Open Access Article
Open Access ArticleAn in situ technique for (U–Th–Sm)/He and U–Pb double dating
N. J.
Evans
*a,
B. I. A.
McInnes
a,
B.
McDonald
a,
M.
Danišík
a,
T.
Becker
b,
P.
Vermeesch
c,
M.
Shelley
d,
E.
Marillo-Sialer
e and
D. B.
Patterson
f
aAuscope GeoHistory Facility, John de Laeter Centre, The Institute for Geoscience Research (TIGeR), Applied Geology/Applied Physics, Curtin University, Perth, WA, Australia. E-mail: noreen.evans@curtin.edu.au
bNanochemistry Research Institute, Department of Chemistry, Curtin University, Perth, WA, Australia
cDepartment of Earth Sciences, University College London, UK
dLaurin Technic Pty Ltd., Canberra, ACT, Australia
eSchool of Earth Sciences, The University of Melbourne, Parkville, Victoria, Australia
fPatterson Instruments Ltd., Austin, TX, USA
First published on 1st June 2015
Abstract
We report on a new laser-based technique for rapid, quantitative and automated in situ double dating (U–Pb and (U–Th–Sm)/He) of minerals, for applications in geochronology, thermochronology and geochemistry. In situ laser microanalysis offers several advantages over conventional bulk crystal methods in terms of spatial resolution, productivity, and safety. This new approach/methodology utilizes an interoperable and integrated suite of analytical instruments including a 193 nm ArF excimer laser system, quadrupole ICP-MS, quadrupole helium mass spectrometry system and swappable flow-through and ultra-high vacuum analytical chambers. We describe the analytical protocols for zircon analysis including grain mounting in Teflon, parameters for parent and daughter isotopic measurement, and standard development, and provide a freeware application for determining (U–Th–Sm)/He ‘pairwise’ ages from analytical data. The in situ double dating method described is applied to the Ellendale lamproite pipe and country rocks, Western Australia and successfully replicates conventional U–Pb and (U–Th–Sm)/He age variations determined previously by conventional techniques.
Introduction
(U–Th–Sm)/He is a popular technique for studying a variety of geological processes.1 The method involves the measurement of 4He, the daughter product of U, Th and Sm radioactive decay. Helium is quantitatively retained by minerals at low temperature, but is gradually lost from the mineral lattice by diffusion at elevated temperatures. Some minerals are more retentive of He than others (e.g., closure temperatures for zircon ≈180 °C (ref. 2 and 3) versus apatite ≈70 °C (ref. 4 and 5)). When integrated with other techniques such as U–Pb dating on the same mineral, complete rock time-temperature histories from 900 °C to 40 °C can be resolved.Conventional (U–Th–Sm)/He dating involves microscopy observation of minerals of interest to characterize individual grains (clarity, morphology, presence/absence of inclusions/cracks/deformation, measurements of grain physical dimensions for alpha correction6–8), loading selected grains into Pt or Nb microvials, extraction and quantification of total 4He content, grain dissolution for parent isotope measurement and, finally, age calculation.5 As comprehensively described by Tripathy-Lang, et al.,9 the limitations of conventional whole grain (U–Th–Sm)/He dating methods include the inability to avoid inclusions, crystallographic defects or ‘bad neighbors’ that can introduce excess 4He,10–12 the uncertainties related to the alpha ejection correction (e.g., parent isotope zonation, potential inaccuracies of grain measurement, natural abrasion and breakage of grains6,8,13–16), the length of time it takes to do a single analysis, and the safety issues associated with the use of aggressive acids such as hydrofluoric, nitric or perchloric for grain dissolution.17 These issues, combined with advances in laser ablation techniques have prompted a foray into in situ (U–Th–Sm)/He dating with encouraging results.9,18–21
The earlier studies at Arizona State University applied ultraviolet laser microprobe analysis to monazite18,19 and later, a combination of secondary ionization mass spectrometry (SIMS) for parent isotope measurement and microprobe for He determination on other U-bearing phases.9,20 Recent work21 has introduced the notion of a ‘pairwise’ approach where standards of known age are compared to samples of interest using laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) for U, Th and He abundance. In situ (U–Th)/He dating permits us to target a selected area within a grain, thereby providing better spatial resolution than conventional methods (allowing us to avoid inclusions, crystallographic defects or zonation) and eliminating of the need for an alpha correction.18,19 The increased productivity (less time required to generate an age relative to conventional methods), ability to circumvent the multiple-handling steps previously required for double dating (SHRIMP/LA-ICP-MS U–Pb and conventional (U–Th–Sm)/He),22,23 and improved worker safety (does not require grain dissolution, thereby avoiding the use of acids) are added advantages.
As noted, previous approaches to in situ (U–Th–Sm)/He dating have utilized laser microprobe + SIMS9,18–20,24 and laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS)21 for parent and daughter measurements. However, none of these approaches have integrated in situ (U–Th–Sm)/He and U–Pb dating on single crystals to obtain double dates. U–Pb and (U–Th–Sm)/He double dating has proven useful for sediment provenance and recycling studies22,25 and in exploration applications (e.g., diamond exploration) where thermal processes are indicative of prospectivity.23,26 These types of studies require large datasets (preferably >100 ages) for proper statistical evaluation.27 In response to this need, we have developed the protocols for a novel analytical approach, suitable to rapid, automated in situ (U–Th–Sm)/He and U–Pb (+trace elements) single crystal double dating of zircon and other U-rich accessory phases. This innovation integrates several analytical instruments including (1) a 193 nm excimer laser equipped with an ultra-high vacuum (UHV) cell; (2) an ultra-high vacuum system with a 4He mass spectrometer, and (3) an ICP-MS. Items (1) and (2) are commercially known as the RESOchron instrument.
Methods
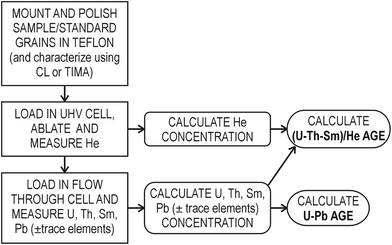
Our workflow for in situ dating is similar to that described previously9,18,19,21 but with some differences in approach and instrumentation. The steps (Fig. 1) involve mounting, polishing and characterizing crystals, He extraction and measurement, pit volume determination, parent isotope analysis and age calculation, as outlined below.1. Sample preparation – mounting and polishing
Zircon grains were mounted into Teflon using methods similar to those adopted for fission track etching.28 The zircon grains were mounted with the c-axis parallel to the mount surface in order to maximize the surface area available for laser ablation. Zircon grains of known and unknown age (‘standards’ and ‘unknowns’, respectively) were arranged in a grid on Kapton polyimide film on a clean glass thin section slide and firmly pressed into a 25 mm round piece of Teflon (DuPont PFA, Type 6000LP) preheated on a hot plate at 230 °C until softened. We found that this type of Teflon does not excessively degas and allows us to reach the desired ultra-high vacuum pressure (i.e. <10−9 mbar) after 8 hours of pumping (usually overnight). When cold, the Teflon disc with embedded zircon grains was removed from between the glass slides and the Kapton was removed. Grains were ground to 4π geometry and polished using 1000 and 2000 grit SiC papers, and 12 μm, 6 μm, 3 μm and 1 μm diamond suspension, sequentially, in an effort to expose the interior surface of the grains and to achieve as flat a surface as possible. Similar to the methods employed by Tripathy-Lang, et al.,9 we examined polished zircon using a range of microscopy methods including reflected and transmitted light, scanning electron microscopy (backscattered electron (SEM-BSE), energy dispersive X-ray spectroscopy (SEM-EDS) and cathodoluminescence (CL)) imaging techniques in order to characterize the distribution of parent nuclides, reveal internal structures that could impact pit volume measurement (discussed below) and identify suitable areas for laser ablation.2. Helium extraction and analysis
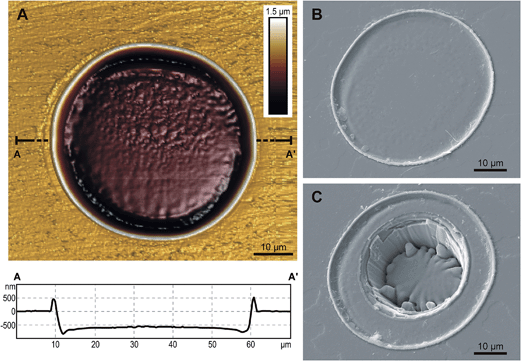
Helium extraction using laser microprobes or short-wavelength pulsed lasers has been shown to generate little or no heating of the surrounding material.18–21,24 There are several potential approaches to in situ helium extraction. The approach developed at Arizona State University9,18,19 employs a small pit (10–20 μm wide and 5–10 μm deep) for helium extraction and a larger pit overtop for U and Th (see Fig. 1b in Tripathy-Lang et al.9). Our experimental approach employed wide, shallow 4He pits followed by a narrower, deeper ablation for U, Th, Sm, Pb (+trace elements); Fig. 2, see details below. We found that this approach improves the accuracy of helium pit volume measurements, a key factor in accurate age determination.18For helium extraction, Teflon containing polished zircon was loaded into the UHV chamber connected to a RESOchron 4He analysis system, and pumped to <10−9 mbar. The standard operating procedure used in this study was to extract 4He from zircon using a 50 μm diameter laser spot (RESOlution M-50A-LR incorporating a Compex 102 excimer laser) directed at the polished zircon surface and ablated for 2 s at 5 Hz and 2–3 J cm−2 fluence. The beam diameter can be altered depending on grain size, expected age and 4He content. The laser settings above typically result in a <2 μm deep ablation pit with a flat bottom. Laser spots were placed >20 μm from the edge of the grain in order to avoid issues related to alpha ejection. Gas from the ablated sample was purified using hot and cold Ti–Zr getters, spiked with 3He, and expanded to the quadrupole mass spectrometer (Pfeiffer PrismaPlus™) where it was analyzed for 65 s. 3He/4He ratios, corrected for HD and 3H by monitoring mass 1, were measured using a Channeltron detector operated in static mode. 4He was determined by isotope-dilution using 3He spike and 4He gas standards of known volume, analyzed throughout the run.
Ablation of zircon was always bracketed by a line blank (4He measured over the same expanded volume as a sample or gas standard, but without any ablation). A typical analysis sequence included a gas standard followed by at least three line blanks (to assure complete removal of gas standard memory effects), a zircon standard, a line blank, an unknown zircon, a line blank, a zircon standard, a line blank, an unknown zircon, a line blank, etc. with a gas standard run every 5 zircon ablations. The line blank used for 4He blank correction was typically in the range of 0.0012 ncc and the typical variation of the line blank throughout an analytical session was 1.2%. The magnitude of 4He in the line blank was two orders of magnitude lower than 4He in a typical sample analysis. The limit of detection was calculated as the 4He concentration in the line blank plus three times the standard deviation on ten sequentially measured line blanks, yielding a value of 0.002 ncc. Reproducibility of 4He gas standards during this study was better than 0.04% and 0.1% (both 1σ) on a daily and long-term basis, respectively. Uncertainty on individual 4He measurements (typically 0.2–2.5%) was calculated as the square root of the sum of the squares of the following: (i) precision of the 4He of the gas standard; (ii) precision of the 4He/3He of the sample after blank subtraction, and (iii) precision of the gas standard volume calibration, which in our case is 0.1%.
3. Pit volume measurement
The volume of the pit from which 4He was extracted is required in order to calculate the concentration of 4He.18 Choice/selection of the method for helium pit volume measurement will depend on the instrumentation available and the depth of the ablated pit. In our work, CLM is used to determine 4He pit volume for pits deeper than ∼3 μm and AFM is used on shallower 4He pits. All ages presented in this paper were generated using AFM pit volumes.4. U, Th, Sm and Pb measurement
After pit volume measurements, Teflon mounts were transferred to the Laurin Technics M50A flow-through cell for a second ablation to determine U, Th, Pb and Sm contents (in addition to a range of trace elements, if desired) using an Agilent 7700s ICP-MS. The CompexPro 102 has a pulse width of 25 ns and the effective cell volume of the Laurin Technic M50A cell is 1 cm3. The laser ablation spot was placed inside the previously ablated 4He pit as shown in Fig. 2 and samples and standards were treated identically. Isotopes were measured in time-resolved mode and the following elements were monitored for 0.07 s each: 49Ti, 91Zr, 147Sm, 202Hg, 204Pb, 206Pb, 207Pb, 208Pb, with 28Si, 29Si, 232Th, and 238U monitored for 0.03 s each. Following a 10 s period of background analysis, samples were spot ablated for 30 s at a 7 Hz repetition rate using a 33 μm diameter beam and laser energy of 2.5 J cm−2. The sample cell was flushed by ultrahigh purity He (0.68 L min−1) and N2 (2.8 mL min−1) and high purity Ar was employed as the plasma carrier gas (flow rate 0.98 L min−1). International glass standard NIST 610 was used as the primary standard to calculate elemental concentrations (using 29Si as the internal standard element) and to correct for instrument drift. Mass spectrometer tuning was also performed on NIST 610 with a constant U–Th ratio of 1 obtained in each run. For U–Pb age determination, the primary age standard was 91500 (1062.4 ± 0.4 Ma (ref. 29)) with Plešovice (337.13 ± 0.37 Ma (ref. 30)), GJ-1 (601 ± 1.3 Ma (ref. 31)), and M257 (561.3 ± 0.3 Ma (ref. 32)) used as secondary age standards. The mass spectra were reduced using the trace element and U_Pb_Geochronology3 data reduction schemes in iolite.33 Precision was better than 5% for most elements based on repeated analyses of secondary internal standards. 206Pb–238U ages calculated for all zircon age standards, treated as unknowns, were within 3% of the accepted value.5. Age calculation
Currently there are two approaches used to calculate (U–Th–Sm)/He ages using in situ methods. In the first (absolute) approach, the age is calculated from the measured concentrations of U, Th, (Sm) and He using the following equation:18 | (1) |
(1) Whereas the original method combined the samples and standards on a one-by-one basis, we combined several standard measurements together in a single block. This was possible because the RESOchron is equipped with a 3He spike tank, making it immune to the sensitivity drift that was a concern in the magnetic sector mass spectrometer used by Vermeesch.21
(2) Whereas the calculations in the original method were performed on the raw data files, the modified method uses the processed elemental concentrations as input. This better fits the natural workflow of the method, that aims to determine trace elemental compositions as well as (U–Th–Sm)/He and U–Pb ages, however, a glass standard is required, introducing potential sources of uncertainty.34
The measurements of U, Th, Sm and the ablation pit volume have associated systematic errors. These can be grouped into a single calibration factor, κ:
 | (2) |
Although the maximum likelihood calculations used to determine κ are relatively straightforward to carry out, the details of taking the partial derivatives are rather tedious. We have implemented the method in a user-friendly browser-based calculator to facilitate the application of the κ-calibration method. The spreadsheet-like app is written in HTML and JavaScript and can either be downloaded and run offline or used as an online web service. The calculator is freely available at http://resochronometer.london-geochron.com and an example of input required can be found in Table 3.
Results and discussion
1. Empirical observations
Considering that one of the advantages of in situ (U–Th–Sm)/He dating methods is the ability to analyze zircon grains or portions of grains that have crystal defects, such as fractures,9 the structures mentioned above, if undetected, have the potential to decrease the accuracy of age determinations. However, once identified, the number of rejected analyses will increase, thus reducing, to a certain extent, the internal precision of the analytical session and, for detrital studies, potentially biasing the results of age interpretations. Detailed examination of target grains using a range of microscopy methods and applying reasonable but higher uncertainties to pit volumes where features were noted, will minimize the effect of these uncertainties.
| U (ppm) | 2σ | Th (ppm) | 2σ | Sm (ppm) | 2σ | |
|---|---|---|---|---|---|---|
| a All analyses were performed using a 33 μm beam, 30 second ablation, 7 Hz, 2–3 J cm−2 fluence (measured at sample surface) with NIST610 as the primary reference material and 28Si as the internal reference isotope. Nasdala43 obtained U and Th values of 288 ± 3 and 122 ± 1 for RB140 and 556 ± 24 and 59 ± 4 for B188, respectively. | ||||||
| B188 (RESOchron, 2nd ablation pit) (n = 30) | 529 | 18 | 58.6 | 2.2 | 1.4 | 0.2 |
| B188 (LA-ICP-MS) (n = 54) | 542 | 11 | 60.6 | 1.4 | — | — |
| RB140 (RESOchron, 2nd ablation pit) (n = 13) | 287 | 24 | 126 | 11 | 2.4 | 0.3 |
| RB140 (LA-ICP-MS) (n = 19) | 273 | 13 | 120 | 5.4 | — | — |
| Sample | (U–Th–Sm)/He traditionala (Ma) | 2σ (Ma) | (U–Th–Sm)/He RESOchronb (Ma) | 2σ (Ma) | 206Pb–238U LA-ICP-MSc (Ma) | 2σ (Ma) | 206Pb–238U RESOchron (Ma) | 2σ (Ma) |
|---|---|---|---|---|---|---|---|---|
| a Measured using methods in Evans.17,23 b Determined using pairwise dating methods with B188 used as the standard against RB140 and vice versa with 2σ uncertainty calculated as the standard deviation on raw ages. c Determined using conventional LA-ICP-MS methods described in the text on grains that had not been ablated first for 4He measurement. d Age from Nasdala et al., 2004 where B188 and RB140 SHRIMP 206Pb–238U ages were 559 ± 8 and 566 ± 3 Ma, respectively. | ||||||||
| B188 | 434.7 (n = 27) | 22 | 444 (n = 14) | 23 | 566 | 9 | 560 | 8 |
| RB140 | 437d | 20 | 422 (n = 13) | 16 | 563 | 11 | 557 | 8 |
| Sample | U (ppm) | 2σ (ppm) | Th (ppm) | 2σ (ppm) | Sm (ppm) | 2σ (ppm) | He (ncc) | 2σ (ncc) | Pit vol (μm3) | ± (μm3) | Known He age (Ma) | 2σ (Ma) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a All pit volumes measured by AFM. Uncertainty on AFM pit volume was 2.4% as described in the text. | ||||||||||||
| B188-1 | 525 | 35 | 59 | 4 | 1.5 | 0.4 | 0.217 | 0.0004 | 1455 | 34.9 | 435 | 22 |
| B188-2 | 544 | 26 | 60 | 3 | 1.4 | 0.3 | 0.202 | 0.0004 | 1216 | 29.2 | 435 | 22 |
| B188-3 | 541 | 22 | 60 | 3 | 1.7 | 0.3 | 0.195 | 0.0004 | 1212 | 29.1 | 435 | 22 |
| B188-4 | 578 | 28 | 64 | 4 | 1.5 | 0.4 | 0.204 | 0.0004 | 1266 | 30.4 | 435 | 22 |
| B188-5 | 591 | 34 | 65 | 3 | 1.8 | 0.4 | 0.221 | 0.0004 | 1340 | 32.2 | 435 | 22 |
| B188-6 | 503 | 22 | 55 | 3 | 1.2 | 0.3 | 0.198 | 0.0004 | 1231 | 29.5 | 435 | 22 |
| B188-7 | 532 | 32 | 60 | 5 | 1.6 | 0.4 | 0.214 | 0.0004 | 1393 | 33.4 | 435 | 22 |
| B188-8 | 700 | 120 | 78 | 14 | 1.9 | 0.6 | 0.177 | 0.0004 | 1095 | 26.3 | 435 | 22 |
| B188-9 | 523 | 11 | 59 | 2 | 1.4 | 0.3 | 0.219 | 0.0004 | 1474 | 35.4 | 435 | 22 |
| B188-10 | 524 | 9 | 59 | 1 | 1.2 | 0.2 | 0.239 | 0.0005 | 1613 | 38.7 | 435 | 22 |
| B188-11 | 508 | 11 | 57 | 1 | 1.3 | 0.3 | 0.240 | 0.0005 | 1639 | 39.3 | 435 | 22 |
| B188-12 | 510 | 10 | 58 | 1 | 1.3 | 0.2 | 0.213 | 0.0004 | 1443 | 34.6 | 435 | 22 |
| B188-13 | 500 | 9 | 58 | 1 | 1.4 | 0.3 | 0.217 | 0.0004 | 1509 | 36.2 | 435 | 22 |
| B188-14 | 511 | 9 | 58 | 1 | 1.3 | 0.3 | 0.245 | 0.0005 | 1689 | 40.5 | 435 | 22 |
| RB140-1 | 298 | 12 | 133 | 6 | 3.2 | 0.6 | 0.119 | 0.0002 | 1363 | 32.7 | 437 | 20 |
| RB140-2 | 303 | 15 | 136 | 6 | 2.5 | 0.5 | 0.102 | 0.0002 | 1135 | 27.2 | 437 | 20 |
| RB140-3 | 303 | 16 | 136 | 8 | 2.8 | 0.5 | 0.111 | 0.0002 | 1264 | 30.3 | 437 | 20 |
| RB140-4 | 277 | 12 | 124 | 6 | 2.4 | 0.4 | 0.131 | 0.0003 | 1503 | 36.1 | 437 | 20 |
| RB140-5 | 288 | 15 | 125 | 6 | 2.3 | 0.4 | 0.113 | 0.0002 | 1292 | 31 | 437 | 20 |
| RB140-6 | 298 | 17 | 132 | 7 | 2.8 | 0.4 | 0.114 | 0.0002 | 1285 | 30.8 | 437 | 20 |
| RB140-7 | 285 | 6 | 130 | 3 | 2.2 | 0.3 | 0.133 | 0.0003 | 1634 | 39.2 | 437 | 20 |
| RB140-8 | 273 | 6 | 126 | 3 | 2.4 | 0.3 | 0.137 | 0.0003 | 1660 | 39.8 | 437 | 20 |
| RB140-9 | 266 | 6 | 123 | 3 | 2.2 | 0.3 | 0.122 | 0.0002 | 1498 | 36 | 437 | 20 |
| RB140-10 | 262 | 6 | 121 | 3 | 2.1 | 0.4 | 0.125 | 0.0003 | 1434 | 34.4 | 437 | 20 |
| RB140-11 | 276 | 7 | 128 | 4 | 2.4 | 0.4 | 0.122 | 0.0002 | 1502 | 36 | 437 | 20 |
| RB140-12 | 271 | 5 | 125 | 2 | 2.2 | 0.4 | 0.133 | 0.0003 | 1602 | 38.4 | 437 | 20 |
| RB140-13 | 278 | 6 | 129 | 3 | 2.5 | 0.4 | 0.122 | 0.0002 | 1466 | 35.2 | 437 | 20 |
| Ellendale pipe 1 | 270 | 16 | 90 | 6 | 1.3 | 0.3 | 0.006 | 0.0001 | 1370 | 32.9 | ||
| Ellendale pipe 2 | 250 | 12 | 100 | 4 | 2.0 | 0.4 | 0.006 | 0.0002 | 1446 | 34.7 | ||
| Ellendale pipe 3 | 136 | 5 | 186 | 8 | 3.9 | 0.6 | 0.003 | 0.0001 | 1469 | 35.3 | ||
| Ellendale pipe 4 | 202 | 10 | 134 | 7 | 2.7 | 0.4 | 0.005 | 0.0001 | 1390 | 33.4 | ||
| Ellendale pipe 5 | 198 | 9 | 177 | 10 | 4.3 | 0.6 | 0.005 | 0.0001 | 1430 | 34.3 | ||
| Ellendale pipe 6 | 642 | 25 | 354 | 16 | 14.5 | 2.0 | 0.012 | 0.0003 | 1374 | 33 | ||
| Ellendale pipe 7 | 322 | 22 | 116 | 7 | 8.8 | 1.1 | 0.007 | 0.0002 | 1429 | 34.3 | ||
| Ellendale pipe 8 | 700 | 42 | 138 | 6 | 5.0 | 0.7 | 0.012 | 0.0003 | 1353 | 32.5 | ||
| Ellendale pipe 9 | 209 | 8 | 118 | 5 | 7.3 | 0.9 | 0.004 | 0.0001 | 1450 | 34.8 | ||
| Ellendale pipe 10 | 418 | 25 | 112 | 8 | 2.2 | 0.4 | 0.007 | 0.0002 | 1413 | 33.9 | ||
| Ellendale pipe 11 | 215 | 11 | 136 | 7 | 2.2 | 0.4 | 0.006 | 0.0002 | 1163 | 27.9 | ||
| Ellendale pipe 12 | 241 | 6 | 225 | 6 | 4.4 | 0.5 | 0.006 | 0.0002 | 1840 | 44.2 | ||
| Ellendale pipe 13 | 330 | 8 | 43 | 1 | 1.8 | 0.5 | 0.008 | 0.0002 | 1496 | 35.9 | ||
| Ellendale pipe 14 | 103 | 3 | 80 | 2 | 1.1 | 0.3 | 0.003 | 0.0001 | 1840 | 44.2 | ||
| Ellendale country rock-1 | 304 | 12 | 153 | 6 | 2.3 | 0.4 | 0.099 | 0.001 | 1225 | 29.4 | ||
| Ellendale country rock-2 | 71 | 5 | 92 | 7 | 2.3 | 0.4 | 0.030 | 0.0003 | 1204 | 28.9 | ||
| Ellendale country rock-3 | 69 | 6 | 34 | 2 | 1.8 | 0.4 | 0.034 | 0.0003 | 1306 | 31.3 | ||
| Ellendale country rock-4 | 144 | 7 | 109 | 5 | 1.3 | 0.3 | 0.059 | 0.0006 | 1216 | 29.2 | ||
| Ellendale country rock-5 | 76 | 4 | 218 | 12 | 18.7 | 1.4 | 0.073 | 0.0007 | 1249 | 30 | ||
| Ellendale country rock-6 | 499 | 18 | 138 | 5 | 0.9 | 0.2 | 0.198 | 0.002 | 1327 | 31.8 | ||
| Ellendale country rock-7 | 174 | 15 | 150 | 11 | 1.9 | 0.4 | 0.068 | 0.0007 | 1113 | 26.7 | ||
| Ellendale country rock-8 | 95 | 7 | 63 | 4 | 3.2 | 0.6 | 0.067 | 0.0007 | 1423 | 34.1 | ||
| Ellendale country rock-9 | 462 | 36 | 73 | 4 | 2.7 | 0.5 | 0.143 | 0.0014 | 1429 | 34.3 | ||
| Ellendale country rock-10 | 81 | 6 | 92 | 9 | 6.4 | 1.1 | 0.068 | 0.0007 | 1258 | 30.2 | ||
| Ellendale country rock-11 | 387 | 22 | 686 | 34 | 22.1 | 1.8 | 0.154 | 0.0015 | 1284 | 30.8 | ||
| Ellendale country rock-12 | 91 | 7 | 43 | 2 | 1.5 | 0.3 | 0.126 | 0.0013 | 1619 | 38.8 | ||
| Ellendale country rock-13 | 153 | 5 | 144 | 5 | 4.0 | 0.5 | 0.066 | 0.0013 | 1584 | 38 | ||
| Ellendale country rock-14 | 106 | 4 | 114 | 3 | 2.2 | 0.4 | 0.047 | 0.0009 | 1812 | 43.5 | ||
| Ellendale country rock-15 | 341 | 6 | 196 | 5 | 2.9 | 0.5 | 0.184 | 0.0037 | 1533 | 36.8 | ||
| Ellendale country rock-16 | 156 | 3 | 205 | 4 | 6.7 | 0.5 | 0.078 | 0.0016 | 1678 | 40.3 | ||
| Ellendale country rock-17 | 257 | 24 | 169 | 18 | 3.7 | 0.5 | 0.085 | 0.0017 | 1694 | 40.7 | ||
| Sample | 206Pb–238U (Ma) | 2σ (Ma) | ‘Pairwise’ (U–Th–Sm)/He (Ma) | 2σ (Ma) |
|---|---|---|---|---|
| a ‘Pairwise’ (U–Th–Sm)/He age calculated using B188 standard. b ‘Pairwise’ (U–Th–Sm)/He age calculated using RB140 standard. | ||||
| RB140-1a | 557 | 18 | 410 | 18 |
| RB140-2a | 555 | 18 | 414 | 21 |
| RB140-3a | 553 | 18 | 406 | 22 |
| RB140-4a | 563 | 19 | 441 | 20 |
| RB140-5a | 559 | 15 | 425 | 22 |
| RB140-6a | 566 | 15 | 417 | 24 |
| RB140-7a | 568 | 12 | 400 | 13 |
| RB140-8a | 558 | 13 | 421 | 13 |
| RB140-9a | 556 | 13 | 428 | 14 |
| RB140-10a | 536 | 14 | 463 | 16 |
| RB140-11a | 559 | 13 | 413 | 14 |
| RB140-12a | 560 | 15 | 429 | 14 |
| RB140-13a | 555 | 16 | 418 | 14 |
| B188-1b | 565 | 14 | 442 | 34 |
| B188-2b | 563 | 13 | 473 | 20 |
| B188-3b | 565 | 15 | 462 | 27 |
| B188-4b | 574 | 11 | 435 | 28 |
| B188-5b | 551 | 14 | 435 | 31 |
| B188-6b | 560 | 12 | 495 | 30 |
| B188-7b | 557 | 13 | 450 | 32 |
| B188-8b | 554 | 13 | 361 | 17 |
| B188-9b | 565 | 9 | 441 | 22 |
| B188-10b | 566 | 11 | 440 | 21 |
| B188-11b | 559 | 9 | 448 | 22 |
| B188-12b | 582 | 19 | 449 | 22 |
| B188-13b | 553 | 9 | 448 | 21 |
| B188-14b | 552 | 10 | 442 | 21 |
| Ellendale pipe 1a | 1270 | 26 | 22.1 | 1.4 |
| Ellendale pipe 2a | 598 | 18 | 25.5 | 1.5 |
| Ellendale pipe 3a | 599 | 26 | 18.8 | 1 |
| Ellendale pipe 4a | 607 | 17 | 26.2 | 1.4 |
| Ellendale pipe 5a | 1175 | 29 | 21.5 | 1.1 |
| Ellendale pipe 6a | 368 | 11 | 19.8 | 1 |
| Ellendale pipe 7a | 2757 | 48 | 21.2 | 1.6 |
| Ellendale pipe 8a | 2167 | 97 | 19.7 | 1.3 |
| Ellendale pipe 9a | 578 | 19 | 18.6 | 0.9 |
| Ellendale pipe 10a | 620 | 16 | 17.1 | 1.2 |
| Ellendale pipe 11a | 1196 | 28 | 34.6 | 2.1 |
| Ellendale pipe 12a | 895 | 17 | 18.3 | 0.9 |
| Ellendale pipe 13a | 1122 | 21 | 23.6 | 1.1 |
| Ellendale pipe 14a | 1147 | 27 | 18.6 | 1 |
| Ellendale country rock-1a | 1756 | 28 | 371 | 17 |
| Ellendale country rock-1a | 1613 | 46 | 418 | 25 |
| Ellendale country rock-3a | 1066 | 40 | 524 | 39 |
| Ellendale country rock-4a | 1133 | 25 | 444 | 21 |
| Ellendale country rock-5a | 1161 | 43 | 696 | 32 |
| Ellendale country rock-6a | 558 | 13 | 434 | 19 |
| Ellendale country rock-7a | 1169 | 27 | 449 | 34 |
| Ellendale country rock-8a | 875 | 33 | 650 | 42 |
| Ellendale country rock-9a | 1830 | 34 | 325 | 25 |
| Ellendale country rock-10a | 1208 | 40 | 793 | 49 |
| Ellendale country rock-11a | 1811 | 28 | 341 | 17 |
| Ellendale country rock-12a | 1727 | 54 | 1119 | 74 |
| Ellendale country rock-13a | 1528 | 28 | 350 | 15 |
| Ellendale country rock-14a | 1601 | 32 | 305 | 13 |
| Ellendale country rock-15a | 1601 | 23 | 478 | 17 |
| Ellendale country rock-16a | 1015 | 26 | 355 | 13 |
| Ellendale country rock-17a | 304.8 | 9.2 | 265 | 23 |
 | ||
| Fig. 4 In situ U–Pb and (U–Th–Sm)/He ages for B188 and RB140 (squares and circles, respectively). Area inside shaded box indicates range of ages obtained on separate aliquots of B188 and RB140 using conventional methods (LA ICP-MS for U–Pb and conventional, whole grain gas extraction and dissolution (U–Th–Sm)/He techniques; Table 2). Prior to application of pairwise age calculation, standard analyses falling outside the range obtained using traditional methods should be closely examined and discarded if debris or structures that cause pit volume inaccuracies are detected. | ||
2. Application: diamondiferous lamproite exploration
U–Pb and (U–Th–Sm)/He double dating has previously been applied to Australian diamond deposits23,26 after recognition of the fact that xenocrystic zircon from kimberlitic and lamproitic intrusions have a unique age profile which is determined by the thermal resetting of zircon helium ages during the emplacement of the lamproite pipe in the upper crust. Zircon from the E9 lamproite at the Ellendale diamond mine (Western Australia) has been well characterised by conventional SHRIMP U–Pb and whole grain (U–Th–Sm)/He methods and, along with the surrounding country rock zircon, provides a convenient natural laboratory to test the viability of the in situ double dating methods described here. The zircon (U–Th–Sm)/He age of 21 Ma for the emplacement of the diamondiferous Ellendale lamproite was corroborated by phlogopite 40Ar/39Ar techniques, and is distinct from the 300–1500 Ma helium ages determined from detrital zircon originating from the regional sandstone country rock (sample located 20 km from the E9 pipe). In contrast, the range of SHRIMP U–Pb ages for the lamproite and detrital zircons in the sandstone were statistically indistinguishable.23 Grains for pairwise in situ U–Pb and (U–Th–Sm)/He dating were selected from samples previously dated in the Evans, et al.23 study. Fig. 5 demonstrates that the in situ U–Pb and (U–Th–Sm)/He double dating technique effectively reproduced the age distribution patterns observed using conventional (U–Th–Sm)/He and SHRIMP U–Pb dating. Although none of the 2–3 Ga (U–Pb age) grains were identified in the country rock samples, this is most likely an artefact of the small number of grains analysed here for demonstration purposes. In the original study, 55 zircon grains were double dated using conventional methods, as opposed to just 17 using in situ techniques.The implications of Fig. 5 are that our in situ (U–Th–Sm)/He and U–Pb double dating technique is a viable tool for rapid, cost-effective analysis of a large number of grains, mitigating several of the primary obstacles to establishing double dating as a potentially viable diamond exploration tool. In addition, sediment provenance and landscape evolution studies9,22,25,44 that require large numbers of double dates for legitimate statistical treatment and identification of significant age populations will benefit from this powerful tool.
Conclusions
We have demonstrated a new and effective analytical system for in situ U–Pb and (U–Th–Sm)/He double dating of zircon. The key dependencies in this approach are the need for accurate measurements of the volume of the ablation pits from which radiogenic helium has been extracted and a standard reference zircon with homogeneous He concentration and a known (U–Th–Sm)/He age. The determination of ablation pit volume is simplified by generating shallow craters (<2 μm deep) with cylindrical geometry and, while the development of zircon reference standards is ongoing, Sri Lanka zircon B188 (435 ± 22 Ma) is our current choice as a primary standard for the pairwise dating technique. We have provided the community with a freeware application (http://resochronometer.london-geochron.com) for in situ (U–Th–Sm)/He age calculation.The successful application of in situ zircon (U–Th–Sm)/He and U–Pb to double dating at the Ellendale diamond mine demonstrates that geological this method is will provide a critical improvement over conventional methods of double dating when large numbers of analyses are required.
Acknowledgements
This project has been supported by the Australian Education Investment Fund and Curtin University via the AuScope Australian Geophysical Observation System project. Sri Lanka zircon was kindly provided by A. Kennedy (Curtin University). We are grateful for the support of B. Godfrey, E. Roberts, D. Gibbs, C. Gabay, A. Norris and M. Hamel throughout the development of the instrumentation and protocols. Solution U, Th and Sm analysis for conventional (U–Th–Sm)/He dating was performed at TSW Analytical, Perth. We are grateful to Elaine Miller (John de Laeter Centre, Microscopy and Microanalysis Facility, Curtin University) for help with SEM imaging. P. Vermeesch is financially supported by NERC grant #NE/K003232/1 and ERC grant #259505 (‘KArSD’). We thank R. Ickert, M. Tremblay and K. Hodges for comprehensive and constructive reviews of an earlier version of the manuscript and three anonymous reviewers for their insightful comments.References
- P. W. Reiners, T. A. Ehlers and P. K. Zeitler, in Thermochronology, ed. P. W. Reiners and T. A. Ehlers, Rev. Mineral. Geochem., 2005, vol. 58, ch. 1, pp. 1–18 Search PubMed.
- P. W. Reiners, T. L. Spell, S. Nicolescu and K. A. Zanetti, Geochim. Cosmochim. Acta, 2004, 68, 1857–1887 CrossRef CAS.
- K. V. Hodges, in Treatise on Geochemistry, ed. H. D. Holland and K. K. Turekian, Elsevier, Oxford, 2nd edn, 2014, vol. 4, ch. 8, pp. 281–308 Search PubMed.
- P. K. Zeitler, A. L. Herczeg, I. McDougall and M. Honda, Geochim. Cosmochim. Acta, 1987, 51, 2865–2868 CrossRef CAS.
- K. A. Farley, J. Geophys. Res., 2000, 105, 2903–2914 CrossRef CAS.
- K. A. Farley and B. I. A. McInnes, Rev. Mineral. Geochem., 2002, 47, 819–844 CrossRef CAS.
- K. A. Farley, R. A. Wolf and L. T. Silver, Geochim. Cosmochim. Acta, 1996, 60(21), 4223–4229 CrossRef CAS.
- T. A. Ehlers and K. A. Farley, Earth Planet. Sci. Lett., 2003, 206, 1–14 CrossRef CAS.
- A. Tripathy-Lang, K. V. Hodges, B. Monteleone and M. Van Soest, J. Geophys. Res., 2013, 118, 1333–1341 Search PubMed.
- C. Spiegel, B. Kohn, D. Belton, Z. Berner and A. Gleadow, Earth Planet. Sci. Lett., 2009, 285(1), 105–114 CrossRef CAS.
- M. Danišík, K. Pfaff, N. J. Evans, C. Manoloukos, S. Staude, B. McDonald and G. Markl, Chem. Geol., 2010, 278(1–2), 58–69 CrossRef.
- C. Gautheron, L. Tassan-Got, R. A. Ketcham and K. J. Dobson, Geochim. Cosmochim. Acta, 2012, 96, 44–56 CrossRef CAS.
- A. G. C. A. Meesters and T. J. Dunai, Chem. Geol., 2002, 186, 333–344 CrossRef CAS.
- A. G. C. A. Meesters and T. J. Dunai, Chem. Geol., 2002, 186, 347–363 CrossRef.
- J. K. Hourigan, P. W. Reiners and M. T. Brandon, Geochim. Cosmochim. Acta, 2005, 69, 3349–3365 CrossRef CAS.
- N. J. Evans, B. I. A. McInnes, A. P. Squelch, P. J. Austin, B. J. McDonald and Q. Qu, Chem. Geol., 2008, 257, 101–113 CrossRef CAS.
- N. J. Evans, J. P. Byrne, J. T. Keegan and L. E. Dotter, J. Anal. Chem., 2005, 60(12), 1159–1165 CrossRef CAS.
- J. W. Boyce, K. V. Hodges, W. J. Olszewski, M. J. Jercinovic, B. D. Carpenter and P. W. Reiners, Geochim. Cosmochim. Acta, 2006, 70, 3031–3039 CrossRef CAS.
- J. W. Boyce, K. V. Hodges, D. King, J. L. Crowley, M. Jercinovic, N. Chatterjee, S. A. Bowring and M. Searle, Geochem., Geophys., Geosyst., 2009, 10, Q0AA0, DOI:10.1029/2009GC002497.
- M. C. Van Soest, B. D. Monteleone, K. V. Hodges and J. W. Boyce, Geochim. Cosmochim. Acta, 2011, 75, 2409–2419 CrossRef CAS.
- P. Vermeesch, S. C. Sherlock, N. M. W. Roberts and A. Carter, Geochim. Cosmochim. Acta, 2012, 79, 140–147 CrossRef CAS.
- I. H. Campbell, P. W. Reiners, C. M. Allen, S. Nicolescu and R. Upadhyay, Earth Planet. Sci. Lett., 2005, 237, 402–432 CrossRef CAS.
- N. J. Evans, B. I. A. McInnes, B. J. McDonald, M. Danišík, F. Jourdan, C. Mayers, E. Thern and D. Corbett, Miner. Deposita, 2013, 48, 413–421 CrossRef CAS.
- M. C. Van Soest, B. D. Monteleone, J. W. Boyce and K. V. Hodges, AGU Fall Meeting Abstracts, San Francisco, 2008, p. B2161 Search PubMed.
- J. M. Rahl, P. W. Reiners, I. H. Campbell, S. Nicolescu and C. M. Allen, Geology, 2003, 31, 761–764 CrossRef CAS.
- B. McInnes, N. J. Evans, B. J. McDonald, P. Kinny and J. Jakimowicz, Lithos, 2009, 112S, 592–599 CrossRef CAS.
- P. Vermeesch, Earth Planet. Sci. Lett., 2004, 224, 441–451 CrossRef CAS.
- P. Zaun and G. A. Wagner, Nucl. Tracks Radiat. Meas., 1985, 10, 303–307 CAS.
- M. Wiedenbeck, P. Allé, F. Corfu, W. L. Griffin, M. Meier, F. Oberli, A. Von Quadt, J. C. Roddick and W. Spiegel, Geostand. Newsl., 1995, 19(1), 1–23 CrossRef CAS.
- J. Sláma, J. Košler, D. J. Condon, J. L. Crowley, A. Gerdes, J. M. Hanchar, M. S. A. Horstwood, G. A. Morris, L. Nasdala, N. Norberg, U. Schaltegger, B. Schoene, M. N. Tubrett and M. J. Whitehouse, Chem. Geol., 2008, 249, 1–35 CrossRef.
- A. R. C. Kylander-Clark, B. R. Hacker and J. M. Cottle, Chem. Geol., 2013, 345, 99–112 CrossRef CAS.
- L. Nasdala, W. Hofmeister, N. Norberg, J. M. Martinson, F. Corfu, W. Dörr, S. L. Kamo, A. K. Kennedy, A. Kronz, P. W. Reiners, D. Frei, J. Košler, Y. Wan, J. Götze, T. Häger, A. Kröner and J. W. Valley, Geostand. Geoanal. Res., 2008, 32(3), 247–265 CrossRef CAS.
- C. Paton, J. C. Hellstrom, B. Paul, J. D. Woodhead and J. M. Hergt, J. Anal. At. Spectrom., 2011, 26, 2508–2518 RSC.
- P. Sylvester, in Laser Ablation in the Earth Sciences: Current Practices and Outstanding issues, ed. P. Sylvester, Mineralogical Association of Canada Short Course, 2008, vol. 40, ch. 5, pp. 67–78 Search PubMed.
- L. P. Black and B. L. Gulson, AGSO J. Aust. Geol. Geophys., 1978, 3, 227–232 CAS.
- E. Marillo-Sialer, J. D. Woodhead, J. Hergt, A. Greig, M. Guillong, A. Gleadow, N. J. Evans and C. Paton, J. Anal. At. Spectrom., 2014, 29, 981–989 RSC.
- J. Woodhead, J. Hergt, S. Meffre, R. R. Large, L. Danyushevsky and S. Gilbert, Chem. Geol., 2009, 262, 344–354 CrossRef CAS.
- A. J. Pedraza, S. Jesse, Y. F. Guan and J. D. Fowlkes, J. Mater. Res., 2001, 16, 3599–3608 CrossRef CAS.
- K. Kolasinski, Curr. Opin. Solid State Mater. Sci., 2007, 11, 76–85 CrossRef CAS.
- F. Sanchez, J. L. Morenza, R. Aguilar, J. Delgado and M. Varela, Appl. Phys. Lett., 1996, 69, 620 CrossRef CAS.
- F. Sanchez, J. L. Morenza and V. Trtik, Appl. Phys. Lett., 1999, 75, 3303 CrossRef CAS.
- P. W. Reiners and S. Nicolescu, ARHDL Report 1, http://www.geo.arizona.edu/~reiners/arhdl/arhdl.htm, 2006.
- L. Nasdala, P. W. Reiners, J. I. Garver, A. K. Kennedy, R. A. Stern, E. Balan and R. Wirth, Am. Mineral., 2004, 89, 219–231 CrossRef CAS.
- P. W. Reiners, in Thermochronology, ed. P. W. Reiners and T. A. Ehlers, Rev. Mineral. Geochem., 2005, vol. 58, ch. 6, pp. 151–179 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |