Direct elemental analysis of honeys by atmospheric pressure glow discharge generated in contact with a flowing liquid cathode
Krzysztof
Greda
a,
Piotr
Jamroz
a,
Anna
Dzimitrowicz
b and
Pawel
Pohl
*a
aWroclaw University of Technology, Faculty of Chemistry, Division of Analytical Chemistry, Wybrzeze Stanislawa Wyspianskiego 27, 50-370 Wroclaw, Poland. E-mail: pawel.pohl@pwr.wroc.pl; Fax: +48-71-320-2494; Tel: +48-71-320-2494
bWroclaw University of Technology, Faculty of Chemistry, Institute of Physical and Theoretical Chemistry, Wybrzeze Stanislawa Wyspianskiego 27, 50-370 Wroclaw, Poland
First published on 17th September 2014
Abstract
Miniaturized atmospheric pressure glow discharge sustained in a compact discharge cell in contact with a flowing liquid cathode was used for the elemental analysis of honeys by optical emission spectrometry. A simplified sample preparation procedure was proposed and samples of honeys were only dissolved in water and acidified with HCl to a concentration of 0.10 mol L−1. The resulting 1.0% m/v in the case of K and Na and 5.0% m/v in the case of Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn solutions of honeys were directly introduced into the discharge cell acting as the liquid cathode of the discharge. To eliminate matrix effects coming from fructose and glucose, a non-ionic surfactant (Triton X-405) was added to the solutions and this resulted in improved signals of studied elements. For calibration, simple (for K and Na) and matrix-matching (for other elements) standard solutions were used. The method was proved to give reliable results and applied in the analysis of 16 commercial white- to amber-colored honeys with limits of detection at levels of 1.0 (Ca), 0.7 (Cu), 2.5 (Fe), 0.5 (K), 0.02 (Li), 0.2 (Mg), 1.8 (Mn), 0.04 (Na), 0.1 (Rb) and 0.2 (Zn) μg g−1.
1. Introduction
Miniaturized low power direct (dc) or alternating current (ac) atmospheric pressure glow discharge (APGD) generated in contact with a flowing liquid cathode (FLC) has rapidly gained popularity and significance, as evidenced by original studies published in recent 2 years.1–14 Important developments reported for this promising and original excitation source used in analytical optical emission spectrometry (OES) relied on replacing a solid metallic anode with miniature flow Ar or He1,2 jets or more commonly by changing the chemical composition of FLC solutions.3–10 In the latter case, it has been established that adding low molecular weight carboxylic acids, i.e., formic6–8 or acetic10 acids, to FLC solutions, results in the substantial enhancement of signals of some elements, e.g., 5- (ref. 7 and 8) or 78-fold6 for Pb, 5- (ref. 10) or 10-fold8 for Hg, 8- (ref. 8) or 13-fold6 for Ag and 17-fold for Cd.6 Responses of other elements, namely Ca, Co, Cu, Fe, Mg, Mn and Ni, were established to increase as well but enhancements observed for them were at a level of 2.7,8 A similar effect was also achieved by adding non-ionic (Triton X-405, Triton X-114)4,5,9 or ionic (cetyltrimethylammonium chloride, CTAC)3 surfactants. The presence of these substances in FLC solutions was responsible for the enhancement of signals of various elements in a magnitude similar to that reported in the case of formic and acetic acids, i.e., 7- (ref. 3) or 10-fold5,9 for Pb, 6-fold for Cu,5,9 5-fold for Hg and Mn,3,5,9 and 2–4-fold for other elements (Ca, Cd, Co, Cr, Cs, Fe, Li, Mg, Ni, Rb, Sr, and Zn).3,5,9Aforementioned suitable signal enhancements were presumed to be a consequence of an increase in the boiling point and a decrease in the surface tension of FLC solutions.3–10 Alterations of physicochemical properties of these aqueous solutions possibly led to changes in the rates of sputtering of analytes and vaporization of water.7,10 According to visual observations made by Schwartz et al.,12 mentioned changes could promote formation of small solution droplets that were easily pulled from the surface of the FLC and increase electrospray-like transport of analytes into the discharge. Indeed, as indicated by a spectroscopic diagnostic of dc-APGD generated in contact with FLC solutions containing non-ionic surfactants,9 the addition of these substances resulted in an expedient increase in the sputtering rate of elements and a decrease in the vaporization rate of water. In these conditions, APGD was stably operated in a less water vapor saturated atmosphere and hence, the electron number density in the near-cathode region of the discharge was higher. However, it appears that the mechanism of action of carboxylic acids and surfactants is not entirely the same. Apart from increases in the signals of elements, surfactants present in FLC solutions led to a reduction of the background level in the vicinity of analytical lines of elements and its fluctuation.3–5,9 What is more, the morphology of the background spectrum was simplified due to a substantial reduction of intensities of emission bands of OH, NO and N2 molecules.3–5,9 In a consequence, limits of detection (LODs) of elements were much improved because of lower background fluctuations and higher signals of their analytical lines. Unfortunately, in the case of formic and acetic acids added to solutions of the FLC, the morphology of background emission spectra acquired in the presence or absence of these acids in FLC solutions was practically the same and thus, the improvement of LODs of elements corresponded only to those noted for signals of these elements.6–8,10
To the best of our knowledge, APGD generated in contact with the FLC, as an alternative excitation source in OES, was applied so far in the elemental analysis of different environmental samples, tissues and food including tuna fish,10 aquatic plants,10 human hair,3 tea,14 stream sediments,3 coal fly ashes,4 soils,5 and spruce needles,5 that were wet digested or extracted. Direct elemental analysis with this method concerned only ground,4 tap,6 natural,6 mineral,14 and pond5 waters.
The information about the elemental composition in honey is an important issue considering the quality, safety and nutritional value of this functional food product and nutrient. Measurements of total concentrations of some selected major (Ca, K, Mg, Na) and minor (Cu, Fe, Mn, Zn) elements in honey help in assessing its geographical and/or botanical origins and wholesomeness.15,16 Due to a high content of carbohydrates, direct elemental analysis of honey encounters many problems related to the heterogeneity of samples and matrix effects.17,18 Possible chemical and physical interference accompanying spectrochemical analysis of honey is commonly eliminated by mineralizing samples using dry and wet ashing procedures. Simplified analysis of honey, samples of which are only dissolved in water or acidic solutions prior to measurements, is uncommon. Such analysis can certainly decrease the time of sample preparation and the risk related to the loss of elements at high temperature and/or contamination of analyzed samples. Considering the effect of non-ionic and ionic surfactants in APGD generated in contact with the FLC on increasing recorded signals of elements as well as reducing the background level and improving its stability,3–5,9 it could be expected that these substances would particularly be desirable in the elemental analysis of undigested samples of honey, containing mainly an organic matrix rich in carbohydrates. Here, due to the unique ability to extinguish molecular emission spectra and quench intensities of OH, N2, and NO bands, the use of surfactants could simplify the emission spectra of solutions of analyzed samples.
To verify this research hypothesis, the suitability of dc-APGD sustained in a modified compact discharge cell in contact with a small-sized FLC for direct elemental analysis of honey was examined. To the best of our knowledge, no studies on the elemental analysis of honeys using OES with excitation in dc-APGD generated in contact with the FLC have been reported to date. Initially, the effect of a honey-like matrix on intensities of atomic emission lines of studied elements (Ca, Cu, Fe, K, Li, Mg, Mn, Na, Rb and Zn) was studied and established to be destructive for the performance of the system. As a remedy, a non-ionic surfactant (Triton X-405) was used as a modifier of the FLC, resulting in boosting of intensities of analytical lines of studied elements and simplifying the morphology of emission spectra of dc-APGD fed with solutions of honey. The analytical performance of the developed dc-APGD-OES method, with the modified FLC by adding Triton X-405, was assessed. The method was used for analysis of 16 commercial weight- to amber-colored honeys for the content of Ca, Cu, Fe, K, Li, Mg, Mn, Na, Rb and Zn. The reliability of the results was verified by using a recovery study as well as a reference method, i.e., flame atomic emission spectrometry (FAES) in the case of K and Na, and another calibration strategy, i.e., standard solution additions in the case of Ca, Mg, Mn, Rb and Zn.
2. Experimental
2.1. Reagents, solutions and samples
Double distilled water was used throughout. TraceCERT grade single-element 1000 mg L−1 standard solutions of Ca, Cu, Fe, K, Li, Mg, Mn, Na, Rb and Zn were supplied by Sigma-Aldrich Chemie GmbH (Germany). A 70% m/v water solution of the polyethylene glycol tert-octylphenyl ether (Triton X-405, C14H22O(C2H4O)40) non-ionic surfactant was also provided by Sigma-Aldrich. J.T. Baker ACS grade reagents, meeting ACS reagent chemical requirements for trace elemental analysis, were used, i.e., solutions of 30% m/m H2O2, 37% m/m HCl and 65% m/m HNO3. Analytical grade D-fructose (>98%) and D-glucose (>98%) were provided by Avantar Performance Materials (Poland).By studying the effect of the honey-like matrix on the response of minor and trace elements of honey, i.e., Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn, 5.0% m/v (g per 100 mL) solutions of this matrix were prepared, assuming that an average concentration of fructose and glucose in honeys is similar and equals in total to 80% m/m. The mentioned 5.0% m/v honey-like matrix solutions contained Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn at a concentration of 0.5 mg L−1, fructose and glucose, each at a concentration of 20 g L−1, and the sum of K and Na at concentrations increasing up to 100 mg L−1 (the ratio of concentrations of K to Na was close to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was derived from the data provided by initial analysis of studied honeys for the content of K and Na by FAES). Triton X-405 was added to solutions of standards and samples to a final concentration of 4.0 mmol L−1 that corresponded to its 5 times critical micelle concentration (5× CMC), which was 0.81 mmol L−1 as given by Hait and Moulik.19 All solutions were acidified with concentrated HCl to a concentration of 0.1 mol L−1.
1, which was derived from the data provided by initial analysis of studied honeys for the content of K and Na by FAES). Triton X-405 was added to solutions of standards and samples to a final concentration of 4.0 mmol L−1 that corresponded to its 5 times critical micelle concentration (5× CMC), which was 0.81 mmol L−1 as given by Hait and Moulik.19 All solutions were acidified with concentrated HCl to a concentration of 0.1 mol L−1.
Sixteen white- to amber-colored commercial honeys from 3 of the greatest producers and/or distributors of bee products in Poland, i.e., CD Inc. (X), Huzar™ Ltd. (Y) and Sadecki Bartnik® (Z), were selected for the study and included acacia (A), buckwheat (B), heather (H), lime (L), multiflower (M) and rape (R) honeys.
2.2. dc-APGD-OES
A fully open-to-air discharge system of a simplified compact design (see Fig. 1) was used throughout for sustaining dc-APGD in contact with a FLC. The system was similar to the one reported in previous studies,5,9,14 but a couple of changes were introduced. Most notably, a W rod, the tip of which was sharpened, replaced the Mo rod with a rounded end. Such an arrangement of the anode was found to give slightly higher intensities of analytical lines of all studied elements and higher values of signal to background ratios (SBRs) for these lines as compared to a former system. In addition, dancing and flickering of the discharge column, possible in the case of the rounded tip, were eliminated. Using a 2-channel peristaltic pump, solutions of the FLC were delivered to the discharge system through a quartz capillary (ID 2.0 mm) at a flow rate of 1.8 mL min−1. The capillary was inserted into a graphite tube (ID 4.0 mm) in such a way that its edge was 2.0 mm below the edge of the graphite tube. Overflowing liquid was collected in a cavity formed at the end of an outer glassy carbon tube, holding the graphite tune and housing the whole cathode compartment. Solutions treated by the discharge were drained out using another peristaltic pump. Both electrodes were vertically oriented. A gap between them, i.e., the anode tip and the surface of the FLC solution, was 5 mm. The discharge was maintained after applying a voltage of 1500 V from a dc power supply to the graphite tube (the electric ground) and the W rod anode (the positive potential). This resulted in a current flow of 45 mA through electrodes, additionally stabilized by a 5 kΩ ballast resistor. The discharge could also be stably operated using lower (down to 0.6 mL min−1) and higher (up to 3.0 mL min−1) flow rates of solutions of the FLC.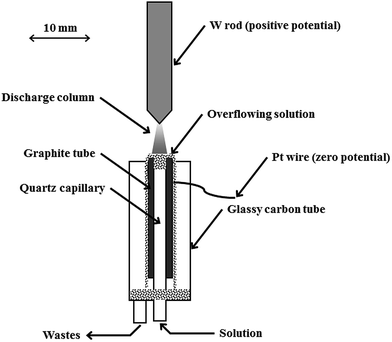 | ||
| Fig. 1 A schematic diagram of a compact discharge cell for dc-APGD generated in contact with a small-sized flowing liquid cathode (FLC). | ||
An achromatic UV lens was used to collect the radiation emitted from the near-cathode region of the discharge. An unmagnified image of this region was focused on the entrance slit (100 μm) of a 320 mm focal length single 1200 grooves mm−1 holographic grating monochromator Triax 320 (Horiba-Jobin Yvon). The exit slit of the monochromator was 100 μm. The resolved radiation was detected using a Hamamatsu R-928 photomultiplier biased at a voltage of −700 V. The output signal of the photomultiplier was amplified using a single photon counting acquisition system SpectraAcq2 integrated with the monochromator. A SpectraMax/32 for Windows software (Instruments SA, Inc.), version 3.2, was used to control the operation of the monochromator, record profiles of emission lines of the studied elements and process the data, i.e., read out intensities of analytical lines and the background in the vicinity of these lines. For the OES acquisition, an integration time of 500 ms was used. Profiles of the most prominent and free from spectral interference atomic emission lines of studied elements, i.e., Zn at 213.9 nm, Fe at 248.3 nm, Mg at 285.2 nm, Cu at 324.8 nm, Mn at 403.1 nm, Ca at 422.7 nm, Li at 670.8 nm, Na at 589.0 nm, K at 766.5 nm and Rb at 780.0 nm, were recorded with a resolution of 0.02 nm. For each element, profiles of its analytical line were acquired 5 times while single intensities were averaged.
2.3. FAES
A PerkinElmer (MA, USA) single-beam atomic absorption spectrometer, model 1100 B, with a deuterium lamp for background correction was used as a reference method in the measurement of concentrations of K and Na by FAES. The fuel-lean air–C2H2 flame used was maintained in a single-slot 10 cm burner head using a flow rate of air of 8.0 L min−1 and a flow rate of C2H2 of 1.4 L min−1. Solutions of standards and samples were introduced into the flame using a stainless steel nebulizer mounted onto a burner/spray chamber, integrated with an end-cup and a drain assemblage. No flow spoiler was used to increase the sensitivity of measurements. The instrument was operated under conditions recommended by a manufacturer for K and Na and included the wavelength of 766.5 nm (K) and 589.0 nm (Na), the spectral resolution of 0.4 nm (K) and 0.2 nm (Na) and the lamp current of 15 mA. For calibration, 5 simple aqueous standard solutions were used with concentrations within the 0.01–2 mg L−1 range. For each measurement cycle, 3 absorbance read-outs were made over a 3 s integration time and averaged (a time-average integration mode).2.4. Procedures
Prior to FAES measurements of K and Na, samples of selected honeys, i.e., acacia honey from CD Inc. (XA) and heather honey from Sadecki Bartnik (ZH), were wet digested in a open-vessel system. Samples of honeys (2.0 g) were treated with 10.0 mL of concentrated HNO3 and heated on a hot plate at 80–90 °C for 2 h to reduce the volume to about 1 mL. Aliquots left were reconstituted with water and diluted to 20.0 mL. Concentrations of K and Na in such prepared 5.0% m/v sample solutions were determined by FAES against simple standard solutions. Prior to measurements, sample solutions were appropriately diluted. For each honey, 3 parallel samples were prepared and analyzed in addition to respective blank samples.In the case of dc-APGD-OES, 1.0% m/v honey solutions of honeys were prepared by dissolving appropriate amounts of analyzed samples (1.0 g per 100 mL) and acidified with a concentrated HCl solution to a concentration of 0.1 mol L−1. The addition of Triton X-405 was not necessary in this case. In such prepared sample solutions, concentrations of K and Na were determined using 5 simple standard solutions within concentration ranges of 0.5–40 mg L−1 (K) and 0.1–10 mg L−1 (Na). Other elements, i.e., Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn, were determined in 5.0% m/v solutions of honey (5.0 g per 100 mL). As before, these solutions were acidified with HCl and Triton X-405 at a concentration of 4.0 mmol L−1 (its 5× CMC) was added to modify the FLC solution. For quantification, 5 matrix-matching standard additions, containing 40 g L−1 of total glucose and fructose and 30 mg L−1 of total K and Na, were used. For each honey, 3 independent samples were prepared and measured in addition to analysis of respective blank samples.
3. Results and discussion
Honey is a complex sample with a matrix rich in organic matter. Multi-elemental analysis of honey samples by inductively coupled plasma optical emission spectrometry (ICP-OES) is commonly preceded by their calcination using wet or dry ashing.20,21 Simplified sample preparation of honey prior to its spectrochemical analysis, e.g., by dissolving respective samples in water only and acidifying, is infrequently reported,18 although it has certainly a special significance in view of analysis simplicity. As previously reported, the concentration of honey in solutions prepared by dissolving respective samples in water is limited since a high organic content, i.e., >2–5% m/v, may substantially increase the viscosity of solutions and markedly affect the sensitivity of measurements, primarily due to the formation of C-containing compounds.14,20 Even when analyzed solutions contain dissolved honey in the amount of 1–2% m/v, intense matrix effects could be present and hence, simple standard calibration cannot be used.203.1. Effect of the honey-like matrix on the response of elements
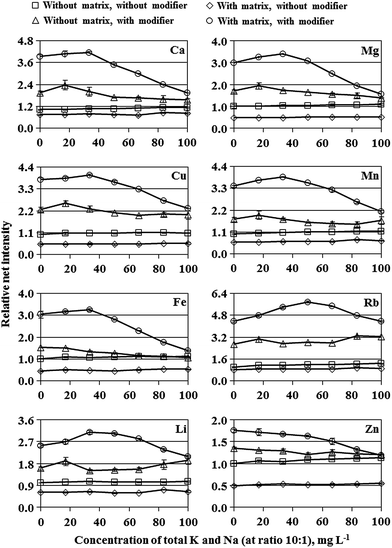
To evaluate the suitability of dc-APGD-OES for determining Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn in solutions of honey samples dissolved only in water (without any pre-digestion), the effect of a honey-like matrix, containing fructose and glucose, was examined at first. Both simple carbohydrates are almost equally present in honey at an average concentration of 0.80 g g−1.22 Therefore, a 5.0% m/v solution of such a honey-like matrix should contain 20 g L−1 of fructose and 20 g L−1 of glucose (40 g L−1 in total). The effect of such a 5.0% m/v honey-like matrix, present in solutions of Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn, on the background corrected (net) intensities of analytical lines of these elements versus an increasing concentration of total K and Na (present in solutions at a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) up to 100 mg L−1 was studied. A blank used for this experiment was a solution containing the honey-like matrix but no elements. For comparison purposes, the effect of the increasing concentration of total K and Na on the response of Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn was also studied in conditions with an added modifier of the FLC, i.e., a non-ionic surfactant Triton X-405 at a concentration of its 5× CMC, and with or without the mentioned 5.0% m/v honey-like matrix in solutions. Net intensities of analytical lines of Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn were measured for 3 independently prepared solutions and averaged. Blanks for this experiment were a solution containing Triton X-405 only or a solution with both the surfactant and the honey-like matrix but with no elements. For simplicity, for each analytical line measured in different experimental conditions, its net intensities were related to the net intensity acquired for a solution without the honey-like matrix, K with Na and Triton X-405, and expressed as the relative intensity.
1) up to 100 mg L−1 was studied. A blank used for this experiment was a solution containing the honey-like matrix but no elements. For comparison purposes, the effect of the increasing concentration of total K and Na on the response of Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn was also studied in conditions with an added modifier of the FLC, i.e., a non-ionic surfactant Triton X-405 at a concentration of its 5× CMC, and with or without the mentioned 5.0% m/v honey-like matrix in solutions. Net intensities of analytical lines of Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn were measured for 3 independently prepared solutions and averaged. Blanks for this experiment were a solution containing Triton X-405 only or a solution with both the surfactant and the honey-like matrix but with no elements. For simplicity, for each analytical line measured in different experimental conditions, its net intensities were related to the net intensity acquired for a solution without the honey-like matrix, K with Na and Triton X-405, and expressed as the relative intensity.
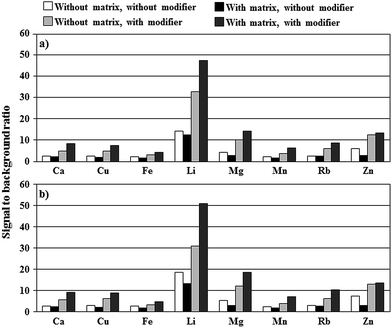
As can be seen from Fig. 2, the presence of fructose and glucose results in decreasing net intensities of analytical lines of studied elements by 50% (Cu, Fe, Mg, Zn), 35% (Li, Mn), 25% (Ca) and 15% (Rb) as compared to net intensities recorded in conditions without the 5.0% m/v honey-like matrix. SBR values for these analytical lines of studied elements were either decreased by 20–40% (Cu, Fe, Li, Mn), 120–140% (Mg, Zn) or remained unchanged (Ca, Rb) (see Fig. 3a). Changes in the response of studied elements versus the concentration of total K and Na increasing up to 100 mg L−1 were rather slight (see Fig. 2). Both, with and without the honey-like matrix in solutions, relative net intensities of analytical lines of studied elements were maximally, i.e., at a concentration of total K and Na of 100 mg L−1, decreased by 10–15% (Ca, Fe, Mg, Mn, Zn) or less than 10% (Cu, Li). An exception was Rb, for which the mentioned increase in the concentration of total K and Na up to 100 mg L−1 led to a rise of the relative net intensity by about 10 or 20%, respectively, in the case of the presence or the absence of the honey-like matrix in solutions.
It was also verified that when the concentration of the honey-like matrix in solutions of studied elements was 2.5 or 10% m/v, which corresponds to the presence of fructose and glucose at a concentration of 40 or 160 g L−1 in total, a decrease in net intensities of analytical lines of studied elements was, correspondingly, about 2 times lower or 2 times higher than that noted for the 5.0% m/v honey-like matrix. This indicated that the suppression of signals of studied elements observed for dc-APGD generated in contact with FLC solutions containing fructose with glucose was strictly related to an increase in the viscosity of these solutions. Considering the morphology of emission spectra of dc-APGD loaded with 2.0, 5.0 and 10% m/v solutions of the honey-like matrix, no emission bands of molecules containing C atoms were identified.
3.2. Effect of the addition of Triton X-405
The addition of Triton X-405 at a concentration of its 5× CMC to solutions of studied elements, but without the 5.0% m/v honey-like matrix, was quite beneficial because it caused an increase in net intensities of analytical lines of studied elements by 1.4- (Zn), 1.5- (Fe), 1.6- (Li), 1.7- (Mg, Mn), 1.9- (Ca), 2.2- (Cu) and 2.7-fold (Rb) as compared to net intensities acquired in conditions with no non-ionic surfactant added to FLC solutions. Observed enhancements corresponded well to those recently reported,5 however, it should be borne in mind that the design of the present discharge system is changed.Surprisingly, the addition of Triton X-405 (at its 5× CMC) to solutions containing the 5.0% m/v honey-like matrix led to much higher enhancements of the net intensities of analytical lines of studied elements than without it. Accordingly, the relative net intensities were equal to 1.8 (Zn), 2.5 (Li), 3.0 (Fe, Mg), 3.4 (Mn), 3.8 (Cu), 3.9 (Ca) and 4.3 (Rb). As can be seen from Fig. 3a, SBR values are also increased in these conditions correspondingly by about 3 (Fe), 4 (Ca, Cu, Li, Mn, Rb), and 5 times (Mg, Zn). When Triton X-405 was added to 5.0% m/v honey-like matrix solutions at lower concentrations, i.e., 1× CMC or 2× CMC, observed enhancements of net intensities of analytical lines of studied elements were lower. At present, the mechanism of a synergistic effect observed for fructose with glucose and the added non-ionic surfactant is difficult to explain, however, it appears that the addition of Triton X-405 to 5.0% m/v honey-like matrix solutions results in increased efficiency of transportation of elements to phases of the discharge. As a result, net intensities of analytical lines of studied elements were enhanced more than 7 (Cu), 6 (Fe, Mg, Mn), 5 (Ca, Rb) and 4 times (Li and Zn) as compared to those recorded in conditions when FLC solutions contained only the 5.0% m/v honey-like matrix, suppressing the response of studied elements.
As can be seen from Fig. 2, an increase in the concentration of total K and Na in solutions to 30 mg L−1 causes a slight enhancement of relative net intensities of analytical lines of all elements except for Zn. Above this concentration, a gradual fall in the response of all elements can be noted. The behavior of SBR values for studied analytical lines was quite similar in these conditions. Fig. 3b gives SBRs for analytical lines of studied elements for conditions when 30 mg L−1 of total K and Na was present in the 5.0% m/v honey-like matrix solution along with the added Triton X-405 modifier. It is evident that the presence of K with Na leads to a slight increase in SBR values, i.e., by about 7% (Fe) to 14% (Rb). At concentrations of total K and Na higher than 30 mg L−1, relative net intensities and SBRs were established to gradually deplete.
In conclusion, it appears that the use of Triton X-405 would enable us to determine studied elements in 5.0% m/v solutions of honeys using external calibration with matrix-matching standard solutions. Considering the morphology of emission spectra of dc-APGD loaded with 5.0% m/v solutions of the honey-like matrix with and without added Triton X-405 (see Fig. 4), it was found that the use of the non-ionic surfactant was responsible for extinguishing emission bands of NO, OH and N2 molecules. Interestingly, in the presence of 10% m/v solutions of the honey-like matrix, the addition of Triton X-405 at its 5× CMC also resulted in enhanced net intensities of analytical lines of studied elements. Accordingly, relative net intensities of these lines were by about 30–40% higher than those acquired for solutions containing the 5.0% m/v honey-like matrix, however, the handling of such viscous solutions was rather inconvenient. Therefore, in further experiments, Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn were determined in analyzed honeys using their 5.0% m/v solutions.
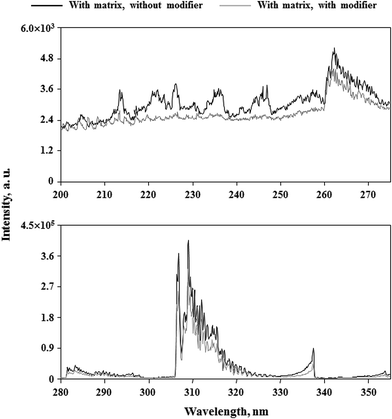 | ||
| Fig. 4 Effect of the addition of Triton X-405 (5× CMC) to the FLC on the morphology of the emission spectrum of dc-APGD loaded with a solution containing 40 g L−1 of fructose and glucose in total. | ||
3.3. Determination of K and Na
Due to the relatively high concentrations of K and Na in analyzed white- to amber-colored honeys as well as sensitivities of analytical lines of K and Na, assessed when measuring 0.5 and 1.0% m/v solutions of the honey-like matrix, the use of more concentrated solutions and Triton X-405 as the FLC modifier was not necessary. Indeed, it was verified that the presence of 1.0% m/v honey-like and honey matrices had no effect on the sensitivities of analytical lines of both elements. The slopes of calibration curves (in a.u. per μg per L) determined for simple water standard solutions, e.g., 27.1 ± 0.2 for the KI 766.5 nm line and 420 ± 12 for the NaI 589.0 nm line, did not statistically differ from the slopes of calibration curves obtained for 1.0% m/v solutions of the honey-like matrix and 1.0% m/v solutions of the honey matrices (acacia XA and heather ZH honeys). However, linearity ranges for both elements were different. In the case of K, calibration curves were linear up to 100 mg L−1 (R2 = 0.9986–0.9999, depending on the matrix), while in the case of Na, the upper linearity range was 40 mg L−1, while the coefficients of determination (R2) for different matrices were varied within 0.9945–0.9967.Because of this, it was decided that 1.0% m/v solutions of analyzed honeys would be prepared for the quantification of their K and Na contents by dc-APGD-OES using simple standard solutions for calibration. To confirm the reliability of results obtained with this methodology, two honeys were selected, i.e., acacia XA and heather ZH, and their samples were analyzed by dc-APGD-OES (after simple water dissolution and acidification with HCl to 0.1 mol L−1) and FAES (after wet oxidative digestion). The results of both analyses are given in Table 1. They correspond well to the results obtained in other Polish honeys.18,23,24 As it can be seen, good agreement between the results obtained with both methods was achieved. Standard errors between concentrations of K and Na determined by both methods were within −6.8 to 2.4%. In addition, a recovery study was carried out. Accordingly, K and Na were added to both selected honeys to double their original concentrations and then, respective samples were prepared and the resulting solutions were measured using dc-APGD-OES against simple standard solutions. Recoveries of added K and Na for both honeys were found quantitative, confirming that results of measurements of K and Na concentrations with the proposed methodology are reliable. The precision was also good since RSDs were in the range of 0.4 to 4.9% in the case of K and from 0.5 to 4.5% in the case of Na. The capability of the method was also evaluated through determining the LODs of K and K. In this case, 1.0% m/v solutions of the honey-like matrix were used.
| Honey | Concentration,c μg g−1 | |||
|---|---|---|---|---|
| Ca | K | Mg | Na | |
| a Results obtained analyzing dissolved samples by dc-APGD-OES with calibration by 3 standard solution additions (Ca and Mg) and digested samples by FAES with calibration by simple standard solutions (K and Na). b Recoveries (in %). XA: 35.0, 200, 10.0 and 10.0 μg g−1 of Ca, K, Mg and Na, respectively, were added. ZH: 50.0, 1000, 10.0 and 20.0 μg g−1 of Ca, K, Mg and Na, respectively, were added. c Average value (n = 3) ± standard deviation. | ||||
| XA | 36.0 ± 1.5 | 184 ± 8 | 11.2 ± 0.6 | 9.29 ± 0.24 |
| XAa | 37.2 ± 1.9 | 180 ± 4 | 11.5 ± 0.3 | 8.92 ± 0.18 |
| XAb | 96.5 ± 3.5 | 101 ± 6 | 96.5 ± 5.5 | 104 ± 9 |
| XB1 | 27.0 ± 3.9 | 91.7 ± 1.5 | 9.26 ± 0.38 | 269 ± 4 |
| XB2 | 43.3 ± 0.5 | 242 ± 10 | 13.1 ± 0.9 | 11.2 ± 0.1 |
| XH | 58.4 ± 1.7 | 564 ± 27 | 22.2 ± 1.1 | 38.7 ± 0.7 |
| XL | 61.8 ± 1.0 | 633 ± 14 | 17.9 ± 0.3 | 96.1 ± 0.5 |
| XM | 63.7 ± 0.2 | 334 ± 76 | 16.6 ± 1.9 | 69.8 ± 1.9 |
| YA | 15.0 ± 0.2 | 225 ± 7 | 6.00 ± 0.15 | 23.2 ± 0.6 |
| YB | 45.6 ± 0.2 | 224 ± 4 | 13.8 ± 1.5 | 25.4 ± 0.3 |
| YL | 74.5 ± 0.4 | 873 ± 22 | 21.4 ± 0.3 | 25.2 ± 0.6 |
| YM | 67.4 ± 0.8 | 412 ± 10 | 16.9 ± 0.3 | 20.4 ± 0.5 |
| ZA | 16.8 ± 1.9 | 195 ± 1 | 6.54 ± 0.29 | 5.50 ± 0.22 |
| ZB | 29.9 ± 0.7 | 334 ± 8 | 14.5 ± 0.1 | 9.95 ± 0.45 |
| ZH | 51.7 ± 3.1 | 1066 ± 26 | 19.4 ± 0.7 | 19.7 ± 0.8 |
| ZHa | 52.9 ± 1.2 | 1040 ± 15 | 20.7 ± 1.6 | 21.1 ± 0.3 |
| ZHb | 99.0 ± 4.0 | 94.9 ± 4.8 | 96.0 ± 7.0 | 108 ± 5 |
| ZL | 58.7 ± 3.3 | 767 ± 23 | 17.8 ± 0.4 | 9.32 ± 0.39 |
| ZM | 50.2 ± 2.3 | 476 ± 9 | 19.5 ± 0.8 | 12.3 ± 0.3 |
| ZR | 54.3 ± 4.7 | 208 ± 8 | 17.1 ± 0.3 | 9.05 ± 0.13 |
A complete set of validation parameters evaluated for dc-APGD-OES, as an alternative method of analysis of honey without necessity of its mineralization, is given in Table 2.
| Element | Sensitivity, a.u. per μg L¬1 | Upper linearity range, mg L−1 | R 2 | Limit of detection (3σ), μg L−1 | Limit of detectiona, μg g−1 | Precisionb, % |
|---|---|---|---|---|---|---|
| a Calculated for 1.0 g (K, Na) or 5.0 g (Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn) of honey dissolved in 100 mL of water. b As relative standard deviation (n = 3) for solutions containing 1.0 mg L−1 of studied elements. c Assessed using 5.0% m/v honey-like matrix-matching standard solutions (40 g L−1 of total glucose and fructose, 30 mg L−1 of total K and Na). d Assessed using 1.0% m/v honey-like matrix-matching standard solutions (8.0 g L−1 of total glucose and fructose). | ||||||
| Cac | 5.18 | 10 | 0.9993 | 49 | 0.98 | 2.0 |
| Cuc | 85.4 | 10 | 0.9973 | 33 | 0.66 | 3.2 |
| Fec | 1.77 | 10 | 0.9991 | 125 | 2.5 | 3.4 |
| Kd | 27.1 | 100 | 0.9990 | 4.6 | 0.46 | 3.7 |
| Lic | 220 | 10 | 0.9969 | 0.75 | 0.015 | 1.7 |
| Mgc | 324 | 10 | 0.9974 | 7.8 | 0.16 | 1.8 |
| Mnc | 4.13 | 10 | 0.9989 | 88 | 1.8 | 0.7 |
| Nad | 420 | 40 | 0.9999 | 0.37 | 0.037 | 2.8 |
| Rbc | 25.6 | 10 | 0.9956 | 5.2 | 0.10 | 3.9 |
| Znc | 32.8 | 5.0 | 0.9959 | 8.0 | 0.16 | 4.8 |
3.4. Determination of Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn
Concentrations of Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn were measured in analyzed honeys using standard solutions with the matrix matching in relation to an average amount of fructose and glucose (40 g L−1 in total) and an average concentration of total K and Na (30 mg L−1) in 5.0% m/v solutions of honeys. The results of this analysis are given in Tables 1 (Ca and Mg) and 3 (Cu, Fe, Li, Mn, Rb and Zn) and they well correspond to those reported for other Polish honeys.18,23,24 Concentrations of Cu and Li in examined honeys were established to be lower than respective LODs of these elements, i.e., 0.7 and 0.02 μg g−1. The precision (as the RSD) of measurements was satisfactorily good and varied in the majority of the cases in the following ranges: 0.3–8.7% (Ca), 6.7–12% (Fe), 0.7–6.9% (Mg), 5.2–6.3% (Mn), 1.8–8.2% (Rb), and 0.8–8.7% (Zn). Occasionally, in cases of 1 to 3 honeys out of 16, these values were higher, i.e., within 11–18%.| Honey | Concentration,d μg g−1 | |||||
|---|---|---|---|---|---|---|
| Cu | Fe | Li | Mn | Rb | Zn | |
| a Results obtained analyzing dissolved samples by dc-APGD-OES with calibration by 3 standard solution additions. b Recoveries (in %). XA: 2.0, 5.0, 1.0, 5.0, 1.0 and 10.0 μg g−1 of Cu, Fe, Li, Mn, Rb and Zn, respectively, were added. ZH: 2.0, 5.0, 1.0, 5.0, 10.0 and 1.0 μg g−1 of Cu, Fe, Li, Mn, Rb and Zn, respectively, were added. c NA = not analyzed. d Average value (n = 3) ± standard deviation. | ||||||
| XA | <0.7 | <2.5 | <0.02 | <1.8 | 0.37 ± 0.02 | 8.36 ± 0.38 |
| XAa | NAc | NAc | NAc | NAc | 0.35 ± 0.03 | 8.86 ± 0.20 |
| XAb | 102 ± 3 | 102 ± 3 | 100 ± 4 | 98.5 ± 3.5 | 100 ± 2 | 92.5 ± 1.5 |
| XB1 | <0.7 | 32.9 ± 2.2 | <0.02 | <1.8 | 0.43 ± 0.02 | 7.15 ± 0.17 |
| XB2 | <0.7 | 9.81 ± 1.16 | <0.02 | <1.8 | 1.04 ± 0.03 | 3.90 ± 0.23 |
| XH | <0.7 | <2.5 | <0.02 | 5.38 ± 0.34 | 5.45 ± 0.27 | 6.02 ± 0.27 |
| XL | <0.7 | <2.5 | <0.02 | <1.8 | 0.92 ± 0.03a | 4.04 ± 0.35 |
| XM | <0.7 | <2.5 | <0.02 | <1.8 | <0.10 | 2.97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11 0.11 |
| YA | <0.7 | <2.5 | <0.02 | <1.8 | 0.36 ± 0.04 | 0.63 ± 0.03 |
| YB | <0.7 | 8.04 ± 1.33 | <0.02 | <1.8 | 0.39 ± 0.07 | 1.36 ± 0.02 |
| YL | <0.7 | <2.5 | <0.02 | <1.8 | 1.06 ± 0.05a | 1.24 ± 0.09 |
| YM | <0.7 | <2.5 | <0.02 | <1.8 | <0.10 | 1.26 ± 0.01 |
| ZA | <0.7 | <2.5 | <0.02 | <1.8 | 0.31 ± 0.01 | 1.51 ± 0.23 |
| ZB | <0.7 | <2.5 | <0.02 | <1.8 | 0.85 ± 0.07 | 19.0 ± 0.8 |
| ZH | <0.7 | <2.5 | <0.02 | 6.90 ± 0.36 | 13.8 ± 1.9 | 0.98 ± 0.12 |
| ZHa | NAc | NAc | NAc | 6.80 ± 1.03 | 9.47 ± 0.88 | 0.97 ± 0.06 |
| ZHb | 109 ± 4 | 105 ± 1 | 140 ± 2 | 102 ± 2 | 145 ± 6 | 102 ± 2 |
| ZL | <0.7 | <2.5 | <0.02 | <1.8 | 0.92 ± 0.02a | 2.66 ± 0.21 |
| ZM | <0.7 | <2.5 | <0.02 | <1.8 | 0.57 ± 0.01 | 10.8 ± 0.2 |
| ZR | <0.7 | <2.5 | <0.02 | <1.8 | <0.10 | 1.23 ± 0.06 |
To verify the validity of the results achieved with the proposed methodology, a recovery study was carried out for acacia XA and heather ZH honeys. Samples were spiked with standard solutions of studied elements to roughly double their original concentrations, then, respective 5.0% m/v sample solutions were prepared and analyzed by dc-APGD-OES against matrix-matching standard solutions. Recoveries found for acacia XA honey were in the range of 92.5% (Zn) to 102% (Cu, Fe). In the case of heather ZH honey, except for Li and Rb, recoveries changed from 96.0% (Mg) to 109 ± 2% (Cu). Higher recoveries were established for Li (140%) and Rb (145%), however, heather ZH honey contains the highest concentration of total K and Na out of all studied honeys. In these conditions, the matrix matching used was inadequate and, likely due to an ionization buffering effect, intensities of atomic emission lines of Li and Rb were enhanced by about 40% (Li) and 45% (Rb). Additionally, concentrations of Ca, Mg, Rb and Zn in acacia XA honey as well as Ca, Mg, Mn, Rb and Zn in heather ZH honey were determined by dc-APGD-OES and the developed methodology but using 3 standard solution additions for calibration (results are given in Tables 1 and 3). As it can be seen, both calibration strategies give comparable concentrations of studied elements in both honeys. This proves that the results of analysis of 5.0% m/v solutions of honeys, resulted from the dissolution of their samples in water only and acidification with HCl, and made by dc-APGD-OES in conditions of Triton X-405 added to them, are dependable. As expected, the only exception was the content of Rb in heather ZH honey, which was by about 45% lower as compared to this determined when matrix-matching standard solutions were used. This was due to the mentioned ionization buffering, and hence, concentrations of Rb in lime XL, YL and ZL honeys, where concentrations of total K and Na in their 5.0% m/v solutions were higher than 30 mg L−1, were determined by the method of 3 standard solution additions. In addition to this calibration method, a higher concentration of total K and Na in matrix-matching solutions or a separate ionization buffer, i.e., CsCl as in flame atomic absorption spectrometry (FAAS), could be used as well, giving accurate results.
Other figures of merit of dc-APGD-OES for Ca, Cu, Fe, Li, Mg, Mn, Rb and Zn are given in Table 2. They demonstrate a good analytical performance of the method proposed in reference to LODs, changing from 0.8 μg g−1 for Li to 125 μg g−1 for Fe, and precision, changing from 0.7% for Mn to 4.8% for Zn.
4. Conclusions
This work demonstrated a compact discharge system for miniaturized dc-APGD generated in contact with the FLC for simplified OES elemental analysis of honey. Using Triton X-405 as a modifier of the FLC, the described dc-APGD excitation source along with the OES detection was successfully used for the direct determination of Ca, Cu, Fe, K, Li, Mg, Mn, Na, Rb and Zn in solutions only prepared by dissolving appropriate samples of honeys in water and acidifying with HCl to a concentration as required for electrical conductivity of the FLC. The methodology proposed enabled us to measure all studied elements with relatively high sensitivity, good precision and fair limits of detection. Giving dependable results in the elemental analysis of the complex honey matrix, the developed method could also be used as a viable alternative for fast and convenient analysis of other sugar-rich samples, i.e., syrups, nectars and juices.Acknowledgements
The work was financed by a statutory activity subsidy from Polish Ministry of Science and Higher Education for the Faculty of Chemistry of Wroclaw University of Technology. Krzysztof Greda is thankful for a fellowship co-financed by European Union within European Social Fund.References
- K. Greda, P. Jamroz and P. Pohl, J. Anal. At. Spectrom., 2014, 29, 893–902 RSC.
- K. Greda, P. Jamroz and P. Pohl, J. Anal. At. Spectrom., 2013, 28, 1233–1241 RSC.
- Z. Zhang, Z. Wang, Q. Li, H. Zou and Y. Shi, Talanta, 2014, 119, 613–619 CrossRef CAS PubMed.
- R. Shekhar, K. Madhavi, N. N. Meeravali and S. Jaikumar, Anal. Methods, 2014, 6, 732–740 RSC.
- K. Greda, P. Jamroz and P. Pohl, Talanta, 2013, 108, 74–82 CrossRef CAS PubMed.
- Q. Xiao, Z. Zhou, H. Zheng, H. He, C. Huang and S. Hu, Talanta, 2013, 106, 144–149 CrossRef CAS PubMed.
- R. Manjusha, M. A. Reedy, R. Shekhar and S. Jaikumar, J. Anal. At. Spectrom., 2013, 28, 1932–1939 RSC.
- T. A. Doroski and M. R. Webb, Spectrochim. Acta, Part B, 2013, 88, 40–45 CrossRef CAS PubMed.
- K. Greda, P. Jamroz and P. Pohl, J. Anal. At. Spectrom., 2013, 28, 134–141 RSC.
- R. Shekhar, Talanta, 2012, 93, 32–36 CrossRef CAS PubMed.
- T. A. Doroski, A. M. King, M. P. Fritz and M. R. Webb, J. Anal. At. Spectrom., 2013, 28, 1090–1095 RSC.
- A. J. Schwartz, S. J. Ray, E. Elish, A. P. Storey, A. A. Rubinshtein, G. C. Y. Chen, K. P. Pfeuffer and G. M. Hieftje, Talanta, 2012, 102, 26–33 CrossRef CAS PubMed.
- K. Gyorgy, L. Bencs, P. Mezei and T. Cserfalvi, Spectrochim. Acta, Part B, 2012, 77, 52–57 CrossRef CAS PubMed.
- P. Jamroz, P. Pohl and W. Zyrnicki, J. Anal. At. Spectrom., 2012, 27, 1032–1037 RSC.
- S. Bogdanov, M. Haldimann, W. Luginbuhl and P. Gallmann, J. Apicult. Res., 2007, 46, 269–275 CAS.
- J. M. Alvarez-Suarez, S. Tulipani, S. Romandini, E. Bertoli and M. Battino, Mediterr. J. Nutr. Metab., 2010, 3, 15–23 CrossRef PubMed.
- P. Pohl, H. Stecka, I. Sergiel and P. Jamroz, Food Anal. Methods, 2012, 5, 737–751 CrossRef.
- P. Pohl, Trends Anal. Chem., 2009, 28, 117–128 CrossRef CAS PubMed.
- S. K. Hait and S. P. Moulik, J. Surfactants Deterg., 2001, 4, 303–309 CrossRef CAS PubMed.
- T. M. F. F. Mendes, S. N. Baccan and S. Cadore, J. Braz. Chem. Soc., 2006, 17, 168–176 CrossRef PubMed.
- M. D. Ioannidou, G. A. Zachariadis and A. N. Anthemidis, Talanta, 2005, 65, 92–97 CAS.
- S. Bogdanov, T. Jurendic, R. Sieber and P. Gallmann, J. Am. Coll. Nutr., 2008, 27, 677–689 CrossRef CAS.
- M. Madejczyk and D. Baralkiewicz, Anal. Chim. Acta, 2008, 617, 11–17 CrossRef CAS PubMed.
- M. Chudzinska and D. Baralkiewicz, Food Chem. Toxicol., 2010, 48, 284–290 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |