Open Access Article
Open Access ArticleA non-chromatographic automated system for antimony speciation in natural water exploiting multisyringe flow injection analysis coupled with online hydride generation – atomic fluorescence spectrometry
Lindomar A.
Portugal
a,
Laura
Ferrer
a,
Antonio M.
Serra
a,
Douglas
Gonçalves da Silva
b,
Sérgio Luis C.
Ferreira
b and
Víctor
Cerdà
*a
aGroup of Analytical Chemistry, Automation and Environment, Department of Chemistry, University of Balearic Island, Cra. Valldemossa 7.5 km, 07122 Spain. E-mail: victor.cerda@uib.es
bInstituto de Química, Universidade Federal da Bahia, Brazil
First published on 10th February 2015
Abstract
A non-chromatographic automated system for the speciation and determination of inorganic and trimethylantimony (TMSb) exploiting multisyringe flow injection analysis (MSFIA) with hydride generation (HG) and atomic fluorescence spectrometry (AFS) is described. A cationic minicolumn was used for retaining the methylated forms of Sb which can generate hydrides, minimizing errors in the inorganic antimony speciation step. The optimization was performed in a multivariate way by employing a three-variable Box–Behnken design and a multiple response strategy. So, this method allows the quantification of Sb using the external calibration with aqueous standards. The method is suitable for monitoring drinking, surface and ground waters according to regulations established by the EU directives for antimony (5.0 μg L−1), and it was applied to the speciation of inorganic antimony and TMSb in several spiked water samples with recoveries close to 100%. The detection limits were 0.03 μg L−1 for Sb(III) and Sb(V) and 0.13 μg L−1 for TMSb. The method was satisfactorily applied to the determination of Sb(III), Sb(V) and TMSb in different water samples collected in Balearic Islands, Spain.
1. Introduction
Antimony is a ubiquitous pollutant distributed in low concentrations in natural water. For this reason it is important to develop sensitive methods for its determination. Antimony is present in the aquatic environment as a result of rock weathering, soil runoff and anthropogenic activities. Because of its chemical properties, antimony is widely used in industry. Among the various industrial applications of Sb compounds, antimony trioxide (Sb2O3) is profusely employed in the production of glassware and ceramics.1 Furthermore, Sb2O3 is added to molten glass as a clarifying agent and is used as a pigment in dyes and paints as well as in the textile industry. Several Sb compounds are used as additives in batteries, metal coatings and in rubber, and others are added to textiles as flame retardants. In 2010 the world mine production of Sb was estimated to be 165![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons.2 Also Sb is a common component of coal and petroleum. Thus Sb is released to the environment by industrial activities. Typical concentrations of dissolved antimony in unpolluted waters are less than 1 μg L−1 . However, in the proximity of anthropogenic sources they can reach up to 100 times the natural levels.3
000 tons.2 Also Sb is a common component of coal and petroleum. Thus Sb is released to the environment by industrial activities. Typical concentrations of dissolved antimony in unpolluted waters are less than 1 μg L−1 . However, in the proximity of anthropogenic sources they can reach up to 100 times the natural levels.3
Generally, the inorganic species of antimony are more toxic than the organic forms, and its compounds were considered as pollutants of priority interest by the Environmental Protection Agency of the United States (USEPA) and by the European Union (Council of the European Communities).3,4 Antimony does not show biological functions and it is easily accumulated in organisms, causing deleterious effects in humans.5 The determination of antimony content is important to protect the health of people and prevent environmental contamination due to its toxicity.5
The development of highly sensitive techniques to identify and/or to quantify Sb species has opened up an increasingly attractive research area for elucidating the fate of Sb in the different environmental compartments. A vast majority of studies have focused on methods based on high-performance liquid chromatography (HPLC), used in conjunction with an element-specific detector. These methods using HPLC separation of Sb species are based on anion-exchange chromatographic methods due to the predominance of Sb anionic species in aqueous environmental samples.6 Since the first proposal of using an anion-exchange column,7 cationic and reversed-phase chromatographic columns have also been evaluated,8 but species separation was not improved compared to anion-exchange methods. In this context, the accurate separation of inorganic Sb(III), Sb(V) and methylated Sb species using a single chromatographic system is notoriously problematic and less often described in the literature. Several HPLC methods have attempted such a purpose, most of them based on the use of strong anion-exchange stationary phases and complexing mobile phases to improve Sb(III) elution.6 Furthermore HPLC systems are complex, involving expensive instruments and dilatory methodologies.
Hydride generation (HG) techniques are widely used for the determination of volatile hydride forming elements in analytical atomic spectrometry to enhance detection power and minimize or eliminate matrix interferences while incurring relatively low additional cost and minimal sophistication.9 Usually, these techniques were combined with atomic absorption spectrometry (HG-AAS),10 atomic fluorescence spectrometry (HG-AFS),11,12 inductively coupled plasma optical emission spectrometry (HG-ICP-OES)13,14 and inductively coupled plasma mass spectrometry (HG-ICP-MS).15,16 Alternative applications using the HG-AFS system can also be used.17
Not only is Sb(III) reduced by NaBH4, but also methylated species of Sb are reduced and Sb(V) is partially reduced.18 This is an important drawback in Sb speciation by HG to obtain accurate results. 8-Hydroxyquinoline was used as a masking reagent for Sb(V) and transition metals during stibine formation.19 For the determination of Sb(V) by HG, it should be previously pre-reduced to Sb(III). Most methodologies for Sb determination have only been applied for the separation or speciation of inorganic species, Sb(III) and Sb(V), without considering the organic species of antimony. This dimethylation leads to the formation of Me2SbH, MeSbH2, SbH3, and Me3Sb, an issue which has been discussed extensively in the literature, but has not yet been unequivocally solved.15 Although non-chromatographic methodologies are an interesting alternative, because they are cheaper, easy to operate and faster than HPLC systems, only a single paper has addressed the use of a non-chromatographic system for antimony speciation. The author studied the influence of a combination of fluoride and iodide as a modifier for the reduction process of Sb(III), Sb(V) and TMSb for stibine generation using flow injection hydride generation coupled to an inductively coupled plasma atomic emission spectrometer (FI-HG-ICP-AES), and the method was applied to orange juice samples.20
Currently, multivariate optimization strategies are very popular in the development of analytical methodologies. The main advantage of their use is the low number of experiments required to achieve the optimal conditions and the indication of possible influences of some variables on others, which is not possible in univariate optimization. The response surface methodology (RSM) can be considered as one of the most important approaches for the multivariate optimization of several analytical procedures.21,22 The selection of a Box–Behnken design as a model for the multivariate optimization of analytical procedures is on account of its growth in the last few years, basically due to its higher efficiency when compared to other second-order designs like the Doehlert matrix and the Central Composite Design and its efficiency is equal to the Doehlert matrix when three factors are studied.21
This paper proposes an automated and non-chromatographic method for the determination and speciation of Sb(III), Sb(V) and TMSb in complex natural water samples by HG-AFS at ng L−1. The method has been applied to various natural water samples collected in Balearic Islands, Spain. Furthermore, the majority of the previously developed methods have been focused on the determination of high levels of antimony. The aim of this work is the determination of antimony at the ng L−1 level in natural waters (coastal water, groundwater, and drinking water), since studies indicate that typical concentrations in unpolluted systems are less than 1 μg L−1.3 In the present study a cationic exchange minicolumn was used in order to retain the methylated forms of Sb which can generate hydrides. In this sense, the retention of trimethylated species contributes to minimizing the errors in the determination of inorganic forms present in the sample.
2. Experimental
2.1. System set-up
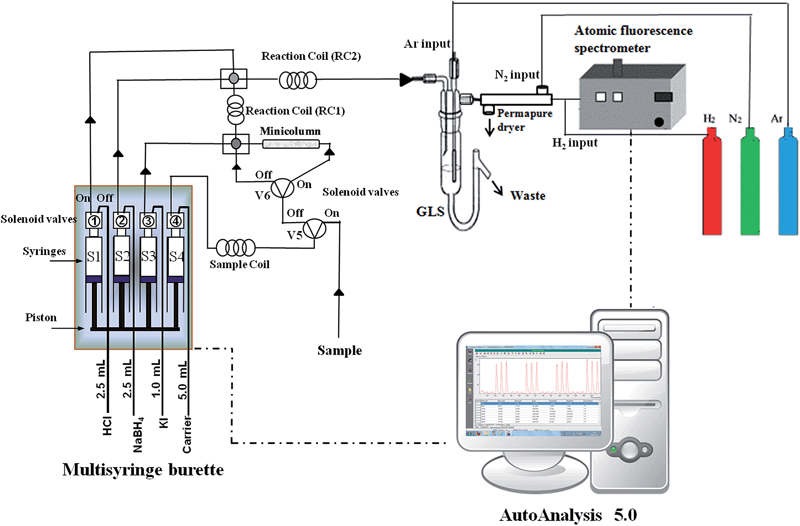
The configuration of the system is presented in Fig. 1. The system consists of a multisyringe burette module with programmable speed (Multiburette 4S, Crison, Alella, Barcelona), employed as a liquid driver. It allows the simultaneous movement of four syringes, which are connected in block to the same stepper motor. Three-way solenoid valves (V1, V2, V3, and V4) (N-Research, Caldwell, NJ, USA) are placed on the head of each syringe with the aim of increasing the versatility and reducing reagent consumption. The “off” position (solenoid disabled) of the head valves connects the syringes to a right channel and the “on” position (solenoid enabled) to a left one. Moreover, the multisyringe has four additional 12 volt outputs, which can control some additional devices.In the proposed system, four syringes were used: S1 (2.5 mL), S2 (2.5 mL), S3 (1.0 mL) and S4 (5.0 mL). The syringes were used as follows: S1 for propulsion of hydrochloric acid solution, S2 to dispense sodium tetrahydroborate solution, S3 to impel the mixture of potassium iodide and ascorbic acid solution to pre-reduce Sb(V) to (Sb(III) and S4 to carry the sample. The acquisition of the peaks was achieved with only one filling of the syringe, increasing the sample frequency. The multisyringe module was equipped with two additional independent solenoid valves (V5) and (V6) (N-Research).
The solenoid valves V1, V2 and V3 control the aspiration and dispensing of the reagents, while V4 and V5 control the sample loading into the holding coil and the sample dispensing to the system. The valve V6 allows the bypass of the sample through the minicolumn. A manifold was constructed with 1.5 mm i.d. (used for the sample aspiration) and 0.8 mm i.d. (used for the rest of the system) PTFE tubes. For the sample loading, the holding coil used was 3 m long with a volume of 5.3 mL.
A drying membrane (Perma Pure Inc., Toms River, NJ) utilizing nitrogen as a purge gas was connected to the outlet of the gas–liquid separator to circumvent entrainment of moisture into the AFS and subsequent quenching of the atomic fluorescence intensity. Water moves through the membrane wall and evaporates into the surrounding air or gas. A non-dispersive atomic fluorescence spectrometer (P.S. Analytical model 10.044, Excalibur detector, PS Analytical) for on line detection equipped with an antimony boosted discharge hollow cathode lamp (primary current 17.5 mA, secondary current 15.0 mA, wavelength 217.6 nm) was used. This spectrometer presents four internal gains and an external fine gain, which allows working on a large concentration range. The fine gain has been adjusted during the optimization, up to a fine gain of 3, which was chosen for the lineal working range, using the internal gain at 100-fold. The transient signals were processed with the peak height. System control, data acquisition and processing of the pump, valves and syringes were performed using the software package Autoanalysis 5.0 (ref. 19) (Sciware Systems, Bunyola, Spain), version 5.0.13.5. A methacrylate minicolumn 5 mm in diameter and 4 cm in length, provided with a porous frit, was used to support the resin (DOWEX® 50 WX8, 100–200 mesh) for TMSb retention.
2.2. Standard solutions and reagents
All chemicals and reagents used were of analytical-grade or higher purity. Ultrapure water (18.2 MΩ cm−1, Millipore, Watford, UK) was used throughout the study. Glassware and plasticware were cleaned by soaking in 10% (v/v) nitric acid and rinsed with ultra-pure water prior to use.A stock standard solution (1000 mg L−1) of Sb(III) was prepared by dissolving antimony potassium tartrate (Carlo Erba, Italy) in 3.0 mol L−1 HCl (Scharlau, Spain) solution. A stock standard solution of Sb(V) was prepared by dissolving potassium pyroantimonate acid (Carlo Erba, Italy) in 3.0 mol L−1 HCl (Scharlau) solution. The solutions were stable for at least 3 months at 4 °C.
A stock solution (1000 mg L−1) of trimethylantimony(V) bromide (Sigma-Aldrich, Germany) was prepared in Milli-Q water and stored in a polyethylene bottle at 4 °C for six months.
A 6% w/v sodium tetrahydroborate solution (Scharlau) in sodium hydroxide 0.2 mol L−1 (Scharlau) was prepared daily.
The 8-hydroxyquinoline stock solution at 1% (w/v, yellowish color), was prepared by dissolving 1.0 g of 8-hydroxyquinoline (AnalaR*, A. R.) in 10 mL of methanol (99.8% Caledon), and then diluted to 100 mL with HCl 10% (w/v).
A stock solution of potassium iodide 50% w/v containing L-ascorbic acid 10% w/v was prepared by dissolving 25.0 g of KI (Scharlau) and 5.0 g of L-ascorbic acid (Scharlau) in 50 mL of ultra-pure water.
A mass of 0.2360 ± 0.0020 g of the cation exchange resin DOWEX® 50W-X8 was used (polystyrene-divinylbenzene with the sulfonic functional group, 100–200 mesh).
2.3. Sample collection and treatment
The water samples were collected and filtered through 0.45 μm cellulose acetate membrane filters immediately after sampling, and acidified to pH 2.0 with hydrochloric acid and stored at 4 °C. The bottles were previously washed with a 10% v/v nitric acid–water solution and afterward with ultrapure water. Before analyses, samples were placed with 8-hydroxyquinoline 0.05% (w/v) and HCl 10% (w/v) and analyzed before 24 h.2.4. Analytical procedure
The analytical procedure for the determination and speciation of Sb can be summarized as follows in three steps:(1) In the first step, Sb(III) is determined. The sample (2.0 mL) is loaded in the sample coil through S4 with V4 and V5 in the “on” position. The sample is then dispensed at 5 mL min−1 with V4 in the “on” position and V5 in the “off” position. At this time V6 switches to the “on” position allowing the sample to pass through the minicolumn and thus TMSb is retained. Then, a sample plug is mixed with HCl (1.0 mL) and NaBH4 (1.0 mL) solutions (2.5 mL min−1) in the reaction coil 2 (RC2). The mixture is impelled to a gas–liquid separator (10 mL min−1), where stibine (H3Sb) is delivered to the AFS-detector by Ar gas at 300 mL min−1, before passing through the Perma Pure dryer with N2 at a 300 mL min−1 flow rate. In this step, Sb(V) did not show any sign of fluorescence emission due to the use of 8-hydroxyquinoline as a complexing agent and the absence of the pre-reducing agent (KI).
(2) In the second step, the total inorganic fraction is determined, i.e. Sb(III) and Sb(V). The procedure is very similar to step 1, but 0.4 mL of KI are added (1.0 mL min−1) in the reaction coil 1 (RC1) in order to pre-reduce Sb(V) to Sb(III). Later, the mixture is merged in RC2 with HCl and NaBH4 solutions. The Sb(V) concentration is calculated by subtracting the Sb(III) concentration previously obtained. In this step, although the mechanism is still unclear, it appears that the pre-reducing solution (KI) breaks the complex formed by the association of Sb(V) and 8-hydroxyquinoline. A similar behavior was previously reported,19 using a mixture of 0.1% 8-hydroxyquinoline + 2.0% KI and achieving a recovery close to 100% for a mixture of Sb(III) + Sb(V).
(3) In the last step, the total antimony is determined, i.e. inorganic species and TMSb. In this step, V6 is switched “off” allowing bypass to the minicolumn. Thus, the total antimony is determined and the TMSb concentration is obtained by the subtraction of previous inorganic fraction concentrations.
3. Results and discussion
3.1. Optimization of the hydride generation system
The optimization of the analytic fluorescence procedure was performed in two steps. First, a two-level full factorial design23 was implemented involving the following factors: sodium tetrahydroborate (NaBH4) concentration (in the range from 0.1 to 0.5% w/v) in sodium hydroxide (NaOH) 0.05 mol L−1; potassium iodide (KI) reagent concentration (from 10 to 15% w/v); and hydrochloric acid concentration (from 1.0 to 5.0 mol L−1). The flow gas parameter (Ar, N2 and H2 were used as in the previous paper24,25 and preliminary studies); the sample flow rate and the acid sample were fixed according to the limitations of the column retention for TMSb. The evaluation of this factorial design demonstrated that for these experimental conditions, factors such as NaBH4, KI and HCl reagent concentration are significant for antimony hydride generation for a 95% significance level and require a final optimization. The ANOVA table showed lack of fit and significant curvature. The curvature test was applied to the results obtained from the full factorial design to evaluate the system's behavior in the central point region. The maximum condition into the region can be identified by curvature test. Eqn (1) was used for the calculation:26| Curvature = RFD − RCP | (1) |
Analysis of the results suggested a negative curvature. This reveals the existence of an analytical region of maximum fluorescence signal near the central point of the experimental conditions.
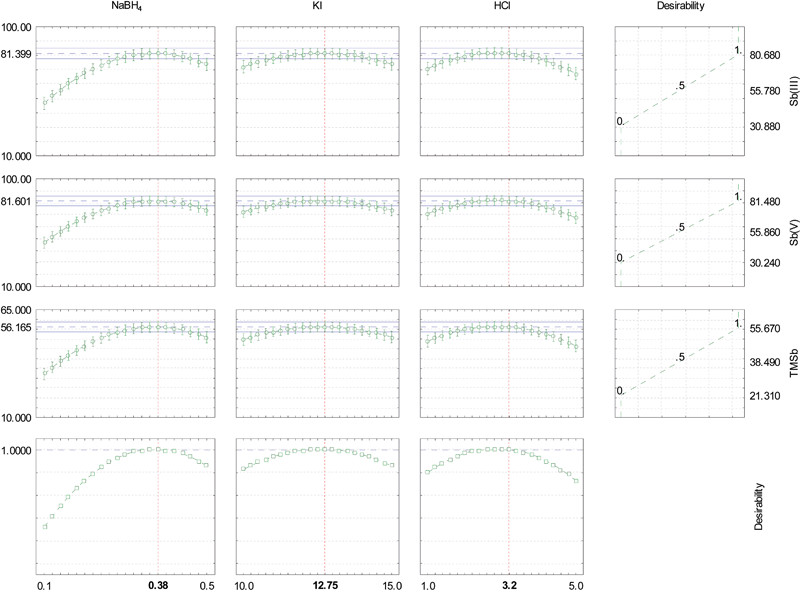
The best analytical performance for the online speciation and detection of critical values in the antimony speciation was achieved by applying the Box–Behnken design for the chemical variables: NaBH4 concentration (% w/v), KI concentration (% w/v) and HCl concentration (mol L−1) (Table 1). This design required fifteen experiments which were performed in a random manner to avoid any systematic error. The response of analytical interest was the fluorescence intensity (peak height) of the species Sb(V), Sb(III) and TMSb obtained in each step. To perform multiple response optimization for speciation and determination of the three antimony species, a mathematical–statistical tool developed by Derringer, which is based on the use of a desirability function, was used. This feature allowed us to combine in a single response (overall desirability) three distinguishing features of each species studied. The use of desirability functions for multiple response optimization experiments was proposed by Derringer and Suich.27 To obtain overall desirability, individual desirability of all responses should be determined (in this case, fluorescence intensity of Sb(III), Sb(V) and TMSb). Thus, each response yi (i = 1, 2, …, m) is transformed into a scale-free value, which is called an individual desirability function (di), where 0 ≤ di ≤ 1, with 0 for an unacceptable response and 1 for a desirable response. The value of di increases as the desirability of the corresponding response increases. The individual desirability function was calculated according to eqn (2), which was used to maximize the analytic responses for Sb(III), Sb(V) and TMSb. In this equation yi is the Sb(III), Sb(V) or TMSb fluorescence intensity; L and H are the lower and upper fluorescence intensities, observed in the experiments for the antimony species. The overall desirability (D) was calculated by determining the geometric mean of individual desirabilities (eqn (3)).
 | (2) |
| D = (d1 × d2 × d3…dk)1/k | (3) |
| Exp. | NaBH4 | KI | HCl | Analytical signal (AU) | |||
|---|---|---|---|---|---|---|---|
| Sb(III)a | Sb(V)a | TMSbb | D | ||||
| 1 | — | — | 0 | 48.82 | 49.01 | 33.69 | 0.3623 |
| 2 | + | — | 0 | 66.24 | 66.60 | 45.71 | 0.7099 |
| 3 | — | + | 0 | 31.83 | 32.14 | 21.96 | 0.0237 |
| 4 | + | + | 0 | 62.04 | 62.00 | 42.81 | 0.6237 |
| 5 | — | 0 | — | 35.82 | 36.11 | 24.72 | 0.1041 |
| 6 | + | 0 | — | 65.39 | 65.71 | 45.12 | 0.6927 |
| 7 | — | 0 | + | 30.88 | 30.24 | 21.31 | 0.0000 |
| 8 | + | 0 | + | 59.99 | 60.28 | 41.39 | 0.5851 |
| 9 | 0 | — | — | 53.50 | 54.12 | 36.91 | 0.4581 |
| 10 | 0 | + | — | 64.66 | 64.17 | 44.61 | 0.6728 |
| 11 | 0 | — | + | 54.08 | 54.11 | 37.32 | 0.4659 |
| 12 | 0 | + | + | 61.57 | 61.47 | 42.48 | 0.6139 |
| 13 | 0 | 0 | 0 | 77.65 | 78.21 | 53.58 | 0.9382 |
| 14 | 0 | 0 | 0 | 80.68 | 79.00 | 55.67 | 0.9836 |
| 15 | 0 | 0 | 0 | 79.80 | 81.48 | 55.06 | 0.9882 |
The individual and overall desirability profiles for Sb(III), Sb(V) and TMSb are calculated according to eqn (2) and (3). Table 1 shows the overall desirability profile (D). Fig. 2 shows the predicted values and desirability analyzed using the real values of the independent variables and the data processed by the STATISTICA software28 for a confidence level of 95%.
The system showed critical values with maximum solution in the central point region (Table 1 and Fig. 2) and a desirability equal to 1.0, for a confidence level of 95%. These values were: NaBH4 0.38% (w/v), HCl 3.2% (v/v) and KI 12.75% (w/v). The ANOVA table shows an adjusted model for the three antimony species with low error and the correlation between observed and predicted values.
3.2. Speciation methodology
Preliminary results and those reported in the literature29 showed the difficulties encountered in developing a methodology for the determination of the three species using anionic chromatographic methods. Such difficulties may be related to the fact that soluble trimethylated antimony can mainly exist as uncharged or cationic ([(CH3)3SbOH]+) species following the dissolution of TMSb in aqueous solution. Thus, its retention in the column cannot be explained as a single anion exchange process, whereas in aqueous solutions, Sb(III) exists as a neutral species at pH around 8, e.g. Sb(OH)3, or as a complex ion di-negatively charged, e.g. [Sb2(C4O6H2)2]2− in the presence of tartrate or EDTA, while Sb(V) exists as a mono-negatively charged species, i.e. [Sb(OH)6]−.1,6,30 So, in this work we decided to determine antimony species (Sb(III), Sb(V) and TMSb) in water samples, using a cation exchange resin for the retention of TMSb. In this way, the analytical error is avoided since the TMSb species generates hydrides.31L-Cysteine has been recognized as a pre-reductant for some years to reduce Sb(V) to Sb(III). However, it is known that its use yields a high value of the analytical signal of the blank. Besides, when L-cysteine is used as a masking agent, it will inevitably reduce the original Sb(V) to Sb(III), making speciation impossible. Therefore, potassium iodide was selected as a pre-reductant for the reduction of Sb(V) to Sb(III) and total Sb determination. Sb(III) and Sb(V) were determined in the absence and presence of potassium iodide. Fig. 3 shows the analytical signals obtained for the three species in study in the presence and absence of KI, without the use of the minicolumn. For this reason, it was decided to use KI because of the high efficiency of speciation of the inorganic forms of Sb. Besides, one study reports that Sb(V) cannot be completely reduced to Sb(III) without a pre-reduction step.32
The compound 8-hydroxyquinoline was used as a masking agent in order to avoid any modification in the oxidation state of Sb(III) to Sb(V).19
3.3. Analytical performance
Using the optimized experimental conditions, limits of detection (LODs) (3δ/s) and quantification (LOQs) (10δ/s) were calculated for Sb(III), Sb(V) and TMSb in water samples following the IUPAC recommendation.33 The LODs and LOQs of Sb(III) and Sb(V) are 0.03 and 0.13 μg L−1, respectively, while for TMSb they are 0.09 and 0.4 μg L−1, respectively. The sample injection throughput obtained was 30 h−1. The precision was evaluated through the relative standard deviation (RSD, %) for the 10 replicate measurements of Sb(III), Sb(V) and TMSb. Calibration was done by comparing the slope of the curve obtained with Sb(V) in aqueous standards with that obtained for analyte addition to the natural water samples. The statistical comparison allows us to determine the similitude between the slopes, with determination coefficients R2 > 0.99. Therefore, it can be concluded that the proposed method can quantify Sb species using external calibration with aqueous standards.In order to investigate the effect of the inorganic Sb species over the TMSb determination, two curves of TMSb were obtained: one for TMSb and other for TMSb in the presence of 1.0 μg L−1 Sb(V) both in aqueous medium (pH = 2.0) using 8-hydroxyquinoline as a masking agent and KI as a pre-reduction reagent for Sb(V). The slopes for both curves showed no significant difference, with the determination coefficient R2 > 0.99 for a confidence level of 95%, as shown in Fig. 4. This indicates that TMSb can be determined in the presence of the inorganic form of Sb (assay concentrations similar to those found in fresh water), using a masking agent.
 | ||
| Fig. 4 Calibration curves for TMSb in aqueous medium (pH = 2) in the absence and presence of 1.0 μg L−1 Sb(V). Both solutions were prepared in 8-hydroxyquinoline and KI. | ||
The analytical parameters used to determine the three studied species are shown in Table 2, and the instrument working conditions are summarized in Table 3.
| Sample flow rate (mL min−1) | 5.0 |
| Sample volume (mL) | 2.0 |
| NaBH4 (mL min−1) | 2.5 |
| NaBH4 (mL) | 1.0 |
| KI (mL min−1) | 1.0 |
| KI (mL) | 0.4 |
| HCl (mL min−1) | 2.5 |
| HCl (mL) | 1.0 |
| Argon flow rate (mL min−1) | 300 |
| Hydrogen flow rate (mL min−1) | 35 |
| Dryer gas (N2) flow rate (mL min−1) | 300 |
| Lamp current primary (mA) | 17.5 |
| Lamp current boost (mA) | 15.0 |
| Injection throughput (inj. h−1) | 30 |
| Gain | 100 |
| Fine gain | 1 |
| Signal type | Peak height |
The LOD, procedure used, sample matrix and chemical forms of antimony determined were compared between the proposed procedure and those of other procedures for antimony determination (Table 4).
| Procedure | Sample | LOD (μg L−1) | Reference | ||
|---|---|---|---|---|---|
| Sb(III) | Sb(V) | TMSb | |||
| a Chromatographic technique. b Non-chromatographic technique. HPLC: high performance liquid chromatography; HG: hydride generation; AFS: atomic fluorescence spectrometry; FI: flow injection; ICP: inductively coupled plasma; AES: atomic emission spectrometry; MS: mass spectrometry; ETV: electrothermal vapourization; AAS: atomic absorption spectrometry; MSFIA: multisyringe flow injection analysis. | |||||
| HPLC-HG-AFSa | Water | 0.26 | 0.09 | 0.04 | 35 |
| HPLC-HG-AFSa | Urine | 0.19 | 0.18 | 0.12 | 11 |
| HPLC-HG-AFSa | Soil | 0.07 | 0.07 | 1.0 | 36 |
| HPLC-HG-AFSa | Seawater | 0.07 | 0.13 | 0.13 | 37 |
| FI-HG-ICP-AESb | Orange juice, soil extracts | 1.2 | 1.4 | 1.1 | 20 |
| HG-ICP-MSb | Seawater | 0.013 | 0,021 | — | 38 |
| ETV-ICP-AESb | River water, tap water, pond water, urine | 0.09 | 0.09 | — | 39 |
| FI-HG-AASb | Natural water | 0.05 | 0.06 | — | 40 |
| HG-ICP-AESb | River water, effluent samples | 0.09 | 0.9 | — | 41 |
| MSFIA-HG-AFSb | Ground water, seawater, drinking water | 0.03 | 0.03 | 0.13 | This work |
3.4. Validation of the proposed method and its application in water samples
Since no certified reference materials exist for antimony speciation, the validation was performed by an addition/recovery test (IUPAC, 2002).34 Hence, in order to establish the trueness of the proposed MSFIA-HG-AFS system for antimony speciation, real samples were spiked at trace level concentrations. Recoveries of drinking waters, coastal seawaters and groundwater doped with 0.2 and 0.4 μg L−1 ranged from 90% to 110% regardless of the sample matrix complexity (see Table 5). Therefore, it was demonstrated that the automated MSFIA-HG-AFS system for antimony speciation is reliable and provides unbiased data for environmental analysis. The antimony species and total antimony were quantified by employing MSFIA-HG-AFS in water samples collected in Balearic Islands.| Sample | Sb(III) (n = 3) | Sb(V) (n = 3) | TMSb (n = 3) | Total Sb (n = 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spiked (μg L−1) | Found (μg L−1) | Rec (%) | Spiked (μg L−1) | Found (μg L−1) | Rec (%) | Spiked (μg L−1) | Found (μg L−1) | Rec (%) | ||
| a GW: groundwater. b SW: seawater. c DW: drinking water. | ||||||||||
| GWa-B | — | 0.23 ± 0.09 | — | — | <LOQ | — | — | <LOQ | — | 0.23 ± 0.10 |
| 0.2 | 0.44 ± 0.06 | 105 | 0.20 | 0.19 ± 0.04 | 95 | 1.00 | 0.91 ± 0.10 | 92 | ||
| 0.4 | 0.58 ± 0.06 | 90 | 0.40 | 0.42 ± 0.07 | 105 | 2.00 | 2.14 ± 0.08 | 104 | ||
| SWb-PP | — | 0.29 ± 0.10 | — | — | 0.27 ± 0.10 | — | — | <LOQ | — | 0.56 ± 0.20 |
| 0.2 | 0.48 ± 0.09 | 95 | 0.20 | 0.49 ± 0.03 | 110 | 1.00 | 0.93 ± 0.10 | 93 | ||
| 0.4 | 0.67 ± 0.08 | 95 | 0.40 | 0.71 ± 0.05 | 110 | 2.00 | 2.17 ± 0.10 | 108 | ||
| SW-PX | — | 0.23 ± 0.08 | — | — | 0.22 ± 0.08 | — | — | <LOQ | — | 0.45 ± 0.16 |
| 0.2 | 0.47 ± 0.10 | 110 | 0.20 | 0.43 ± 0.08 | 105 | 1.00 | 0.97 ± 0.12 | 97 | ||
| 0.4 | 0.61 ± 0.05 | 95 | 0.40 | 0.59 ± 0.06 | 105 | 2.00 | 2.20 ± 0.09 | 110 | ||
| SW-SJD | — | 0.12 ± 0.04 | — | — | 0.20 ± 0.06 | — | — | <LOQ | — | 0.32 ± 0.10 |
| 0.2 | 0.29 ± 0.06 | 91 | 0.20 | 0.43 ± 0.07 | 98 | 1.00 | 0.97 ± 0.13 | 97 | ||
| 0.4 | 0.48 ± 0.05 | 90 | 0.40 | 0.59 ± 0.04 | 105 | 2.00 | 2.10 ± 0.10 | 105 | ||
| SW-CB | — | 0.12 ± 0.09 | — | — | 0.29 ± 0.10 | — | — | <LOQ | — | 0.42 ± 0.09 |
| 0.2 | 0.34 ± 0.10 | 110 | 0.20 | 0.48 ± 0.06 | 95 | 1.00 | 1.07 ± 0.09 | 107 | ||
| 0.4 | 0.50 ± 0.07 | 95 | 0.40 | 0.70 ± 0.06 | 103 | 2.00 | 2.10 ± 0.08 | 105 | ||
| DWc-GC | — | 0.13 ± 0.10 | — | — | 0.27 ± 0.08 | — | — | <LOQ | — | 0.39 ± 0.18 |
| 0.2 | 0.32 ± 0.08 | 95 | 0.20 | 0.45 ± 0.04 | 90 | 1.00 | 0.89 ± 0.14 | 90 | ||
| 0.4 | 0.49 ± 0.08 | 90 | 0.40 | 0.66 ± 0.04 | 98 | 2.00 | 2.13 ± 0.08 | 106 | ||
4. Conclusions
A new non-chromatographic automated method based on MSFIA coupled to HG-AFS for antimony speciation was described.The maximum efficiency was obtained thanks to the combination of multivariate design optimization with multiresponse tools.
The proposed method provides several advantages such as a high degree of automation, an elevated precision (RSD < 5%), and low limits of detection that allow Sb speciation analysis in environmental waters. Besides, a high injection frequency together with the minimization of sample and reagent volumes makes this method an efficient and environmentally friendly tool for antimony species evaluation.
The proposed method was successfully applied to several kinds of water samples, achieving recoveries of 90–110%.
Acknowledgements
Lindomar Portugal thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (Brazil) for providing the post-doctoral studies scholarship (PDE) to carry out research work at the University of the Balearic Islands. This work was funded by the Spanish Ministry of Economy and Competitivity (CTQ2013-47461-R project) and by the Balearic Government (43/2011 cofinanced by FEDER funds).References
- M. Krachler, H. Emons and J. Zheng, Trends Anal. Chem., 2001, 20, 79 CrossRef CAS.
- D. E. Guberman, Antimony, in Mineral Commodity Summaries, U.S. Geological Survey, Reston, Virginia, 2014, pp. 18–19 Search PubMed.
- M. Filella and N. Belzile, Earth-Sci. Rev., 2002, 57, 125 CrossRef CAS.
- M. He, X. Wang, F. Wu and Z. Fu, Sci. Total Environ., 2012, 421, 41 CrossRef PubMed.
- K. Bencze, Antimony, in Handbook on Metals in Clinical and Analytical Chemistry, ed. H. G. Seiler, A. Sigel and H. Sigel, Marcel Dekker, New York, 1994, pp. 227–236 Search PubMed.
- R. Miravet, E. Hernández-Nataren, A. Sahuquillo, R. Rubio and J. F. López-Sánchez, Trends Anal. Chem., 2010, 29, 28 CrossRef CAS PubMed.
- P. Smichowski, Y. Madrid, M. B. Calle-Gutiñas and C. Cámara, J. Anal. At. Spectrom., 1995, 10, 815 RSC.
- J. Lintschinger, I. Koch, S. Serves, J. Feldmann and W. R. Cullen, Fresenius' J. Anal. Chem., 1997, 359, 484 CrossRef CAS.
- J. Dedina and D. L. Tsalv, Hydride Generation Atomic Absorption Spectrometry, Wiley, New York, 1995 Search PubMed.
- A. Erdem and A. E. Eroglu, Talanta, 2005, 68, 86 CrossRef CAS PubMed.
- W. Quiroz, H. Arias, M. Bravo, M. Pinto, M. G. Lobos and M. Cortés, Microchem. J., 2011, 97, 78 CrossRef CAS PubMed.
- A. Bellido-Martín, J. L. Gómez-Ariza, P. Smichowsky and D. Sánchez-Rodas, Anal. Chim. Acta, 2009, 649, 191 CrossRef PubMed.
- A. Ilander and A. Vaisänën, Anal. Chim. Acta, 2011, 689, 178 CrossRef CAS PubMed.
- P. Qiu, C. Ai, L. Lin, J. Wu and F. Ye, Microchem. J., 2007, 87, 1 CrossRef CAS PubMed.
- M. Krachler and H. Emons, J. Anal. At. Spectrom., 2001, 16, 20 RSC.
- J. Bowman, B. Fairman and T. Catterick, J. Anal. At. Spectrom., 1997, 12, 313 RSC.
- P. Montesinos, M. L. Cervera, A. Pastor and M. Guardia, Talanta, 2003, 60, 787 CrossRef.
- A. D'Ulivo, L. Lampugnani, G. Pellegrini and R. Zamboni, J. Anal. At. Spectrom., 1995, 10, 669 RSC.
- T. L. Deng, Y. W. Chen and N. Belzile, Anal. Chim. Acta, 2001, 432, 239 CrossRef.
- N. Ulrich, Anal. Chim. Acta, 2000, 417, 201 CrossRef CAS.
- S. L. C. Ferreira, R. E. Bruns, G. D. Matos, M. M. David, G. C. Brandão, E. G. P. da Silva, L. A. Portugal, P. S. dos Reis, A. S. Souza and W. N. L. dos Santos, Anal. Chim. Acta, 2007, 597, 179 CrossRef CAS PubMed.
- M. A. Bezerra, R. E. Santelli, E. P. Oliveira, L. S. Vilar and L. A. Escaleira, Anal. Chim. Acta, 2007, 597, 179 CrossRef PubMed.
- R. E. Bruns, I. S. Scarminio and B. de Barros Neto, Statistical Design—Chemometrics, Elsevier, Amsterdam, 2006 Search PubMed.
- A. M. Serra, J. M. Estela and V. Cerdà, J. Anal. At. Spectrom., 2012, 27, 1858 RSC.
- N. V. Semenova, L. O. Leal, R. Forteza and V. Cerdà, Anal. Chim. Acta, 2005, 530, 113 CrossRef CAS PubMed.
- D. G. da Silva, L. A. Portugal, A. M. Serra, S. L. C. Ferreira and V. Cerdà, Food Chem., 2013, 137, 159 CrossRef CAS PubMed.
- G. Derringer and R. Suich, J. Qual. Technol., 1980, 12, 214 Search PubMed.
- StatSoft Inc, Statistica 8.0, StatSoft Inc, Tulsa, USA, 2007 Search PubMed.
- M. J. Nash, J. E. Maskall and S. J. Hill, Analyst, 2006, 131, 724 RSC.
- A. R. Kumar and P. Riyazuddin, Int. J. Environ. Anal. Chem., 2007, 87, 469 CrossRef CAS.
- M. Dodd, S. L. Grundy, K. J. Reimer and W. R. Cullen, Appl. Organomet. Chem., 1992, 6, 207 CrossRef CAS.
- B. Alegria, R. Barbera and R. Farre, Int. J. Environ. Anal. Chem., 1990, 38, 65 CrossRef.
- G. L Long and J. D. Winefordner, Anal. Chem., 1983, 55, 712 CrossRef.
- IUPAC, Pure Appl. Chem., 2002, 74(5), 835–855 Search PubMed.
- A. Sayago, R. Beltrán, M. A. F. Recameles and J. L. Gómez-Ariza, J. Anal. At. Spectrom., 2002, 17, 1400 RSC.
- W. Quiroz, D. Olivares, M. Bravo, J. Feldman and A. Raab, Talanta, 2011, 84, 593 CrossRef CAS PubMed.
- I. D. Gregori, W. Quiroz, H. Pinochet, F. Pannier and M. Potin-Gautier, J. Chromatogr. A, 2005, 1091, 94 CrossRef PubMed.
- A. C. Fornieles, A. G. Torres, E. V. Alonso, M. T. S. Cordero and J. M. C. Pavon, J. Anal. At. Spectrom., 2011, 26, 1619 RSC.
- Y. J. Li, B. Hu and Z. C. Jiang, Anal. Chim. Acta, 2006, 576, 207 CrossRef CAS PubMed.
- F. Y. Zheng, S. H. Qian, X. Q. Li, L. X. Huang and L. X. Lin, Anal. Sci., 2006, 22, 1319 CrossRef CAS.
- A. A. Menegário, P. Smichowski, P. S. Tonello, G. Polla, E. P. Oliveira and R. E. Santelli, Anal. Chim. Acta, 2008, 625, 131 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |