Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Speciation of chromium by dispersive liquid–liquid microextraction followed by laser-induced breakdown spectrometry detection (DLLME–LIBS)
Ivanise
Gaubeur
*a,
Miguel Ángel
Aguirre
b,
Nikolay
Kovachev
b,
Montserrat
Hidalgo
*b and
Antonio
Canals
b
aHuman and Natural Sciences Center – Federal University of ABC, Av. dos Estados, 5001, 09210-971, Santo André, São Paulo, Brazil. E-mail: ivanise.gaubeur@ufabc.edu.br; Tel: +55 11 4996 0187
bDepartment of Analytical Chemistry and Food Science and University Materials Institute – University of Alicante, Apdo. 99, Alicante E-03080, Spain. E-mail: montserrat.hidalgo@ua.es
First published on 9th November 2015
Abstract
In this study, an analytical methodology based on a combination of dispersive liquid–liquid microextraction with laser-induced breakdown spectrometry was evaluated for simultaneous pre-concentration, speciation and detection of Cr. The microextraction procedure was based on the injection of appropriated quantities of 1-undecanol and ethanol into a sample solution containing the complexes formed between Cr(VI) and diethyldithiocarbamate (DDTC). The main experimental factors affecting the complexation and the extraction of metal (pH, DDTC concentration, extractant and volume of disperser solvents) were optimized using a multivariate analysis consisting of two steps: a Plackett–Burman design followed by a Circumscribed Central Composite Design (CCCD). Under optimum microextraction conditions, the analytical figures of merit of the proposed methodology were assessed. The method was finally applied to the analysis of a certified reference material hard drinking water (ERM® CA011a), yielding results in good agreement with the certified value.
1 Introduction
Nowadays, it is widely known that the mobility, bioavailability and toxicity of trace metals in environmental systems not only depend on their concentrations, but critically on their chemical form. Among the various metals in the environment, for instance in soils or superficial and underground water, which may pose toxicity to mankind, we can find chromium. Due to the use of this element in a number of products and industrial activities (e.g. pigments, leather dyeing, electrotyping, wood preservation, and catalysts), it can be dispersed into the environment, causing serious damage.1–5Chromium can be found in two main forms, trivalent and hexavalent. Cr(III) is an essential trace element and is widely used in nutritional supplements as it plays an important role in maintaining living organisms, for example, controlling glucose as well as being involved in the metabolism of lipids and proteins.3,4 However, studies have shown that some Cr(III) compounds may have genotoxic effects on cell cultures under certain conditions.6 Cr(VI) is considered much more toxic than Cr(III) and is classified as a carcinogenic element.7 The main effects associated with the primary exposure of Cr(VI) compounds are respiratory, gastrointestinal, immunologic, hematologic, reproductive and developmental. The difference between toxicological effects from Cr(III) and Cr(VI) is rather complex and is also related to the chemical characteristics of each species such as the high oxidation potential of Cr(VI), as well as the stability, mobility and bioavailability of such species in the environment. In addition, the permeability of Cr(VI) in cells is greater than that of Cr(III).8–10
Based on the above mentioned discussion, analytical measurements expressed as total metal content in different aquatic, terrestrial or aerial environments appear to be insufficient for proper assessment of the environmental risk of potentially dangerous species. The rapidly growing interest in the elucidation of the chemical forms, in addition to the quantitative estimation of specific elements, has resulted in the development of a new generation of analytical methodologies that are able to perform what is defined as speciation analysis. Hyphenation of chromatographic or extraction techniques, for species separation, with spectrometric techniques is the usual analytical strategy for speciation analysis, and among the different spectrometric techniques, inductively coupled plasma mass spectrometry (ICP-MS), inductively coupled plasma optical emission spectrometry (ICP-OES), and electrothermal atomic absorption spectrometry (ETAAS) are most widely employed.10–12 To date, very limited studies have been focused on the use of Laser-Induced Breakdown Spectrometry (LIBS) for speciation analysis.13
Among the various procedures used for extraction and preconcentration of metals, aiming at speciation for later detection, we can indicate dispersive liquid–liquid microextraction (DLLME), which consists of the rapid injection of a mixture of extractant and disperser solvents into a sample solution. This procedure results in the formation of a cloudy solution, in which fine droplets of the extractant solvent are dispersed in the sample, thus making it possible to carry out both extraction and preconcentration within a rapid single stage.14 In addition to its rapidity and simplicity, the use of very small organic solvent volumes in DLLME represents an added advantage over conventional liquid–liquid extraction methodologies, because it makes the procedure less harmful to the environment, with reduced generation of residue, resulting in a reduction in the cost and time devoted to related analysis.
In this study, the speciation of chromium in water samples by combination of DLLME with LIBS is presented for the first time. The analytical methodology evaluated here is based on the selective complexation of Cr(VI) with diethyldithiocarbamate (DDTC) in acidic medium, followed by the extraction of Cr(VI) chelates in 1-undecanol using a DLLME procedure. The resulting microvolumes of the analyte-enriched organic solvent are finally analyzed by LIBS. The total Cr content is obtained using the same procedure, after previous oxidation of Cr species in the sample. Experimental factors affecting the chelation and extraction of Cr(VI) were optimized using a multivariate approach. Finally, the proposed methodology was applied to the quantification of Cr in a certified reference material, to assess the analytical capabilities of the method.
2 Experimental
2.1 Instrumentation
The sample, reagents and final solutions were weighed using an analytical balance (model AX423, Sartorius, Madrid, Spain). A pH meter with a combined glass electrode was used for pH measurements. A centrifuge (model 2690/5, Nahita Centrifuges, Beriain, Spain) was used to accelerate the phase separation. The disperser and extractant solvent mixture was injected into the sample using a 1000 μL syringe (Gastight®, Hamilton Co, Reno, Nevada, USA).For LIBS analysis, the laser-induced plasmas were generated in air at atmospheric pressure by focusing a 10 Hz pulsed Nd-YAG laser (model HYL101 Handy-YAG, Q-switched, Quanta System S.P.A., Varese, Italy) on the sample to analyze it. The laser was operated in single-pulse mode, emitting at its fundamental wavelength (i.e. 1064 nm) with energy 130 mJ per pulse and 10 ns FWHM pulse width. The laser beam was focused on the samples using a N-BK7 plano-convex lens with 100 mm focal length (model KPX094AR.33, Newport Corporation, Irvine, USA). Plasma emission was directly collected with a five-furcated optical fiber (5 × 400 μm fiber optic cable, model FC5-UV400-2, Avantes, Eerbeek, Netherlands), and was imaged on the entrance slit of a five-channel spectrometer with spectral coverage from 200 nm to 844 nm (model AVS-Rackmount-USB2 housing equipped with five preconfigured AvaSpec-ULS2048-USB2-RM channels, Avantes, Apeldoorn, The Netherlands), wherein the plasma light was spectrally resolved and detected. A delay system consisting of two pulse generators (digital delay/pulse generator, model DG 535, Stanford Research Systems, Inc. and 1–50 MHz pulse/function generator, model 8116A, Hewlett Packard/Agilent Technologies, Santa Clara, USA) was used for synchronization of laser firing and data acquisition. Spectra were obtained 1.3 μs after plasma generation, with 1 ms acquisition time. Cr I (357.869 nm) was the emission line evaluated in this study. LIBS spectra were processed using the spectroscopic software LIBS++®, v. 3.12.4.1., IPCF-CNR (Pisa, Italy).
2.2 Reagents and solutions
All solutions were prepared with analytical grade chemicals and deionized water obtained from a Milli-Q system (Millipore, Bedford, USA). A 1.0% (w/w) DDTC stock solution was prepared daily by dissolving appropriate amounts of reagent ≥99.0% (Sigma Aldrich, St. Louis, USA). Ethanol (Sigma Aldrich, St. Louis, USA) was used as a disperser solvent and 1-undecanol (Acros Organics, Geel, Belgium) as an extractant solvent. Chromium(VI) standard solutions were prepared by appropriate dilutions of 10% K2CrO4 aqueous stock solution (Scharlau, Sentmenat, Spain). Sulfuric acid (Merck, Darmstadt, Germany) and KMnO4 (Scharlau, Sentmenat, Spain) solutions were used for pH adjustment and as an oxidant reagent, respectively.2.3 DLLME procedure and LIBS analysis
To extract the analyte by DLLME, a fixed amount of sample (1000 mg) or standard solution was transferred to a 10 mL glass tube.For Cr( VI ) determination, 522 mg of chelating agent DDTC 1.0% (w/w) and 900 mg of H2SO4 (1.0 mol L−1) were added to the sample and the mixture was filled with deionized water up to 9.000 g.
For total Cr determination, after acidification with 900 mg of 1.0 mol L−1 H2SO4, 2 droplets of 0.050 mol L−1 KMnO4 solution were added. The resulting mixture was heated at 45 °C for 15 minutes to ensure the oxidation of Cr species.15 After cooling, 522 mg of chelating agent DDTC 1.0% (w/w) was added and the mixture was filled with deionized water up to 9.000 g.
After the steps described above, with the aim of either Cr(VI) or total Cr determination, a mixture of 50 μL of extractant solvent (1-undecanol) and 156 μL of disperser solvent (ethanol) was injected into the sample using a glass syringe. Phase separation was then achieved by centrifugation at 3000 rpm for 3 min.
LIBS analysis of the resulting analyte-enriched organic solvent was carried out using the surface-enhanced LIBS methodology (SENLIBS) already described elsewhere.16–20 To this end, 10 μL of the organic extract was transferred to an aluminum substrate, heated to dryness and analyzed by the LIBS experimental system described above (Section 2.1).
The Cr(III) concentration in the samples was evaluated from the difference between the total Cr and Cr(VI) concentrations found using the above mentioned procedure.
2.4 Optimization of DLLME experimental parameters
The DLLME procedure was optimized by multivariate analysis consisting of two steps: (i) a Plackett–Burman design (screening) followed by (ii) a Circumscribed Central Composite Design (CCCD) (optimization) using the NemrodW statistical software (NemrodW® v.2007/2010, LPRAI, Marseille, France). Each step involved 12 microextraction experiments, which were carried out randomly to minimize the effect of uncontrollable variances. The optimization studies were carried out using a standard solution containing 500 μg kg−1 Cr(VI) in deionized water. A LIBS emission signal obtained from the analysis of the organic solvents resulting from the microextraction procedures was used as a response variable.3 Results and discussion
3.1 Optimization of experimental parameters
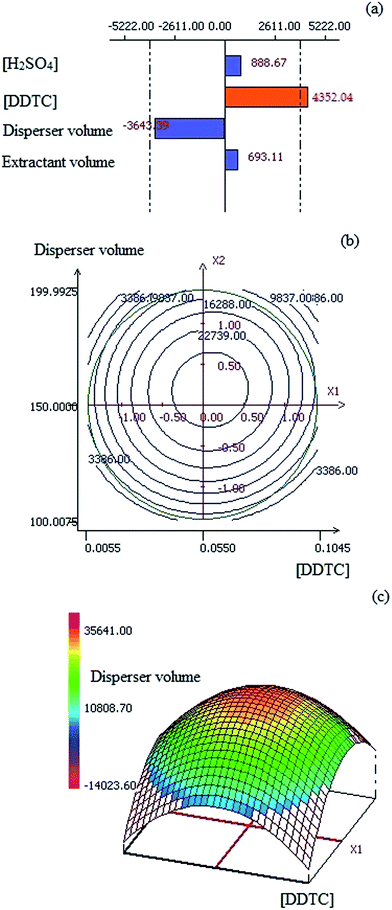
Table 1 shows the experimental factors and levels used in the exploratory planning, leading to a matrix with 12 experiments (Plackett–Burman design). The results obtained from this screening study are shown in the Pareto chart in Fig. 1(a). In this chart, bars to the right indicate a positive influence on the DLLME procedure when increasing the value of the experimental factor, whereas bars to the left indicate a negative influence. The two vertical lines refer to the reliability level of 95% and the factors with a significant influence on the DLLME procedure go beyond these lines. As observed, the H2SO4 concentration and extractant solvent volume do not have a significant influence on DLLME. Therefore, we decided to maintain the H2SO4 concentration at 0.10 mol L−1 and the 1-undecanol volume at 50 μL. On the other hand, the DDTC concentration seems to be an important factor with respect to Cr(VI) microextraction and increasing its concentration is beneficial to DLLME.| Plackett–Burman | ||
|---|---|---|
| Experimental factor | Level | |
| Low (−1) | High (+1) | |
| H2SO4/(mol L−1) | 0.01 | 0.1 |
| [DDTC]/(%, w/w) | 0.01 | 0.1 |
| Disperser volume/(μL) | 100 | 200 |
| Extractant volume/(μL) | 50 | 100 |
| Circumscribed central composite design | |||||
|---|---|---|---|---|---|
| Experimental factor | Level | Star points (α = 1.4142) | |||
| Low (−1) | Central (0) | High (+1) | −α | +α | |
| [DDTC]/(%, w/w) | 0.020 | 0.055 | 0.090 | 0.0055 | 0.105 |
| Disperser volume/(μL) | 114 | 150 | 185 | 100 | 200 |
The volume of disperser solvent was a factor that did not go beyond the reference line but remained close to it. For this reason, the disperser solvent volume was also investigated in the following step. The DDTC concentration and disperser solvent volume were studied at five levels using a Circumscribed Central Composite Design (CCCD). Table 1 shows various levels selected in the CCCD, leading to a matrix with 12 experiments based on 4 repetitions of the central point. The results of this study are given in Fig. 1(b) and (c) as a contour plot and a response surface, respectively, showing the variation in the LIBS emission signal as a function of DDTC concentration and disperser solvent volume.
It can be observed in Fig. 1(b) that the disperser solvent volume and DDTC concentration meet an optimal value for Cr(VI) extraction at 156 μL and 0.058% (w/w), respectively. The quantity of DDTC added to the sample should be high enough to ensure quantitative complexation of the analyte. In general, an excess is necessary to guarantee the formation of a fair amount of the target analyte complex, even in the presence of interfering species.20–22 The volume of disperser solvent should be controlled to ensure adequate extractant solvent dispersion, thus leading to the formation of fine droplets that are responsible for the extraction efficiency in DLLME. However, an excess of disperser solvent may increase the solubility of the previously formed hydrophobic analyte complex in the aqueous phase and the dilution of the organic phase, thus resulting in a lower extraction efficiency.
Based on the results shown above, the DLLME experimental conditions selected for chromium speciation were: DDTC concentration 0.058% (w/w), H2SO4 concentration 0.10 mol L−1, 50 μL of 1-undecanol as extractant solvent and 156 μL of ethanol as disperser solvent.
3.2 Analytical figures of merit: LIBS and DLLME–LIBS
Analytical figures of merit of the DLLME–LIBS methodology (i.e. sensitivity, limit of detection (LOD), limit of quantification (LOQ) and repeatability) were evaluated to assess the analytical capability of this procedure with respect to determination of Cr in water samples. In addition, analytical figures of merit characterizing the direct LIBS analysis of the aqueous samples for Cr determination (i.e. LIBS analysis of the samples without applying the DLLME procedure prior to LIBS detection) were also evaluated, with the aim of assessing the advantages provided by the use of the proposed preconcentration step for quantification of Cr by the LIBS technique.Calibration curves were obtained, in triplicate, with both LIBS and DLLME–LIBS methodologies. In the DLLME–LIBS methodology, Cr(VI) was extracted from five aqueous calibration standard solutions with concentrations increasing up to 300 μg kg−1. Afterward, the analyte-enriched solvents resulting from the extractions were analysed by LIBS as indicated in Section 2.1, that is, 10 μL of solvent were placed on an aluminum substrate, heated to dryness and analysed by LIBS. In the LIBS methodology, calibration was performed by analyzing five Cr(VI) aqueous calibration standards with concentrations increasing up to 1000 μg kg−1. In this case, however, 10 μL of each aqueous standard were directly heated to dryness on the aluminium substrate and were analysed by LIBS (i.e. without any previous microextraction step). In all cases, LIBS analysis was carried out by averaging the LIBS signal obtained from four single laser shots in different positions on the same dry residue.
Table 2 shows the analytical figures of merit of both LIBS and DLLME–LIBS procedures. Sensitivity was derived from the slope of the calibration graphs. The LOD calculation was based on three times the standard deviation of ten blank determinations (deionized water for LIBS and 1-undecanol for DLLME–LIBS), whereas the LOQ was based on ten times the standard deviation of ten blank determinations. Repeatability (RSD, relative standard deviation) was estimated from 10 independent measurements (i.e. ten independent extractions of a 200 μg kg−1 Cr(VI) standard solution followed by LIBS detection of the resulting analyte-enriched solvents) by DLLME–LIBS and 10 independent measurements of a 800 μg kg−1 Cr(VI) standard solution in deionized water by LIBS.
| Parameters | Cr I (357.869 nm) | |
|---|---|---|
| LIBS | DLLME–LIBS | |
| a Number of calibration points, n = 5. b Value ± standard deviation. c Relative standard deviation, n = 10, [Cr(VI)] 800 μg kg−1 (LIBS) and 200 μg kg−1 (DLLME–LIBS). d Sensitivity of DLLME–LIBS/sensitivity of LIBS. | ||
| Linear range/(μg kg−1) | 0.0–1000 | 0.0–300 |
| R 2 | 0.9741 | 0.9859 |
| Sensitivity/(cts kg μg−1)a,b | 6.4 ± 3.3 | 204 ± 74 |
| LOD/(μg kg−1) | 68 | 3.1 |
| LOQ/(μg kg−1) | 227 | 10 |
| Repeatability (RSD%)c | 17 | 18 |
| Relative sensitivityd | 32 | |
As can be observed, the use of the DLLME procedure prior to LIBS analysis (DLLME–LIBS method) results in a 32-fold increase in sensitivity compared to the direct LIBS analysis of the solutions (LIBS method). Such a high increase in sensitivity leads to about a 22-fold decrease in the detection and quantification limits compared to the LIBS method, the LOD and LOQ being 3.1 μg kg−1 and 10 μg kg−1, respectively, obtained with DLLME–LIBS methodology.
3.3 Analysis of a certified reference material
The method accuracy was evaluated from the analysis of a certified reference material of hard drinking water CRM (ERM® CA011a) containing Ca, Mg, K and Na as majority elements, with concentrations ranging from about 5 to 90 mg kg−1, depending on the element, and many other minority concomitant metals (Zn, Ni, Mn, Pb, Fe, Cu, Cd, and Al) in concentrations ranging from about 5 to 2000 μg kg−1. From this analysis, the Cr in the sample was found to be 44 ± 7 μg kg−1 in the form of Cr(III), which was in good agreement with the certified value (48 ± 3 μg kg−1). Percent recovery, calculated by comparison with the Cr(III) certified value, was 92 ± 14%. From this result, it can be argued that, at least for samples with matrix compositions similar to that of the analyzed CRM, no matrix effects resulting from the presence of concomitant metals seem to influence quantification of Cr.The low precision obtained in the analysis, as already pointed out elsewhere,19 can be mainly attributed to the low repeatability of the LIBS measurement. Samples are heated to dryness on an aluminum substrate prior to LIBS analysis, which results in an inhomogeneous distribution of the analyte on the dry residue being interrogated by the laser, and thus a low repeatability even for replicate LIBS measurements performed at different positions on the same residue. This inconvenience can be solved using a different strategy for the predictable and homogeneous deposition of the liquid sample on the aluminum substrate, which is currently being performed in our laboratory with very promising results.
Determination of the Cr in different samples after extraction procedures, such as cloud point extraction (CPE), DLLME, ultrasound-assisted DLLME, ionic liquid DLLME, salt-assisted liquid–liquid microextraction with ionic liquid (SALLME-IL), solidified floating organic drop microextraction (SFODME) or hollow fiber liquid phase microextraction (HF-LPME), combined with spectrometric techniques has also been carried out by other authors.2,15,23–27 As shown in Table 3, a procedure combining LIBS with DLLME leads to detection limits of the same order than the ones obtained using different instrumental techniques, except in the case of GFAAS detection, as expected. The DLLME procedure proposed in this study is simple, avoiding the solidification step to separate the analyte-enriched organic drop from the aqueous solution, the magnetic stirring for organic dispersion and the heating step commonly needed in CPE. In addition, the simple and compact LIBS instrumentation, when compared to the more complicated and voluminous FAAS, GFAAS or ICP-OES equipment, can be considered a very attractive feature characterizing the proposed DLLME–LIBS methodology, being a further step toward the development of analytical methodologies that can be performed in miniaturized and portable systems for in-field early-warning and monitoring.
| Sample treatment/spectrometric technique | Experimental conditions | LOD | Reference |
|---|---|---|---|
| a APDC: ammonium pyrrolidinedithiocarbamate; DPC 1,5-diphenylcarbazide; [C4mim][BF4] 1-butyl-3-methylimidazolium tetrafluoroborate; [C8MIm][NTf2] 1-octyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide; TTA 2-thenoyltrifluoroacetone; PMBP 1-phenyl-3-methyl-4-benzoylpyrazol-5-one. | |||
| Dispersive liquid–liquid microextraction (DLLME)/FAAS | Chelating reagent: APDC | 0.07 μg L−1 (Cr(VI)) | 2 |
| Extractant solvent: carbon tetrachloride, 60 μL | 0.08 μg L−1 (Crtotal) | ||
| Disperser solvent: ethanol, 2.00 mL | |||
| Sample volume: 25 mL | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Salt-assisted liquid–liquid microextraction with ionic liquid (SALLME-IL)/FAAS | Chelating reagent: DPC | 1.25 μg L−1 | 15 |
| Extractant solvent: [C4mim][BF4], 150 μL | |||
| Oxidant reagent: KMnO4 | |||
| Sample volume: 10 mL | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Ionic liquid dispersive liquid–liquid microextraction (IL-DLLME)/GFAAS | Chelating reagent: APDC | 2 ng L−1 | 23 |
| Extractant solvent: [C8MIm][NTf2], 33 μL | |||
| Sample volume: 10 mL | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Solidified floating organic drop microextraction (SFODME)/GFAAS | Chelating reagent: TTA | 0.006 μg L−1 | 25 |
| Extractant solvent: 1-undecanol, 30 μL | |||
| Reducing reagent: hydroxylamine hydrochloride | |||
| Sample volume: 10 mL | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Cloud point extraction (CPE)/ICP-OES | Chelating reagent: PMBP | 0.81 μg L−1 | 26 |
| Surfactant: triton X-100, 1.0 mL | |||
| Reducing reagent: ascorbic acid | |||
| Sample volume: 10 mL | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Dispersive liquid–liquid microextraction (DLLME)/LIBS | Chelating reagent: DDTC | 3.1 μg kg−1 | This work |
| Extractant solvent: 1-undecanol, 50 μL | |||
| Disperser solvent: ethanol, 156 μL | |||
| Oxidant reagent: KMnO4 | |||
| Sample volume: 9 mL | |||
A number of different liquid phase microextraction (LPME) procedures have already been used by our group to increase the LIBS sensitivity for liquid sample analysis.16–20 A single drop microextraction procedure (SDME), using APDC (pyrrolidinedithiocarbamate) as a complexing agent and toluene as an extractant solvent, was combined with LIBS detection for the quantification of Cr, Cu, Mn, Ni and Zn in water samples.19 The SDME-LIBS method resulted in a 2.5-fold increase in sensitivity and a 2.9-fold decrease in the LOD when compared to the direct LIBS analysis of the samples for Cr determination (LIBS method). The same metals were analyzed using the combination of DLLME with LIBS (DLLME–LIBS).18 In this study, using APDC and tetrachloromethane as complexing agent and extractant solvent, respectively, the sensitivity and the LOD for Cr determination were further improved (i.e. 4.8 and 4.5 times, respectively, as obtained with the LIBS method).
The DLLME–LIBS method proposed in this study is a significant improvement on previous methods with respect to Cr quantification,18,19 showing that adequate selection of the microextraction procedure to be used prior to LIBS detection, along with working conditions (e.g. complexing agent and extractant solvent) are critical in improving the analytical performance of this spectrometric technique for liquid sample analysis. In this study, the use of DDTC as a complexing agent was based on the previous study proposed by Yanagisawa et al.,28 which demonstrated the possibility of Cr speciation by selective extraction of Cr(VI) in an acidic medium. However, DPC (1,5-diphenylcarbazide) is the most frequently used complexing agent for Cr(VI) determination and is also recommended by the US Environmental Protection Agency (EPA methods 7196a and 218.7) and the International Organization for Standardization (ISO 11083). As an example, Balasubramanian and Pugalenthi29 proposed the determination of total Cr by ICP-OES, FAAS and UV-visible spectrophotometry methods. With the use of DPC as a colorimetric agent for the spectrophotometric method, the authors obtained a detection limit of 5 μg L−1. Because studies on different and more efficient microextraction conditions are currently in process by our research group, to further improve the analytical capabilities of LPME–LIBS methodologies, a comparison between DPC and substituted dithiocarbamates as complexing agents for Cr speciation by DLLME–LIBS procedures seems to be an unavoidable next step.
4 Conclusions
For certain type of samples, such as those of environmental interest, determination of the total content of some metals is frequently insufficient to correlate metal concentration to possible toxicological effects or environmental risks, especially for those metals displaying different behavior depending on their oxidation state such as Cr(III) and Cr(VI) ions. The combination of microextraction procedures, which not only makes possible speciation but also analyte preconcentration, with spectrometric detection techniques, has therefore been of particular interest over the last few years for metal speciation purposes.The study presented here is a breakthrough in hyphenating a microextraction procedure based on DLLME modality with LIBS detection for speciation and determination of Cr at low concentrations in liquid samples. The DLLME–LIBS methodology developed in this study allows not only speciation of Cr but also quantification of this element at a concentration level as low as 10 μg kg−1. This LOD value is well below the level of concentration established by the Environmental Protection Agency (EPA), 100 μg L−1,30 or the European Drinking Water Directive of The European Union Council (EUC), 50 μg L−1,31 for Cr in drinking water.
Compared to direct LIBS analysis of the samples (i.e. LIBS method), the addition of the proposed DLLME step for analyte enrichment prior to spectroscopic measurement improves sensitivity by a factor of 32 and decreases the LOD and LOQ by a factor of 22. These results represent great progress over studies previously performed with the aim of improving the analytical capability of LIBS by hyphenation with microextraction procedures.18,19 However, as in previous study, the low repeatability of the LIBS measurement step is still the limiting factor with respect to improving the method accuracy. Studies on new ways of preparing the micro-samples for reproducible LIBS measurements are currently being performed by our research group, to overcome this inconvenience. Therefore, further improvements can be expected by continuing this research line, focusing on the hyphenation of LPME methodologies with LIBS detection (LPME–LIB).
Acknowledgements
National Council for Scientific and Technological Development (Brazil) (CNPq - Science without Borders project no. 245782/2012-5) and São Paulo Foundation Research (FAPESP project no. 2011/19730-3) are acknowledged. This study has been supported by the Government of Spain (CTQ2011-23968) and the Regional Government of Valencia (Spain) (ACOMP/2013/072).References
- N. Unceta, F. Séby, J. Malherbe and O. F. X. Donard, Anal. Bioanal. Chem., 2010, 397, 1097 CrossRef CAS PubMed.
- P. Hemmatkhah, A. Bidari, S. Jafarvand, M. R. M. Hosseini and Y. Assadi, Microchim. Acta, 2009, 166, 69 CrossRef CAS.
- L. Hua, Y. C. Chan, Y. P. Wu and B. Y. Wu, J. Hazard. Mater., 2009, 163, 1360 CrossRef CAS PubMed.
- J. Kotas and Z. Stasicka, Environ. Pollut., 2000, 107, 263 CrossRef CAS PubMed.
- D. Dinda and S. K. Saha, J. Hazard. Mater., 2015, 291, 93 CrossRef CAS PubMed.
- D. A. Eastmond, J. T. MacGregor and R. S. Slesinski, Crit. Rev. Toxicol., 2008, 38, 173 CrossRef CAS PubMed.
- U.S. Environmental Protection Agency, Toxicological review of hexavalent chromium, Washington, DC, 1998, Last access in 07/03/2015, http://www.epa.gov/iris/toxreviews/0144tr.pdf Search PubMed.
- U.S. Department of Health and Human Services, Public Health Service Agency for Toxic Substances and Disease Registry, Toxicological profiles for chromium, Last access in 07/03/2015, http://www.atsdr.cdc.gov/toxprofiles/tp7.pdf.
- P. M. Outridge and A. M. Scheuhammer, Rev. Environ. Contam. Toxicol., 1993, 130, 31 Search PubMed.
- B. Markiewicz, I. Komorowicz, A. Sajnóg, M. Belter and D. Barałkiewicz, Talanta, 2015, 132, 814 CrossRef CAS PubMed.
- V. Gómez and M. P. Callao, TrAC, Trends Anal. Chem., 2006, 25, 1006 CrossRef.
- A. L. Rosen and G. M. Hieftje, Spectrochim. Acta, Part B, 2004, 59, 135 CrossRef.
- C. R. Dockery, J. E. Pender and S. R. Goode, Appl. Spectrosc., 2005, 59, 252 CrossRef CAS PubMed.
- V. Andruch, I. S. Balogh, L. Kocúrová and J. Sandrejová, J. Anal. At. Spectrom., 2013, 28, 19 RSC.
- B. Majidi and F. Shemirani, Microchim. Acta, 2012, 176, 143 CrossRef CAS.
- M. A. Aguirre, S. Legnaioli, F. Almodóvar, M. Hidalgo, V. Palleschi and A. Canals, Spectrochim. Acta, Part B, 2013, 79, 88 CrossRef.
- A. M. Jesus, M. A. A. Pastor, M. Hidalgo, A. Canals and E. R. Pereira-Filho, J. Anal. At. Spectrom., 2014, 29, 1813 RSC.
- M. A. Aguirre, E. J. Selva, M. Hidalgo and A. Canals, Talanta, 2015, 131, 348 CrossRef CAS PubMed.
- M. A. Aguirre, H. Nikolova, M. Hidalgo and A. Canals, Anal. Methods, 2015, 7, 877 RSC.
- I. Gaubeur, M. A. Aguirre, N. Kovachev, M. Hidalgo and A. Canals, Microchem. J., 2015, 121, 219 CrossRef CAS.
- R. Galbeiro, S. Garcia and I. Gaubeur, J. Trace Elem. Med. Biol., 2014, 28, 160 Search PubMed.
- S. Garcia, F. Gerondi, T. R. L. C. Paixão, M. A. Z. Arruda and I. Gaubeur, J. Braz. Chem. Soc., 2015, 26, 490 CAS.
- I. López-García, Y. Vicente-Martínez and M. Hernández-Córdoba, J. Anal. At. Spectrom., 2012, 27, 874 RSC.
- I. López-García, M. Briceño, Y. Vicente-Martínez and M. Hernández-Córdoba, Talanta, 2013, 115, 166 CrossRef PubMed.
- M. R. Moghadam, S. Dadfarnia and A. M. H. Shabani, J. Hazard. Mater., 2011, 186, 169 CrossRef CAS PubMed.
- P. Liang and J. Li, At. Spectrosc., 2005, 26, 89 CAS.
- C. Zeng, Y. Lin, N. Zhou, J. Zheng and W. Zhang, J. Hazard. Mater., 2012, 237, 365 CrossRef PubMed.
- M. Yanagisawa, M. Suzuki and T. Takeuchi, Microchim. Acta, 1973, 61, 475 CrossRef.
- S. Balasubramanian and V. Pugalenthi, Talanta, 1999, 50, 457 CrossRef CAS PubMed.
- United States Environmental Protection Agency, Water: Chromium in drinking water, Last access in 7/18/2015, http://water.epa.gov/drink/info/chromium/index.cfm.
- Council Directive 98/83/EC. of 3 November 1998 on the quality of water intended for human consumption, Off. J. Eur. Communities L 330 (05/12/1998) 0032–0054 Last access in 1/06/2015, http://ec.europa.eu/environment/water/water-drink/legislation_en.html.
| This journal is © The Royal Society of Chemistry 2015 |