Multiplexed microfluidic blotting of proteins and nucleic acids by parallel, serpentine microchannels†
Sha
He‡
ab,
Yi
Zhang‡
a,
Pei
Wang
c,
Xingzhi
Xu
c,
Kui
Zhu
a,
Wenying
Pan
a,
Wenwen
Liu
a,
Kaiyong
Cai
*b,
Jiashu
Sun
*a,
Wei
Zhang
a and
Xingyu
Jiang
*a
aBeijing Engineering Research Center for BioNanotechnology & CAS Key Laboratory for Biological Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: xingyujiang@nanoctr.cn; sunjs@nanoctr.cn
bBiorheological Science and Technology, Ministry of Education, College of Bioengineering, Chongqing University, Chongqing 400044, China. E-mail: kaiyong_cai@cqu.edu.cn
cBeijing Key Laboratory of DNA Damage Response and College of Life Sciences, Capital Normal University, Beijing 100048, China
First published on 7th October 2014
Abstract
This work develops a high-throughput, high-efficiency and straightforward microfluidic blotting method for analyzing proteins and nucleic acids. Sample solutions containing antibodies (for protein detection) or hybridization probes (for nucleic acid detection) are introduced into the parallel, serpentine microchannels to specifically recognize the immobilized targets on the substrate, achieving the identification of multiple targets in multiple samples simultaneously. The loading control, molecular weight markers, and antigen/antibody titration are designed and integrated into the microfluidic chip, thus allowing for the quantification of proteins and nucleic acids. Importantly, we could easily distinguish the adjacent blotting bands inside parallel microchannels, which may be difficult to achieve in conventional blotting. The small dimensions of microfluidic channels also help to reduce the amount of probing molecules and to accelerate the biochemical reaction. Our microfluidic blotting could bypass the steps of blocking and washing, further reducing the operation time and complexity.
Introduction
Blotting is a process of transferring electrophoresis-separated proteins or nucleic acids onto a solid substrate and subsequently probing them with antibodies or hybridization probes.1,2 Despite rapid advances in high-throughput approaches in analyzing biomolecules, blotting is still a popular tool and gold standard for protein and nucleic acid analysis in molecular biology and related fields. It could identify a specific target in a mixture of samples and determine the absolute molecular weight and relative abundance of the target.3 Conventional blotting only allows for the detection of one target each time, and cannot be used for simultaneous analysis of multiple targets, although they have already been separated from each other after the electrophoresis and transfer procedures. To realize multiplexed blotting, various strategies have been reported, especially in the case of Western blot (WB) for proteins. Stripping and reprobing protocols required sequential steps and might weaken the original strength of the interaction, hence the resulting signals.4 Others are based on spectroscopic labeling techniques, such as multicolor quantum dots,5,6 surface-enhanced Raman scattering,7 and so forth. These strategies, although effective in their proposed design, were limited by the possible spectral overlap of the excitation/emission peak position or the complexity related to distinguishing fingerprints of the various spectra when the number of samples was high.Microfluidics is a promising technology for many fields, including small-scale fluid dynamics, biological applications and diagnosis/prognosis of diseases.8–13 In particular, microfluidic systems stand out for biomolecular assays because they allow multiplexation and miniaturization and thus significantly reduce the reaction time and reagents required.12,14–16 The microfluidic systems may overcome the limitations of conventional blotting, including low efficiency, labor-intensiveness, and high cost. There have been some trials on the combination of microfluidic systems and conventional blotting to miniaturize the whole platform.17–19 Previously, we reported a microfluidic WB (μWB) system to provide a simple, effective strategy for multiplexation.17 The μWB has demonstrated its power but it is not directly compatible with multi-lane blotting, which is commonly used in biological labs. Here we dramatically improve the μWB system to make it directly compatible with the most common experimental protocols of multi-lane blotting by applying parallel, serpentine microfluidic channels. We show that this integration is a paradigm-shift design for blotting both proteins and nucleic acids while resolving almost all of the problems in conventional blotting such as lack of multiplexation, high cost, labor-intensiveness, and so forth.
Experimental
Device design and assembly
Our design is inspired by microfluidic micromosaic immunoassay,20–22 which involves perpendicularly introducing a set of different antigens and a set of antibodies in microfluidic channels. By using parallel microfluidic channels perpendicular to the antigen bands to introduce varying antibodies, we could realize multiplexed blotting.23,24 Since the general principles of protein and nucleic acid blotting are similar, we thus use both of them as examples to demonstrate our multiplexed micro-blotting (MMB) analytical systems.The basic design of the MMB analytical system includes two major components (Fig. 1). One is the polyvinylidene fluoride (PVDF) membrane with protein or DNA samples loaded (Fig. 1A) and the other is the polydimethylsiloxane (PDMS) chip with embedded parallel, serpentine microfluidic channels (Fig. 1B, ESI†). We prepared the former through electrophoresis and transfer while fabricating the latter by soft lithography (see the ESI†). The serpentine profile of our microfluidic chip completely avoids the need of three-dimensional (3D) parallel channels, which are often required when multiple parallel targets have to be processed simultaneously.25,26 Compared to 3D microchannels, our design allows for one-step replica molding of the chip to keep the fabrication steps simple.27 As a model demonstration, we fabricated a microfluidic chip containing seven serpentine channels compatible with a five-lane electrophoresis system (Bio-Rad, Mini Protean). Each microfluidic channel has a total length of 30 cm, a width of 400 μm and a height of 500 μm and the interval between neighboring channels is 800 μm (Fig. S1, ESI†). The microfluidic chip is 72.5 mm long, 51.5 mm wide, and 5 mm high. We simply applied a clamp (Fig. S2, ESI†) to assemble the PDMS chip with the PVDF membrane together (Fig. S3, ESI†). The clamp is made of poly(methyl methacrylate) (PMMA) which is rigid enough to hold the chip and the membrane together tightly with the assistance of screws. It is also optically transparent without affecting any optical characterization of the reaction inside the chips.
Protein blotting
The microfluidic network was placed on the membrane with microfluidic channels perpendicular to the protein bands on the membrane. We inserted the pipette tips into the inlets of microfluidic channels and pressed the pipette to push the different types of antibody solutions with various concentrations flowing into the microchannels. By using the PMMA clamp, we found that in this manner the PDMS chip can seal conformally with the PVDF membrane without detectable leakage between any channels. After immunoreaction, the PDMS chip was peeled off the PVDF membrane for observation. For the detailed information about the use of the blocking solution and washing solution, please refer to our previous work.17 It should be noted that we pipetted the antibody solution from the inlet and repeated it from the outlet to eliminate the signal attenuation along the channels. The sample introduction process relies solely on pipettes rather than external tubes or pumps. As a result, the operation of our system is quite straightforward and independent of skilled hands, making MMB easily adaptable to common biological laboratories.For antibody/antigen titration, we first loaded different amounts of cell lysates into four electrophoretic lanes, 20 μg, 10 μg, 5 μg, and 2.5 μg, and immobilized them on the PVDF membrane by electrophoresis and transfer. The chip was placed on the membrane and serially diluted antibody solution that specifically targets Annexin was introduced into every channel. The initial dilution was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and the final dilution was 1
20 and the final dilution was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1280 with a twofold dilution step.
1280 with a twofold dilution step.
Nucleic acid blotting
Two series of sequences were used in the nucleic acid blotting experiment (Table S2†). All of the sequences were synthesized by Sangon Biotech (Shanghai) Co., Ltd. The specific sequences were intentionally terminated with different molecules are of different molecular weights, which can be recognized by the same detection probes, but have separated positions in the result images. We used the Mini-Protean tetra cell (Bio-Rad, Catalog # 165-8025) for DNA blotting. We carried out the nucleic acid blotting experiment as follows: (1) we prepared the acrylamide solution by mixing 80 mL of Acr/Bis (40%, acrylamide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bis-acrylamide, 19
bis-acrylamide, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 20 mL of 10× TBE and 84 g of urea. The volume of the mixture was tuned to 200 mL by adding an appropriate amount of deionized water and the solution was stored at 4 °C; (2) the DNA oligomers were mixed with loading buffers at equal volumes and incubated at 95 °C for 5 min, ensuring that every oligomer was single stranded; (3) we prepared the polyacrylamide gel (acrylamide solution 5 mL, ammonia persulfate 50 μL, TEMED 5 μL) and left it for 30 min under ambient conditions for the gel to condense; (4) we loaded the DNA–buffer mixture into the electrophoresis wells and ran the electrophoresis at 150 V for 60 min; (5) we transferred the DNA from the gel onto the PVDF membranes at 30 V for 3.5 h with a Trans-Blot transfer cell (Bio-Rad). After the nucleic acid transfer, the blotted PVDF membrane was incubated at 37 °C for 1 h to dry; (6) we assembled the DNA-blotted PVDF membrane and the PDMS microfluidic chip with the clamping device and fastened every screw; (7) we introduced designated DNA probes whose sequences were complementary to the DNA that were blotted onto the PVDF membrane; (8) the clamping device was disengaged and the membrane was subjected to three rounds of stringent washes; and (9) we put the membrane in the typhoon scanner and collected the data.
1), 20 mL of 10× TBE and 84 g of urea. The volume of the mixture was tuned to 200 mL by adding an appropriate amount of deionized water and the solution was stored at 4 °C; (2) the DNA oligomers were mixed with loading buffers at equal volumes and incubated at 95 °C for 5 min, ensuring that every oligomer was single stranded; (3) we prepared the polyacrylamide gel (acrylamide solution 5 mL, ammonia persulfate 50 μL, TEMED 5 μL) and left it for 30 min under ambient conditions for the gel to condense; (4) we loaded the DNA–buffer mixture into the electrophoresis wells and ran the electrophoresis at 150 V for 60 min; (5) we transferred the DNA from the gel onto the PVDF membranes at 30 V for 3.5 h with a Trans-Blot transfer cell (Bio-Rad). After the nucleic acid transfer, the blotted PVDF membrane was incubated at 37 °C for 1 h to dry; (6) we assembled the DNA-blotted PVDF membrane and the PDMS microfluidic chip with the clamping device and fastened every screw; (7) we introduced designated DNA probes whose sequences were complementary to the DNA that were blotted onto the PVDF membrane; (8) the clamping device was disengaged and the membrane was subjected to three rounds of stringent washes; and (9) we put the membrane in the typhoon scanner and collected the data.
Results and discussion
To demonstrate the utility of MMB, we firstly used the microfluidic chip to simultaneously detect three different proteins, β-actin, Annexin, and pan 14-3-3, from four lanes in NIH-3T3 cell lysates (Fig. 2). MMB can analyze multiple types of target proteins on many samples simultaneously. The readout for multiple types of target proteins is in a dot array format rather than bands in conventional blotting. In comparison, when identifying multiple target proteins in various samples by conventional blotting, processes such as cutting the protein-loaded membrane along the electrophoresis lanes are necessary, which would lead to separate incubation with different antibodies towards specific target proteins and increase the time and expense needed for one experiment as well as introducing systematic deviations. Besides, even a very slight shift in post-cutting alignment would impair the accuracy of the results if several protein bands with similar molecular weights have to be identified. As to multiple types of target proteins within one specific electrophoresis lane, stripping was a major choice for the sequential determination of different targets. Stripping would certainly weaken the binding between the antigen and the substrate (in our case it was the PVDF membrane) and thus dramatically increase the inaccuracy as the number of detections increases. Our MMB system would easily circumvent both cutting and stripping processes and eliminate all these negative influences from conventional blotting.Apart from simultaneous visualization of multiple targets in multiple samples, our system can provide all useful information that conventional blotting does. As we previously reported, the compartmentalization of different antibodies into separate channels can incorporate the loading controls and internal molecular weight markers into a single blot. Our MMB system here inherited this advantage and further expanded it into every single specific electrophoresis lane. As shown in Fig. 2, we introduced a mixture of three antibodies that targeted β-actin, Annexin and pan 14-3-3 into channel 1 across all four lanes. The clearly separated, regularly arranged three dots acted as internal molecular weight markers. In this case, every lane carried its own internal molecular “ruler” to determine the molecular weight of the target proteins and eliminate false signals. The reason why we emphasize the use of internal molecular weight markers lies in the following three aspects. (i) The electrophoresis/transfer conditions between different batches of experiment cannot be identical in practice. It is desirable to have a designed molecular weight ruler in spatial proximity to the sample since such an arrangement decreases the inaccuracy in measuring molecular weights as a result of the non-uniform electrical field. (ii) The commonly used commercially available pre-stained molecular weight standards often require the addition of a dye. The dye might affect the mobility as well as the actual molecular weight of the marker protein, especially when the size of the marker protein is small.28 (iii) Commercial pre-stained markers are often visible to the naked eye in the electrophoresis and transfer steps, but not so on the X-ray films or scanned digital images in the detection step.29 The internal molecular weight marker incorporated in our protocol, however, is close to the sample in every lane and can be visualized in the same way as the samples do without additional dye, eliminating all three existing problems in conventional blotting.
In addition to qualitative performance referred above, conventional blotting can also determine the relative abundance of target proteins and nucleic acids, which is often important to identify the change in cellular events.3,30 Generally, there are two steps required for achieving quantitative determination: antibody and antigen titration. Antibody titration is used to determine the optimal antibody concentration in the experiment for the best signal-to-noise ratio. Antigen titration can build a calibration curve with acceptable linearity to determine the up-regulation or down-regulation of targets reliably. In conventional blotting, these two steps have to be done with separate, multiple rounds of blotting, but limited amounts of samples sometimes make separate experiments impossible.31 In MMB, however, these two steps can be combined and completed in a single blot (Fig. 3A). On the one hand, seven parallel channels within one lane allow us to simultaneously investigate the effect of a wide spectrum of concentration of antibodies towards the same amount of antigens, while four electrophoretic lanes can be loaded with samples in serial dilution. We could easily locate the saturation area of the fluorescence signal intensity in the fitted sigmoidal curve and use the turning point as the optimized concentration in subsequent experiments (Fig. 3B). The optimized concentration informs the strongest signal that a system was able to achieve with the least amount of antibodies. On the other hand, we applied this optimized concentration of antibodies to titrate serially diluted antigens, and the fluorescence intensity has an excellent linear relationship with the amount of antigens. The resulting calibration curve built here enabled direct, rapid determination of the actual mass of samples (Fig. 3C).
Besides integrating these routine operations, the compartmentalization of antibodies in parallel microfluidic channels helps to clearly discriminate merged protein bands. Target proteins with very close molecular weights within one electrophoresis lane sometimes merge together, appearing as one “fat” band, making each of them undistinguishable. In MMB, in contrast, the target protein bands, even merged together, can be easily identified by channels. The introduction of different antibodies in different channels would divide these bands into microdots via positional information provided by the microchannels. To confirm our hypothesis, we used MMB to analyze four types of proteins, β-actin, Annexin, pan 14-3-3 and GAPDH, simultaneously. Among them Annexin and GAPDH have close molecular weights (43 kDa for β-actin, 36 kDa for Annexin, 35.9 kDa for GAPDH and 30 kDa for pan 14-3-3. It is very difficult to distinguish the difference of 0.1 kDa in molecular weight). In conventional blotting, the signals for these four target proteins displayed as three bands, among which Annexin and GAPDH merged together as a “fat” band with brighter signals (Fig. 4A). This makes it difficult for accurate identification amongst these proteins. When the MMB system is applied, antibodies for β-actin, Annexin, GAPDH and pan 14-3-3 were introduced into separate channels and all signals were clearly confined within its own channels (Fig. 4B), generating a series of micro-dots. As a result, the identification of several neighboring bands could be realized using only one electrophoresis experiment and one detection experiment, dramatically simplifying the labor-intensive process of conventional blotting.
In addition to the capacity for parallel detection of MMB, the featured small sizes of micro-channels could also save the amount of antibody compared with conventional blotting. The extremely small volume of reaction spaces, typically around 40 μL, would surely confine the antibodies in a concentrated manner and promote the spatial efficiency. By applying the same amount of antibodies in these two scenarios, MMB was two to eight times more sensitive than conventional blotting in almost every comparison (Fig. S4, ESI†). We reasoned that size reduction of the micro-channels localized the antibodies and thus increased the concentration of antibodies to benefit the efficiency of reactions. In other words, MMB could exhibit the same performance as conventional blotting does by consuming a much less amount of antibodies.
Generally, large quantities of antibodies and long incubation time would result in measurable non-specific adsorption in conventional blotting, which demands extensive blocking and washing steps to reduce. As a result, it is natural for us to explore whether non-specific adsorption could be mitigated by taking advantage of the reduction of antibody and time in MMB. We attempted skipping the blocking–washing process in MMB and found that it did not have apparent influence on the quality of the results until the saturation time compared to operations with blocking–washing steps (Fig. 5). We can skip blocking–washing steps in microfluidic blotting because (1) the antibody solution we used was of extremely low volume (~40 μL), and thus the amount of antibodies involved in the antibody–antigen recognition process is far less than that in conventional blotting; (2) the physical absorption is a non-targeted, dynamic process, which needs a sufficient amount of time to reach measurable signals from non-specific binding. Non-specific binding is often a problem for conventional blotting because it usually goes with overnight incubation. The reaction time we optimized (~20 min) was also much shorter than that used in conventional blotting, and the incubation was terminated before physical absorption became overwhelming; (3) the antibody solution also contains high concentration of other proteins (serum proteins, milk proteins) besides the designated antibodies. The antibodies will more favorably bind to the corresponding antigens inside the microchannels compared with milk proteins, especially given that the reaction time was very limited; (4) we have several channels to carry out parallel reaction in a single run, and the parallel results can help us to eliminate false-positive outcomes by comparison. The skipping of blocking–washing implied a further reduction of operation time and complexity. Also, we found that one can even reduce the incubation time to 5 min by using high concentration (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 here, 1
20 here, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 in ordinary assays) of antibodies, without inducing any non-specific adsorption. It was worth noting that the antibody consumed in such a manner was still less than that in conventional blotting, simply because of the significant size reduction of reactants and reagents. Given these advantages of skipping blocking, 5 min incubation and less antibodies, MMB would be appealing in potential practical applications in most biological labs. In order to investigate the limitations of the channels for practical use, we extended the design of the chip to work for the ten-lane electrophoresis system (Bio-Rad, Mini Protean, see the ESI,† Fig. S8). We found that this chip can be compatible for easy manipulation as well. Accommodating more lanes means more samples incorporated, therefore indicating that MMB is even more likely to contribute to real-world applications.
500 in ordinary assays) of antibodies, without inducing any non-specific adsorption. It was worth noting that the antibody consumed in such a manner was still less than that in conventional blotting, simply because of the significant size reduction of reactants and reagents. Given these advantages of skipping blocking, 5 min incubation and less antibodies, MMB would be appealing in potential practical applications in most biological labs. In order to investigate the limitations of the channels for practical use, we extended the design of the chip to work for the ten-lane electrophoresis system (Bio-Rad, Mini Protean, see the ESI,† Fig. S8). We found that this chip can be compatible for easy manipulation as well. Accommodating more lanes means more samples incorporated, therefore indicating that MMB is even more likely to contribute to real-world applications.
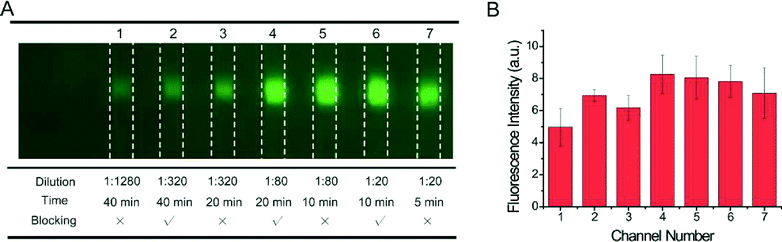 | ||
| Fig. 5 (A) Examination of the blocking conditions for different reaction conditions. (B) Quantification of the corresponding fluorescence intensity. | ||
Furthermore, we showed how MMB would identify the complexation status of phosphatase-4 (PP4), a crucial enzyme in the DNA damage repair process. DNA damage can transform the histone variant H2AX into its phosphorylated form γ-H2AX to hinder the DNA repair. PP4 can precisely control the basal level γ-H2AX and dephosphorylate it into its pristine form, initializing the repair process. It is believed that PP4 forms several multimeric complexes with proteins to carry out its function, but the subunit compositions are not well characterized. A recent study has identified several predicted components using conventional immunoprecipitation/WB assays with 293 T cell lysates, including one central catalytic subunit PP4C and four regulatory subunits PP4R1, PP4R2, PP4R3α, and PP4R3β.32 To identify these proteins, five respective electrophoresis, transfer and blotting steps and membrane cutting/combination were required.33 Herein, we showed that our MMB chip for a ten-lane system would dramatically simplify this process into a single blot. We first loaded five lanes from left to right with PP4R3β, PP4R2, PP4R1, PP4R3α, PP4C, and loaded the sixth lane with non-specific IgG as negative control. The protein-transferred PVDF membrane was assembled with the designed PDMS microfluidic chip before we introduced five antibodies that specifically target each subunit, respectively. The resulting image from this single MMB contains almost all of the crucial information needed for identifying the complexation between different subunits (Fig. 6). For example, in the second lane loaded with PP4R2, there were four dots generated by the immuno-recognition of the PP4R2 antibody, PP4R3α antibody, PP4R3β antibody and PP4C antibody (ESI† Table S1). This indicates that the regulatory subunit PP4R2 would have to interact with PP4R3α and PP4R3β for normal biological functions besides binding the central catalytic component PP4C. More importantly, this intact image would also provide the correct information about their molecular weight without resorting to membrane cut/recombination steps.
To demonstrate the versatility of our MMB system, we performed multiplexed DNA blotting using the same setup. Oligomers with conservative sequences of hepatitis B virus (HVB) or variola virus (VV) were intentionally modified with A10 or EG18 to make them have different molecular weights and they were referred to as target (T) sequences: T-HVB-A10, T-HVB-EG18, T-VV-A10, T-VV-EG18. A mixture of four target sequences was loaded to two electrophoretic lanes and separated electrophoretically. After the targets were transferred to membranes, serpentine microchannels were assembled with the membrane and the detection probes (DP) were introduced into the channels and incubated. We could visualize both HVB and VV targets at the same time, even with different A10 or EG18 terminal modifications (Fig. 7). Since these two sequences are highly conservative to these two diseases, it would be critical for disease control or homeland security to detect these two sequences simultaneously in complex samples. Our setup, due to its intrinsic multiplexation capability, can detect these two targets in two different samples and the capacity for multiplexation can be further increased.
Conclusions
In conclusion, our MMB system would triumph over conventional blotting when aiming at analyzing multiple targets because it provided readout for all targets at the same time in a single image. When doing so, MMB does not require any sophisticated instrumentation or experienced personnel (unlike other high-throughput strategies for the analysis of biomolecules). It can integrate various routine operations such as loading control, internal molecular weight marker and antigen/antibody titration as well as provide higher resolution with reduced time and cost. We believed that our MMB system is a paradigm-shift design in terms of convenience and cost-effectiveness. It could help promote the efficiency of blotting drastically, which might interest biochemical, medical and engineering researchers. We believe that it would become a potential candidate for next-generation analytical tool for proteins and nucleic acids, especially for multiplexed detection in real-world applications.Acknowledgements
We acknowledge financial support from the MOST (2013AA032204, and 2013YQ190467), the NSFC (21475028, 51105086, 21025520, and 81361140345), the Beijing Municipal Science & Technology Commission (Z131100002713024), the Chinese Academy of Sciences (XDA09030305, XDA09030308), and the CAS/SAFEA International Partnership Program for Creative Research Teams.Notes and references
- H. Towbin, T. Staehelin and J. Gordon, Proc. Natl. Acad. Sci. U. S. A., 1979, 76, 4350–4354 CrossRef CAS.
- W. N. Burnette, Anal. Biochem., 1981, 112, 195–203 CrossRef CAS PubMed.
- B. T. Kurien and R. H. Scofield, Methods, 2006, 38, 283–293 CrossRef CAS PubMed.
- A. Sadra, T. Cinek and J. B. Imboden, Anal. Biochem., 2000, 278, 235–237 CrossRef CAS PubMed.
- R. Bakalova, Z. Zhelev, H. Ohba and Y. Baba, J. Am. Chem. Soc., 2005, 127, 9328–9329 CrossRef CAS PubMed.
- W. Chen, D. H. Xu, L. Q. Liu, C. F. Peng, Y. Y. Zhu, W. Ma, A. Bian, Z. Li, Y. Yuan, Z. Y. Jin, S. F. Zhu, C. L. Xu and L. B. Wang, Anal. Chem., 2009, 81, 9194–9198 CrossRef CAS PubMed.
- X. X. Han, Y. Kitahama, Y. Tanaka, J. Guo, W. Q. Xu, B. Zhao and Y. Ozaki, Anal. Chem., 2008, 80, 6567–6572 CrossRef CAS PubMed.
- J. Sun, Y. Xianyu and X. Jiang, Chem. Soc. Rev., 2014, 43, 6239–6253 RSC.
- Y. Zhang, L. Zhang, J. Sun, Y. Liu, X. Ma, S. Cui, L. Ma, J. Xi and X. Jiang, Anal. Chem., 2014, 86, 7057–7062 CrossRef CAS PubMed.
- J. Sun, Y. Gao, R. J. Isaacs, K. C. Boelte, C. P. Lin, E. M. Boczko and D. Li, Anal. Chem., 2012, 84, 2017–2024 CrossRef CAS PubMed.
- J. Sun, M. Li, C. Liu, Y. Zhang, D. Liu, W. Liu, G. Hu and X. Jiang, Lab Chip, 2012, 12, 3952–3960 RSC.
- X. Mu, W. F. Zheng, J. S. Sun, W. Zhang and X. Y. Jiang, Small, 2013, 9, 9–21 CrossRef CAS PubMed.
- Y. Xianyu, K. Zhu, W. Chen, X. Wang, H. Zhao, J. Sun, Z. Wang and X. Jiang, Anal. Chem., 2013, 85, 7029–7032 CrossRef CAS PubMed.
- K. L. Gilroy, S. A. Cumming and A. R. Pitt, Anal. Bioanal. Chem., 2010, 398, 547–554 CrossRef CAS PubMed.
- J. Sun, Y. Xianyu, M. Li, W. Liu, L. Zhang, D. Liu, C. Liu, G. Hu and X. Jiang, Nanoscale, 2013, 5, 5262–5265 RSC.
- Y. Liu, J. Yu, M. Du, W. Wang, W. Zhang, Z. Wang and X. Jiang, Biomed. Microdevices, 2012, 14, 17–23 CrossRef CAS PubMed.
- W. Y. Pan, W. Chen and X. Y. Jiang, Anal. Chem., 2010, 82, 3974–3976 CrossRef CAS PubMed.
- S. Jin, G. J. Anderson and R. T. Kennedy, Anal. Chem., 2013, 85, 6073–6079 CrossRef CAS PubMed.
- G. Hu, Y. Gao and D. Li, Biosens. Bioelectron., 2007, 22, 1403–1409 CrossRef CAS PubMed.
- X. Y. Jiang, J. M. K. Ng, A. D. Stroock, S. K. W. Dertinger and G. M. Whitesides, J. Am. Chem. Soc., 2003, 125, 5294–5295 CrossRef CAS PubMed.
- A. Bernard, B. Michel and E. Delamarche, Anal. Chem., 2001, 73, 8–12 CrossRef CAS PubMed.
- Y. Zhang, X. W. Wang, L. S. Song, C. L. Xu, L. Y. Ma, Z. H. Li, J. H. Xi and X. Y. Jiang, Anal. Methods, 2012, 4, 3466–3470 RSC.
- O. Stoevesandt, M. J. Taussig and M. Y. He, Expert Rev. Proteomics, 2009, 6, 145–157 CrossRef CAS PubMed.
- J. LaBaer and N. Ramachandran, Curr. Opin. Chem. Biol., 2005, 9, 14–19 CrossRef CAS PubMed.
- M. Y. Zhang, J. B. Wu, L. M. Wang, K. Xiao and W. J. Wen, Lab Chip, 2010, 10, 1199–1203 RSC.
- J. R. Anderson, D. T. Chiu, R. J. Jackman, O. Cherniavskaya, J. C. McDonald, H. K. Wu, S. H. Whitesides and G. M. Whitesides, Anal. Chem., 2000, 72, 3158–3164 CrossRef CAS PubMed.
- X. Y. Jiang, Q. B. Xu, S. K. W. Dertinger, A. D. Stroock, T. M. Fu and G. M. Whitesides, Anal. Chem., 2005, 77, 2338–2347 CrossRef CAS PubMed.
- Y. M. Zhang, L. Geng and D. W. Huang, Anal. Biochem., 2009, 390, 206–208 CrossRef CAS PubMed.
- Y. L. Bai, C. G. Koh, M. Boreman, Y. J. Juang, I. C. Tang, L. J. Lee and S. T. Yang, Langmuir, 2006, 22, 9458–9467 CrossRef CAS PubMed.
- Y. Li, J. F. Lu, Y. H. Han, X. X. Fan and S. W. Ding, Science, 2013, 342, 231–234 CrossRef CAS PubMed.
- J. Renart and I. V. Sandoval, Methods Enzymol., 1984, 104, 455–460 CAS.
- J. Liu, L. Xu, J. Zhong, J. Liao, J. Li and X. Xu, Cell Cycle, 2012, 11, 2643–2649 CrossRef CAS PubMed.
- D. Chowdhury, X. Xu, X. Zhong, F. Ahmed, J. Zhong, J. Liao, D. M. Dykxhoorn, D. M. Weinstock, G. P. Pfeifer and J. Lieberman, Mol. Cell, 2008, 31, 33–46 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4lc00901k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |






