densities. The application of DAF to primary pig dorsal root ganglion neurons as a contact-less means of separating these neurons from debris and smaller cells, which results from tissue digestion, is demonstrated.
Experimental results and discussion
Initial experiments were conducted with synthetic particles of various sizes and densities in order to demonstrate the feasibility of the approach. Previous studies have found that these particles are a reasonable surrogate for biological cells.29 Once DAF had been successfully used to discriminate within heterogeneous mixture particles by their sizes and densities in multiple cycles, we progressed to show the capacity of DAF to isolate dorsal root ganglion cells from a mixture of cells and debris as it develops during tissue digestion.Principle of operation
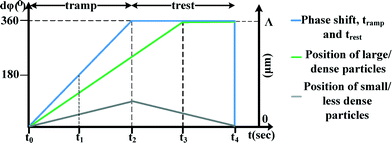
The time variant primary acoustic force combined with the viscous force enables one to discriminate particles according to their physico-mechanical properties (size and density). The acoustic force Fa, eqn (4), is dependent on the particle radius and particle density. Smaller or less dense particles and cells experience a lower acoustic force Fa, with respect to larger or denser particles and cells; therefore, particles and cells of different sizes and different densities will separate. This outlines the fundamental concept utilising the DAF method.The technique relies on a repeated cycling pattern of the phase difference between two excited transducers from 0° to 360°. Within each cycle, the phase is swept completely through 360° over a time tramp and then allowed to rest for a period trest before commencing the next cycle. The interplay between the rate at which the phase is swept and the length of the rest time is at the core of the separation technique. During tramp, under the correct conditions, the particles of interest experience a strong acoustic force compared to the viscous force.
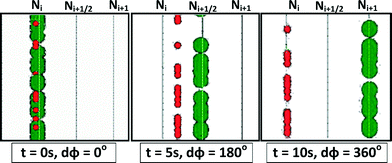
For a system with particles trapped in one of many nodes, N, the particles of interest closely follow the node that traps them and travel from the initial position of the node Ni to the initial position of the next node Ni+1 (Fig. 2 and 3). This controlled manipulation occurs since a phase shift of 360° moves each node exactly one integer node position, a distance Λ (Fig. 1). If at the end of the tramp period, the smaller particles have not travelled more than halfway (Fig. 2 and 3) from their initial position of Ni to the next node position Ni+1, they will relax to their starting position during trest, whereas the particles of interest, which have travelled past the midpoint Ni and Ni+1, will relax towards the next node position Ni+1.
The interplay between acoustic force and viscous drag force is at the heart of the discriminating ability of the DAF method with trest serving as an equilibrating interval, during which particles settle at their nearest nodes before the next cycle begins. Assuming the length of tramp has been selected correctly, the best choice of trest to achieve cell or particle discrimination depends on the balance between the acoustic force, Fa, eqn (4), and the viscous force, Fv, eqn (6). trest and tramp were measured for different sizes of particles, by applying a step change of 180° in phase to one of the transducers.25 In water, polystyrene particles of 6, 10 and 45 μm diameters, respectively, require a time delay of 5, 2 and 0.5 s, respectively, to reach their equilibrium positions. This serves as the basis to establish the timescale for trest and tramp required to discriminate between particles during the experiments. The DAF method can then be optimized to achieve the optimum separation performance in specific applications. These optimum values of tramp and trest are dependent on the viscosity of the medium, as well as the density and size of the particles.
Acoustic separation simulation
The mechanism of particle separation was studied by using a numerical model to predict the particle behaviour under a dynamic acoustic field. The code was developed in Visual Basic from scratch. We modelled particle behaviour under different dynamic acoustic fields, particle properties (size and density), liquid viscosity and flow. The program sums the forces acting on the particles taking into account the primary acoustic force Fa, eqn (4), and the viscous force Fv, eqn (6), as a function of time.The equation of motion of a particle labelled by its position r(t) is given by:
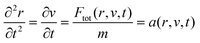 | (1) |
F tot is the sum of Fa and Fv, m is the mass of the particle, and a is the acceleration as a function of particle position r and the relative particle velocity v at time t.
These equations of motion are integrated step by step using the Verlet algorithm,30,31eqn (2) and (3), to produce the movement of the particles.
| r(t + Δt) = 2r(t) − r(t − Δt) + a(t)Δt2 | (2) |
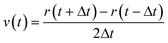 | (3) |
The computer program can simulate the movement of P particles among several types of particles differing in their radius and density. Fig. 2 denotes the predicted particle separation of two classes of particles differing in size. The ratio between tramp and trest is 1, with a tramp = trest = 5 s. As predicted, large and small particles are separated.
Acoustic separation by particle size
To investigate the potential of this method to discriminate particles by size, sets of polystyrene particles (Polysciences Europe, Germany) with varying diameters, (a) 10 and 45 μm and (b) 6 and 10 μm, were subjected to a dynamic acoustic field.The transducers were excited at a frequency of 4.00 MHz with an amplitude of 8 Vpp. At this frequency, the wavelength of the sound waves in water is λ = 370 μm (the velocity of sound velocity in water is 1480 m s−1).32 A schematic of the experimental setup is shown in Fig. 3. The particles agglomerate at the nodes with a separation of λ/2 = Λ = 185 μm.33,34 To estimate the order of magnitude of the acoustic pressure experienced by the particles of different diameters (6, 10, and 45 μm), we recorded the time taken for the particles to agglomerate at the nodes starting from a randomly distributed motion state. These experiments were repeated 5 times at 5 different locations within the device using time-lapse microscopy. The viscous force, Fv, derived from the dimensions and densities of the particles as well as the viscosity of the medium (eqn (6)), allows the acoustic pressure amplitude acting on the particles to be calculated. The acoustic pressure was found to be 91 ± 7 kPa, 62 ± 4 kPa and 48 ± 2 kPa for 6, 10 and 45 μm diameter particles, respectively.
The separation experiments were conducted using two mixtures of particles, each at a particle density of 4.99 × 105 particles mL−1. Mixture A contained 10 and 45 μm diameter particles at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. Mixture B contained 6 and 10 μm diameter particles at a ratio of 1
100. Mixture B contained 6 and 10 μm diameter particles at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. At these concentrations, no aggregation of particles was observed. As predicted, the time variant acoustic field was able to manipulate and, at the same time, separate particles according to their size (Fig. 4). Fig. 4a shows the expected behaviour of the large and small particles over time (black = t0, purple = t1, red = t2, yellow = t3) in relation to the initial position of the nodes (indicated by Ni and Ni+1) and antinodes (indicated by Ni+1/2) of the acoustic landscape. During tramp, the large particles, experiencing larger acoustic force, follow the moving node and thus can be moved from Ni to Ni+1. At the same time, smaller particles, experiencing a smaller acoustic force, cannot follow the moving node and thus move over a smaller distance. During trest, the smaller particles return to their initial position Ni, while the larger particles settle at the next node Ni+1. Fig. 4b shows particle traces as a function of time (71 frames covering 36 s). In this case, the 45 μm diameter particles follow the shifted acoustic field (moving towards the right-hand side), while the 10 μm diameter particles stay close to the position of the original node. The time between frames was 0.5 s. The average movement of small and large particles was 3 ± 1 μm s−1 and 30 ± 1 μm s−1, respectively, during tramp.
100. At these concentrations, no aggregation of particles was observed. As predicted, the time variant acoustic field was able to manipulate and, at the same time, separate particles according to their size (Fig. 4). Fig. 4a shows the expected behaviour of the large and small particles over time (black = t0, purple = t1, red = t2, yellow = t3) in relation to the initial position of the nodes (indicated by Ni and Ni+1) and antinodes (indicated by Ni+1/2) of the acoustic landscape. During tramp, the large particles, experiencing larger acoustic force, follow the moving node and thus can be moved from Ni to Ni+1. At the same time, smaller particles, experiencing a smaller acoustic force, cannot follow the moving node and thus move over a smaller distance. During trest, the smaller particles return to their initial position Ni, while the larger particles settle at the next node Ni+1. Fig. 4b shows particle traces as a function of time (71 frames covering 36 s). In this case, the 45 μm diameter particles follow the shifted acoustic field (moving towards the right-hand side), while the 10 μm diameter particles stay close to the position of the original node. The time between frames was 0.5 s. The average movement of small and large particles was 3 ± 1 μm s−1 and 30 ± 1 μm s−1, respectively, during tramp.
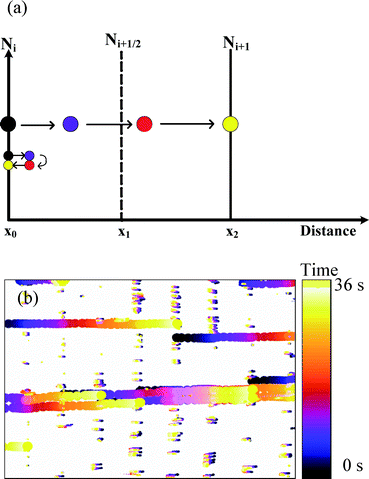 | ||
| Fig. 4 Position of the particles as a function of time (represented by colour). (a) A schematic illustration that shows 4 positions of large and small particles. Larger particles, experiencing larger acoustic force, are transported to the next node Ni+1 at x2, whereas smaller particles, experiencing smaller acoustic force, return to Ni at x0. (b) Experimental data of the 45–10 μm particle mixtures under the dynamic acoustic field (71 frames covering 36 s). The longer trails show the distance traversed by 45 μm diameter particles, whereas the short trails illustrate how 10 μm diameter particles do not traverse the space (see video in the ESI†). | ||
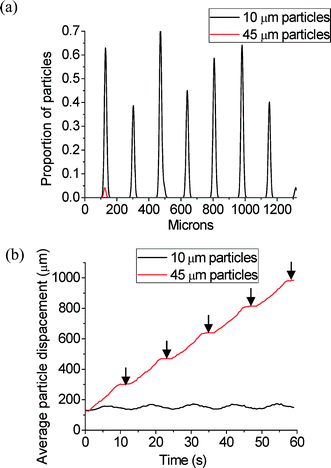
A series of time-lapse images were recorded at regular intervals during separation to produce particle traces. The image stack was then analysed using FIJI35 and the particle positions were extracted. The resulting data (x, y, particle area) was used to calculate the efficiency (eqn (7)) and purity (eqn (8)) of separation for each experiment by computing the particle density projected along the node lines and analysing the time variation of this density. Fig. 5a shows the initial position for 10 and 45 μm diameter particles for seven consecutive nodes. Fig. 5b shows the average displacement of the particles for five cycles of continuous phase shift including the rest period. It can be seen that the large particles successively travel during tramp and stabilise during trest at the next node resulting in an overall displacement over time. Simultaneously, the small particles move only slightly, therefore not crossing the midpoint of the nodes, and then return to their initial node position during trest.
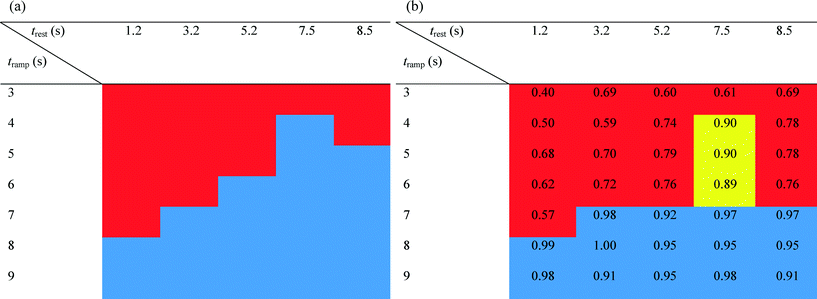
Separation efficiency was simulated using the DAF model outlined above with the same parameters as the experiments. Table 1(a) shows the simulation results. The regions coloured red represent the parameters that did not show particle separation, whereas the particles were successfully separated in the blue region. Table 1(b) shows the experimental results conducted replicating the simulation parameters. The blue regions represent those parameters where >91% separation was achieved and the red regions represent the parameters where <80% separation was achieved. The yellow regions show those parameters where the separation was 80–90% successful. Comparing computational and experimental results, it can be seen that there is a good match between the simulation and the experimental data.
It can be observed that separation performance improves with tramp and trest until it reaches a maximum. This indicates that the acoustic forces that the shifting nodes exert on the particles of interest need a minimum time to overcome inertia and viscous force in order to move the particles from the node Ni to the next node Ni+1. tramp is the critical parameter, since it has the greatest effect on the separation result. The value of trest is less influential on the separation of the entities.
When suitable parameters are selected, the separation process reaches its best experimental performance: with the separation ratio achieving ~100% purity and efficiency when trest = 4 s and tramp = 8 s.
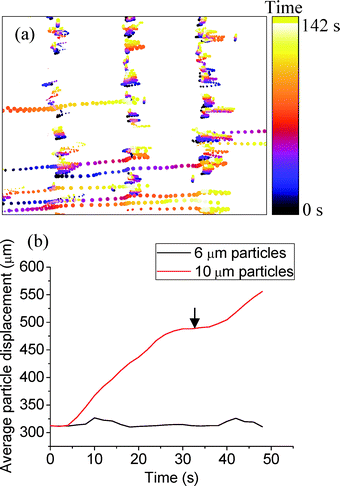
The simulation and experiment were replicated using 6 and 10 μm diameter particles. For these particles, it was found that ~97% of particles separate, with an efficiency and a purity of ~97%. These results were achieved with a value of tramp = 15 s and trest = 15 s. Fig. 6 shows the particle traces as a function of time for 6 and 10 μm diameter particle mixtures (71 frames covering 142 s) under these conditions. The 10 μm diameter particles move from node to node (moving towards the right-hand side), while the smaller particles (6 μm in diameter) remain close to their initial position of the original node. The average movement of the 6 and 10 μm diameter particles was calculated as 2 ± 1 μm s−1 and 10 ± 1 μm s−1, respectively, during tramp.
These above experiments were performed using the smallest gap in size of commercially available particles (10 ± 1 μm and 6 ± 0.6 μm; Polysciences Europe, Germany). Therefore, an experimentally accessible discrimination capability of the acoustic separation device is ±2 μm difference of particle diameter.
Acoustic separation by particle density
In this section, particles of the same diameter but of different densities are separated. The acoustic forces are not solely based on particle size but also depend on density (or particle mass), eqn (5). We tested the separation performance against particle density by using 10 μm diameter polystyrene particles and iron-oxide filled particles with a density of ρ = 1.05 g cm−3 and ρ = 1.41 g cm−3, respectively. The ratio of the concentration of iron-oxide filled and polystyrene particles was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100.
100.
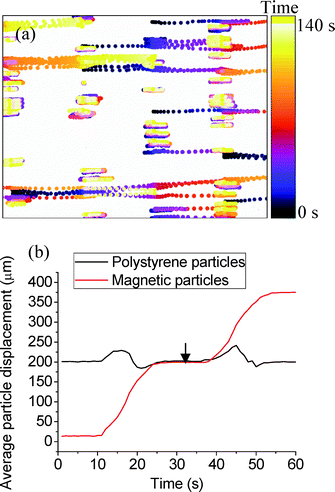
As for previous experiments, Fig. 7 shows particle traces as a function of time for 10 μm diameter iron-oxide filled and polystyrene particle mixtures using DAF (140 frames covering 140 s). It can be seen that the denser particles (iron-oxide filled) are significantly affected by the DAF and continuously travel from left to right, whilst the less dense particles (polystyrene) have a more restricted range of movement close to their starting positions. With these particles, a separation performance of ~99% has been recorded, with an efficiency of ~100% and a purity of ~98% for tramp = 14 s and trest = 14 s. The average movement of the polystyrene and iron-oxide filled particles was calculated as 3 ± 1 μm s−1 and 15 ± 1 μm s−1, respectively, during tramp.
The less dense particles (polystyrene) experience smaller acoustic force (eqn (4) and (5)) and thus cannot follow the DAF, while the denser particles (iron-oxide filled) track the movement of the dynamic acoustic field. Thus, particles of the same diameter but different densities can be sorted with the DAF method.
Acoustic separation of dorsal root ganglion (DRG) cells from a heterogeneous medium
As mentioned, particles used in our previous experiments are a reasonable surrogate for biological cells.29 Since the acoustic force is proportional to the particle volume, the polystyrene particles are thus likely to experience forces of the same order of magnitude as the cells. At higher intensities, ultrasound can create physiological effects; however, several studies have investigated viability and gene expression at intensities sufficient for manipulation in the MHz range and found no observable detrimental effect on cell viability over extended periods of exposure.36–39To assess a potential practical application, we applied the dynamic acoustic field to separate porcine dorsal root ganglion (DRG) neurons from a freshly isolated mixture containing myelin debris and other non-neuronal cells. The neurons would normally be separated based on their hydrodynamic state using centrifugation across a Ficoll gradient.40 The DRG neurons have an average size from 17 to 145 μm, while the myelin debris has a size of approximately from 10 to 15 μm.
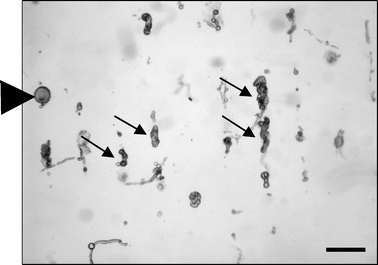
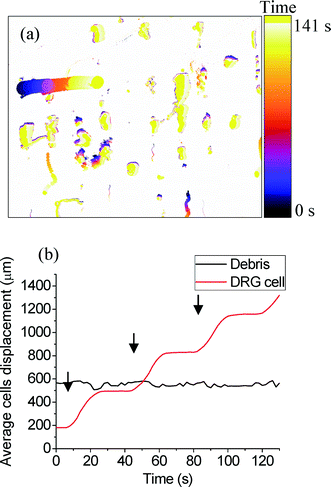
Fig. 8 shows debris (~26 μm) and a single DRG neuron (~85 μm) in the presence of an acoustic standing wave. These entities aligned themselves in vertical lines, agglomerating at the nodes of the acoustic field.41 A dynamic acoustic field was then applied (using a tramp and trest of 5 s), and the resulting time-lapse overlay is represented in Fig. 9a. The static material does not produce a trace, whereas the material that has been displaced shows a trace that moves from left to right. The DRG cell follows the shifted acoustic field (moving towards the right-hand side), while the debris exhibits minimal displacement of the original node. The time between frames was 0.5 s. The average movement of the DRG cell was calculated to be 18.5 ± 1 μm s−1 during tramp. Fig. 9b shows the displacement of the DRG over time.
In these experiments, the DRG cells exhibit similar behaviour to the particles in the previous experiments. Only the large DRG neurons are differentially shifted to the right, while smaller cells and debris remain in their original node.
Conclusion
We have demonstrated a new technique using a dynamic acoustic field to separate particulate materials on the basis of size and density. Separation occurs as long as the balance of the acoustic forces and the viscous force is differentiated between particle types. Since the acoustic forces are a function of size and density of the particles, many types of particles or cells can be separated. A detailed evaluation has been carried out using polystyrene and iron-oxide filled particles, prior to applying the technique to the separation and purification of recovered dorsal root ganglion cells from the myelin cells. The advantages of DAF for the separation of particles include its inherent safety and biocompatibility, the possibility of operating over large distances (centimetres), the high purity (ratio of particle populations) that can be achieved that is up to 100% and the high efficiency (ratio of separated particles over total number of particles of the same type in the sample) that can be up to 100%.Methods
Acoustic radiation force
The primary acoustic force Fa, eqn (4), and viscous force Fv, eqn (6), to which the particles are submitted at time t are given by eqn (4) to (6):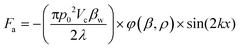 | (4) |
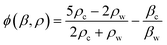 | (5) |
| Fv = −6πηRv | (6) |
The acoustic contrast factor, eqn (5), represented by ϕ in eqn (4), depends on both the particle density (ρc) and its compressibility (βc) in relation to the corresponding properties of the surrounding medium (ρw, βw). The equation of the primary radiation force, Fa, eqn (4), states that the acoustic force applied on the particles is proportional to the acoustic pressure amplitude (ρ0) squared and to the volume of the particles (Vc). The acoustic dynamic field takes advantage of the size dependency of the mechano-physical properties of the micro-entities being sorting, that scales with particle volume, inducing a primary force which is strongly dependent on particle size (r3) and medium viscosity.
Acoustic device
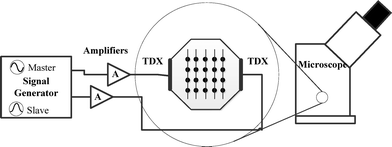
The acoustic device, described elsewhere,25 has been used to demonstrate how the dynamic acoustic field technology can be applied to particle sorting. In this paper, only two opposite transducers were used. The pair of transducer was synchronised using an arbitrary waveform generator (TGA12104, Thurlby Thandar Instruments, UK) allowing independent control of the amplitude, phase and frequency of each channel. The waveform generator was controlled by a general purpose interface bus (GPIB) utilizing a script written in Labview (National Instruments, USA). The signals, created by the waveform generator, were amplified and matched by high-speed buffers (AD811 Analog Devices USA; BUF634T, Texas Instruments, USA), before being fed to the transducers via length matched coax cables.Furthermore, an agar layer was introduced into the device to minimize the streaming42 and maximize the precision in control of the particle movement.
Separation performance
We studied the separation performance in terms of purity and efficiency depending on the ramping time and the resting time of the phase. Separation purity and separation efficiency are two figures of merit that can used to assess separation performance. The separation purity and efficiency can be expressed as: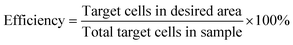 | (7) |
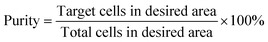 | (8) |
The demonstrated results in Table 1 were calculated using the following formula which is the average of purity and efficiency as described above:
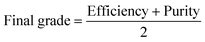 | (9) |
Competing financial interests
The authors declare no competing financial interests.Acknowledgements
We would like to acknowledge the following support which enabled the work presented: A.L.B. holds a Lord Kelvin Adam Smith Fellowship in Sensors Systems, and G.S. holds a Sensor Systems Initiative PhD studentship (Sensors Systems Initiative, University of Glasgow). J.N.R. was supported by an NC3Rs research contract (DRGNEt, contract no. 25752-175161). Part of this work was supported by an EPSRC EFutures grant (RES/0560/7386) and a Royal Society Research grant (RG130493).References
- Diethard Mattanovich and Nicole Borth, Microb. Cell Fact., 2006, 5(1), 12 CrossRef PubMed.
- Dimitri Pappas and Kelong Wang, Anal. Chim. Acta, 2007, 601(1), 26 CrossRef CAS PubMed.
- S. Neethirajan, I. Kobayashi, M. Nakajima, D. Wu, S. Nandagopal and F. Lin, Lab Chip, 2011, 11(9), 1574 RSC.
- R. G. Miller and R. A. Phillips, J. Cell. Physiol., 1969, 73(3), 191 CrossRef CAS PubMed.
- Yan Gao, Wenjie Li and Dimitri Pappas, Analyst, 2013, 138(17), 4714 RSC.
- A. Lenshof and T. Laurell, Chem. Soc. Rev., 2010, 39(3), 1203 RSC.
- Y. Chen, P. Li, P.-H. Huang, Y. Xie, J. D. Mai, L. Wang, N.-T. Nguyen and T. J. Huang, Lab Chip, 2014, 14(4), 626 RSC.
- J. A. Davis, D. W. Inglis, K. J. Morton, D. A. Lawrence, L. R. Huang, S. Y. Chou, J. C. Sturm and R. H. Austin, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(40), 14779 CrossRef CAS PubMed.
- A. A. S. Bhagat, S. S. Kuntaegowdanahalli and I. Papautsky, Microfluid. Nanofluid., 2009, 7(2), 217 CrossRef CAS.
- A. Valero, T. Braschler, N. Demierre and P. Renaud, Biomicrofluidics, 2010, 4(2), 022807 CrossRef PubMed.
- N. Harris, R. Boltryk, P. Glynne-Jones and M. Hill, Phys. Procedia, 2010, 3(1), 277 CrossRef CAS PubMed.
- L. Schmid, D. A. Weitz and T. Franke, Lab Chip, 2014, 14(19), 3710 RSC.
- O. Jakobsson, C. Grenvall, M. Nordin, M. Evander and T. Laurell, Lab Chip, 2014, 14(11), 1943 RSC.
- X. Ding, S.-C. S. Lin, M. I. Lapsley, S. Li, X. Guo, C. Y. Chan, I. K. Chiang, L. Wang, J. P. McCoy and T. J. Huang, Lab Chip, 2012, 12(21), 4228 RSC.
- X. Ding, Z. Peng, S. C. Lin, M. Geri, S. Li, P. Li, Y. Chen, M. Dao, S. Suresh and T. J. Huang, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(36), 12992 CrossRef CAS PubMed.
- J. J. Hawkes, R. W. Barber, D. R. Emerson and W. T. Coakley, Lab Chip, 2004, 4(5), 446 RSC.
- M. Hill, Y. Shen and J. J. Hawkes, Ultrasonics, 2002, 40(1–8), 385 CrossRef CAS.
- D. Carugo, T. Octon, W. Messaoudi, A. L. Fisher, M. Carboni, N. R. Harris, M. Hill and P. Glynne-Jones, Lab Chip, 2014, 14(19), 3830 RSC.
- M. Nordin and T. Laurell, Lab Chip, 2012, 12(22), 4610 RSC.
- F. Petersson, A. Nilsson, C. Holm, H. Jonsson and T. Laurell, Analyst, 2004, 129(10), 938 RSC.
- A. H. J. Yang and H. T. Soh, Anal. Chem., 2012, 84(24), 10756 CrossRef CAS PubMed.
- Yang Liu and Kian-Meng Lim, Lab Chip, 2011, 11(18), 3167 RSC.
- T. Kozuka, T. Tuziuti, H. Mitome, F. Arai and T. Fukuda, in Micromechatronics and Human Science, 2000. MHS 2000. Proceedings of 2000 International Symposium on (IEEE, 2000), p. 201 Search PubMed.
- T. Kozuka, T. Tuziuti, H. Mitome and T. Fukuda, in Micro Machine and Human Science, 1995. MHS '95, Proceedings of the Sixth International Symposium on (IEEE, 1995), p. 179 Search PubMed.
- A. L. Bernassau, C. R. P. Courtney, J. Beeley, B. W. Drinkwater and D. R. S. Cumming, Appl. Phys. Lett., 2013, 102(16), 164101 CrossRef PubMed.
- A. Bernassau, P. MacPherson, J. Beeley, B. W. Drinkwater and D. Cumming, Biomed. Microdevices, 2013, 15(2), 289 CrossRef CAS PubMed.
- A. L. Bernassau, O. Chun-Kiat, M. Yong, P. G. A. Macpherson, C. R. P. Courtney, M. Riehle, B. W. Drinkwater and D. R. S. Cumming, IEEE Trans. Ultrason. Ferroelectr. Freq. Control, 2011, 58(10), 2132 CrossRef PubMed.
- C. R. P. Courtney, C. K. Ong, B. W. Drinkwater, A. L. Bernassau, P. D. Wilcox and D. R. S. Cumming, P. R. Soc. A, 2012, 468, 337 CrossRef.
- J. Shi, D. Ahmed, X. Mao, S.-C. S. Lin, A. Lawit and T. J. Huang, Lab Chip, 2009, 9(20), 2890 RSC.
- L. Verlet, Phys. Rev., 1968, 165(1), 201 CrossRef.
- L. Verlet, Phys. Rev., 1967, 159(1), 98 CrossRef CAS.
- M. Baumann, V. Daanen, A. Leroy and J. Troccaz, in Computer Vision Approaches to Medical Image Analysis, ed. R. R. Beichel and M. Sonka, Springer, Berlin/Heidelberg, 2006, vol. 4241, p. 248 Search PubMed.
- T. Laurell, F. Petersson and A. Nilsson, Chem. Soc. Rev., 2007, 36(3), 492 RSC.
- T. Kozuka, T. Tuziuti, H. Mitome and T. Fukuda, in Micro Machine and Human Science, 1994. Proceedings, 1994 5th International Symposium on (IEEE, 1994), p. 83 Search PubMed.
- J. Schindelin, I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair, T. Pietzsch, S. Preibisch, C. Rueden, S. Saalfeld, B. Schmid, J. Y. Tinevez, D. J. White, V. Hartenstein, K. Eliceiri, P. Tomancak and A. Cardona, Nat. Methods, 2012, 9(7), 676 CrossRef CAS PubMed.
- H. Bohm, P. Anthony, M. R. Davey, L. G. Briarty, J. B. Power, K. C. Lowe, E. Benes and M. Groschl, Ultrasonics, 2000, 38(1), 629 CrossRef CAS.
- D. Bazou, R. Kearney, F. Mansergh, C. Bourdon, J. Farrar and M. Wride, Ultrasound Med. Biol., 2011, 37(2), 321 CrossRef PubMed.
- B. Vanherberghen, O. Manneberg, A. Christakou, T. Frisk, M. Ohlin, H. M. Hertz, B. Onfelt and M. Wiklund, Lab Chip, 2010, 10(20), 2727 RSC.
- Hultstrom, O. Manneberg, K. Dopf, H. M. Hertz, H. Brismar and M. Wiklund, Ultrasound Med. Biol., 2007, 33(1), 145–151 CrossRef CAS PubMed.
- R. Gilabert and P. McNaughton, J. Neurosci. Methods, 1997, 71(2), 191 CrossRef CAS.
- F. Gesellchen, A. L. Bernassau, T. Dejardin, D. R. S. Cumming and M. O. Riehle, Lab Chip, 2014, 14(13), 2266 RSC.
- A. L. Bernassau, P. Glynne-Jones, F. Gesellchen, M. Riehle, M. Hill and D. R. S. Cumming, Ultrasonics, 2014, 54(1), 268 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4lc01153h |
| ‡ G.D.S. did the experiments and analysis of the data. Acoustic technologies were devised by A.L.B. with input from D.R.S.C. M.O.R. and J.N.R. conceived the idea of applying the dynamic acoustic field to dorsal root ganglion cells and extracted the cells from pig tissue. D.R.S.C., M.O.R. and A.L.B. drafted the paper. All authors commented on the final manuscript. |
| This journal is © The Royal Society of Chemistry 2015 |