Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evaluation and analysis of environmentally sustainable methodologies for extraction of betulin from birch bark with a focus on industrial feasibility†
Mikael E.
Fridén
a,
Firas
Jumaah
b,
Christer
Gustavsson
c,
Martin
Enmark
c,
Torgny
Fornstedt
c,
Charlotta
Turner
b,
Per J. R.
Sjöberg
a and
Jörgen
Samuelsson
*c
aUppsala University, Department of Chemistry – BMC, Analytical Chemistry, P.O. Box 599, 75124 Uppsala, Sweden
bLund University, Department of Chemistry, Centre for Analysis and Synthesis, P.O. Box 124, 22100 Lund, Sweden
cKarlstad University, Department of Engineering and Chemical Sciences, SE-651 88 Karlstad, Sweden. E-mail: jorgen.samuelsson@kau.se; Fax: +46 547001460; Tel: +46 547001620
First published on 20th August 2015
Abstract
Betulin from birch bark was extracted using two principally different extraction methodologies – classical Reflux Boiling (RB) and Pressurized Liquid Extraction (PLE). The extraction methods were analyzed based on both recovery and purity as well as for RB industrial feasibility. The purity and recovery for the different extraction methods were analyzed using High Performance Liquid Chromatography (HPLC) coupled with three different detection principles: Diode Array Detection (DAD), Mass Spectrometry (MS) and Charged Aerosol Detection (CAD). The chromatographic purity was determined by all detections whereas the DAD was used also for complementary gravimetric calculations of the purity of the extracts. The MS detection (in MS and MS/MS modes) was mainly used to characterize the impurities. Two steps to increase the purity of RB extracts were evaluated – pre-boiling the bark in water and precipitation by adding water to the extract. Finally, the methods were compared in terms of amounts of betulin produced and solvent consumed. The RB method including a precipitation step produced the highest purity of betulin. However, results indicate that PLE using three cycles with the precipitation step gives similar purities as for RB. The PLE method produced up to 1.6 times higher amount of extract compared to the RB method. However, the solvent consumption (liter solvent per gram product) for PLE was around 4.5 times higher as compared to the classical RB. PLE performed with only one extraction cycle gave results more similar to RB with 1.2 times higher yield and 1.4 times higher solvent consumption. The RB process was investigated on an industrial scale using a model approach and several important key-factors could be identified. The most energy demanding step was the recycling of extraction solvent which motivates that solvent consumption should be kept low and calculations show a great putative energy reduction by decreasing the ethanol concentration used in the RB process to lower than 90%.
Introduction
A vast amount of birch bark is obtained annually as a by-product from the forest industry; a recent estimate for only Sweden – the world's second largest producer of processed forest products – is 1–2 million m3 per year.1 The white outer bark contains high amounts of betulin (up to 30% of dry weight)2,3 together with low amounts of betulinic acid4 which could be used for other purposes than energy production which is currently the most common use.5 Betulin is widely used in cosmetics6 and is also a precursor for the synthesis of betulinic acid7 which has important medical properties, such as antitumor,8,9 anti-inflammatory10,11 and anti-HIV activities.12,13 Therefore, a cost-effective purification process for these compounds with low environmental impact is highly desirable. Life cycle assessment has been used to compare two methods for extraction of betulin from birch bark – leaching into ethanol at ambient temperature and extraction using liquid carbon dioxide (50 bar, 16 °C) with 20 wt% ethanol as a co-solvent.1 The latter was concluded as having a lower environmental impact. However, betulin was not purified in the extraction but just determined in terms of concentration in the ethanol extract.There are numerous studies exploring different extraction techniques for betulin in birch bark, including pressurized liquid extraction (PLE),14 supercritical fluid extraction (SFE),15 microwave assisted extraction (MAE),16 and classical reflux boiling (RB) or leaching.17 While PLE and MAE require special equipment, the increased temperature and pressure usually utilized is believed to allow for more exhaustive extraction of the target compounds as compared to classical RB. On the contrary, SFE is known as a more selective extraction technique.18 None of the published studies have calculated the energy usage necessary to compare different methodologies in terms of betulin production. Furthermore, only a few studies actually did purify betulin from the extract, and if they did, the method for determination of the purity varied largely, resulting in non-comparable or even erroneous results. For more information about the chemistry and general processing of birch bark, the reader is referred to the comprehensive review by P. Krasutsky.19
The required purity of the extracted target compound depends on the intended use of the final product. Plant extracts intended for use in medical products are regulated e.g. by the Food and Drug Administration in the USA and European Medicines Agency in the EU.20,21 Should the same extract be used as a chemical of technical quality, it does not have the same stringent requirements. In fact, “technical quality” does not seem to be a well-defined term; descriptions like “reasonable quality”22 and “do not have an established standard set for quality and impurity levels”23 are used by some major suppliers.
The aim of this study is to find a suitable strategy for producing betulin with an appropriate purity and quality while considering environmental aspects such as solvent and energy consumption. This involves first a careful analytical evaluation of promising and environmentally sustainable extraction principles (RB and PLE). Secondly, utilizing different detection principles such as mass spectrometry (MS), UV/vis spectroscopy (using a diode array detector, DAD) and charged aerosol detector (CAD) coupled with HPLC to determine the purity and recovery of the extraction methods. Finally, the industrial feasibility of the most promising extraction technique is investigated in more detail by establishing mass and energy balances for industrial scale extraction, and solvent recovery processes based on reliable experimental data. Additionally, SFE was tested as a potential extraction method but was not pursued further due to poor performance, see ESI.†
Results and discussion
In this study, a number of screening experiments were performed for extraction of betulin from birch bark utilizing RB and PLE and the results were evaluated based on recovery, purity and solvent consumption. Supercritical Fluid Extraction (SFE) was also tested but was dropped due to high solvent consumption, low recovery and no additional purity compared to PLE and RB, see ESI.† The most promising extraction method for scale-up was thereafter investigated in terms of industrial feasibility and environmental sustainability, taking mass and energy balances into account. In Fig. 1, the birch wood process is presented. The part of the process investigated in this study is marked with dashed lines.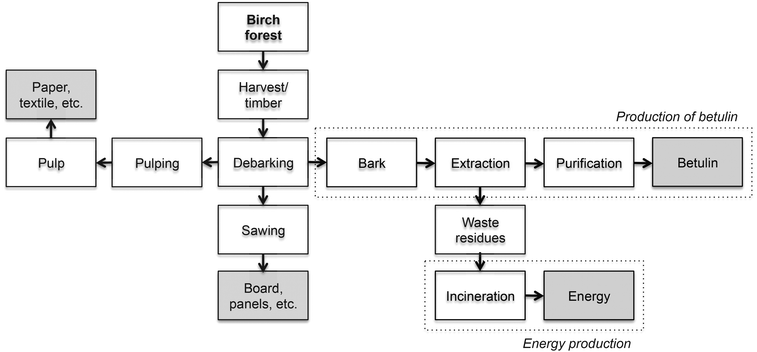 | ||
| Fig. 1 A schematic overview of how birch wood is processed and where our suggested extra process steps fit into the whole process. The investigated part of the process is marked with dashed lines. | ||
Determination of suitable conditions for extraction by reflux boiling
Several aspects were explored prior to the more in depth analytical investigations. First, two different solvents were considered for the RB experiments: ethanol and acetone. Acetone was rejected because it did not provide any apparent advantages regarding yield and purity compared to the more environmentally sustainable ethanol, see Table 1. Secondly, the suitable boiling time in the RB was evaluated based on purity and the extracted amount of betulin, see Table 1. 10 min boiling time was selected because only a minor increase in yield was observed for longer boiling times. Finally, ethanol RB with or without pre-boiling of bark in water to remove potential hydrophilic contaminations was evaluated. In Fig. 2, chromatograms from RB extracts purified by different means are presented. As can be seen, some impurities are removed with the pre-boiling step; 9 out of 42 peaks are removed and some more have been reduced to some extent (DAD data). However, the later precipitation step in the process is much more efficient in removing impurities; 26 out of 42 peaks are completely removed and the remaining peaks have been greatly reduced. Thus pre-boiling in water would not substantially increase the purity of the target compound further in this particular case (see ESI Tables S1–S3† for more information).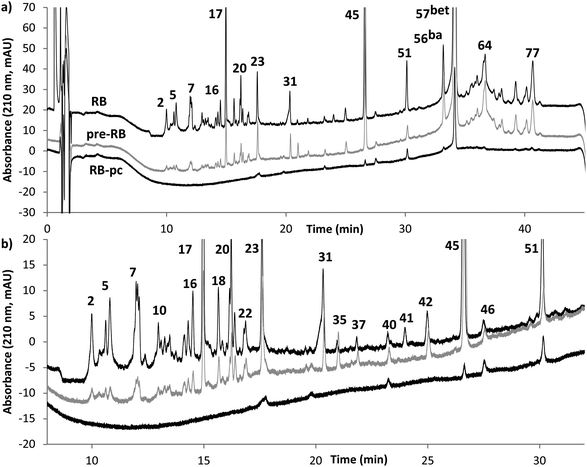 | ||
| Fig. 2 (a) Chromatograms recorded with DAD 210 nm after different means of purifying the extract; no purification (upper black line, RB), pre-boiling in water (grey line, pre-RB) and precipitation (lower black line, RB-pc). For increased clarity RB has been off-set by 20 mAU, and pre-RB by 5 mAU. Betulin is labeled bet and betulinic acid ba. (b) Magnification of part of the chromatograms in (a), with RB off-set by 5 mAU and pre-RB by 3 mAU. Peaks are labeled in chronological order, according to the retention times in ESI Table S7.† | ||
Determining purities and extraction efficiencies of the methods
Determination of purity is not trivial. For example, for UV-measurements it is required that the compounds have chromophoric groups whereas in MS the compound need to be chargeable or be charged to give a signal and for CAD the compound volatility should not be too high (must have lower volatility than the mobile phase). In addition, standard compounds are needed for both analytes and all the impurities. One possible exception is if CAD is used since this detector is considered being more generic, at least for non-volatile compounds. The purities using DAD, CAD, MS and gravimetric as well as the extraction efficiencies and solvent consumption are summarized in Table 2. The principal different methods for estimating the purity gave different results and in general, the more unselective detector CAD gave the highest purity, as discussed further below. The main aim with MS detection was not to evaluate the purity but instead to characterize the impurities in a more qualitative way and see how these patterns change for different extraction methods and operational settings.| Extraction method | Chromatographic purity, DAD (%) | Chromatographic purity, CAD (%) | Chromatographic purity, TIC (%) | Gravimetric purity (%) | Extracted amount (mg betulin per g bark) | Solvent consumption (l solvent per g betulin) |
|---|---|---|---|---|---|---|
| n.d., not determined. | ||||||
| Reflux boiling (n), ethanol | 72.9 ± 7.1 | 80.8 ± 0.5 | 39.5 ± 2.9 | 33.9 ± 3.8 | 51.6 ± 4.1 | 0.194 ± 0.015 |
| Reflux boiling (n), acetone | 76.1 ± 2.7 | 80.0 ± 2.0 | 32.3 ± 2.2 | 33.9 ± 3.4 | 44.6 ± 3.6 | 0.224 ± 0.018 |
| Reflux boiling (2), ethanol | 85.3 ± 3.6 | 87.1 ± 0.4 | 28.6 ± 3.6 | 60.9 ± 1.7 | 47.5 ± 7.5 | 0.210 ± 0.033 |
| Reflux boiling (2), acetone | 86.5 ± 2.6 | 86.5 ± 3.5 | 36.2 ± 1.6 | 59.3 ± 4.3 | 44.5 ± 1.7 | 0.225 ± 0.009 |
| Reflux boiling (3), ethanol | 78.7 ± 2.0 | 85.1 ± 1.5 | 33.5 ± 3.8 | 38.2 ± 3.6 | 48.3 ± 3.2 | 0.207 ± 0.014 |
| Reflux boiling (3), acetone | 80.6 ± 2.0 | 86.8 ± 0.1 | 43.2 ± 3.6 | 46.5 ± 9.5 | 45.2 ± 2.1 | 0.221 ± 0.010 |
| Reflux boiling (b), ethanol | 87.6 ± 2.8 | 86.4 ± 0.1 | 30.8 ± 4.4 | 62.8 ± 8.6 | 48.6 ± 9.4 | 0.206 ± 0.040 |
| Reflux boiling (b), acetone | 85.6 ± 3.8 | 90.0 ± 0.7 | 39.6 ± 2.2 | 60.1 ± 1.8 | 45.1 ± 2.3 | 0.222 ± 0.011 |
| PLE | 72.6 ± 4.0 | 77.9 ± 1.7 | 34.8 ± 7.2 | 17.0 ± 3.1 | 76.7 ± 8.1 | 0.980 ± 0.106 |
| PLE (1st cycle) | 73.3 ± 8.0 | 82.0 ± 1.5 | 57.5 ± 7.3 | 25.4 ± 4.0 | 62.5 ± 9.8 | 0.254 ± 0.040 |
| PLE (p, 1st cycle) | 66.5 ± 3.0 | n.d. | n.d. | 47.4 ± 0.3 | 58.8 ± 0.4 | 0.287 ± 0.002 |
In the ESI† we have detailed the data even more: the total ion chromatograms (TICs) and extracted ion chromatograms (XICs), can be found in Fig. S1† with peak data in Table S3† (RB extracts), Fig. S2† with peak data in Table S4† (PLE) and Fig. S3† with peak data in Table S5† (SFE). Triterpenes previously reported in birch bark are for example betulinic aldehyde, betulone, betulonic acid, betulonic aldehyde and lupeol and β-amyrin.3,19 Among the impurities are compounds showing similar fragmentation pattern as betulin, betulinic acid and the triterpenes mentioned.24 For example peak 17 is assigned as betulonic acid, however the substituent (230 Da, at m/z 685) was only observed in one replicate. Furthermore, two peaks show signals matching betulin with substituents: peaks 14 (betulin with a 26 Da substituent, m/z 469) and 19 (betulin with a 75 Da substituent, m/z 518). Peak 19 also shows a signal which probably is a water adduct (m/z 461) or from the loss of parts of the substituent mentioned above. Detailed MS and MS/MS data of betulin, betulinic acid and some of the major impurities along with possible identities are shown in ESI, Table S6.†
Purities determined by gravimetric analysis (see the procedure in the ESI†) gave lower values than those obtained both by DAD and CAD, indicating that there could be several impurities not detected by the detectors, see Table 2. Many times this is observed because the contaminations are unsuitable for one or more of the detectors, such as lack of a chromophore for DAD or poor ionization for MS, or because they occur at levels too low to give a response which would be integrated (see ESI†). Table 2 shows that the precipitation step results in the highest purity, both for RB and PLE. In order to investigate where the observed extra contaminations in the gravimetric analysis originate from, the precipitate from the RB was dissolved in ethanol, filtered and precipitated again. The analysis of this sample showed to have similar purity both for gravimetric and DAD. This clearly indicates that insoluble material, which was also observed in the filter (probably dust and cellulose), are present in the first precipitate. Because CAD, DAD and MS methods are based on chromatographic separations these contaminations are lost in the sample preparation filtration steps, and as a consequence the estimated purity is overestimated. To improve the process, a finer filter after the leaching step was used (102 Double Ring filter paper). The gravimetric purity with the additional filtration step without water pre-boiling was determined to be 60.2% and 63.1% after the precipitation step. These gravimetric purities are similar to the ones observed with birch bark pre-boiled in water after the precipitation step, see Table 2. Fourier transform infrared spectroscopy (FTIR) analysis showed that the spectral library similarity against wood decreases and similarity against betulin increases with process steps and filtration followed by precipitation had the same quality using birch bark with or without the pre-boiling step. In Fig. S4† photos of dried betulin process fluids or precipitates are presented for morphological comparison.
When the RB extracts purified by precipitation are compared before and after the precipitation step the gravimetric purity is significantly increased while only a slight (not statistically significant) decrease in the extracted amount occurs, based on analysis of variance (ANOVA) (data not shown). This indicates that the purification should be performed on the other extracts as well. ANOVA (data not shown) also suggests that the additional pre-boiling step affects neither the extracted amount nor the final purity to a notable extent (comparing extracts purified only by precipitation to extracts purified by both pre-boiling and precipitation). As a consequence we draw the conclusion that the pre-boiling step could be omitted from the final process without a reduction in purity, assuming precipitation is performed.
The greatest extracted amount was obtained by PLE using three cycles, reaching a total of 79 mg per g bark, see Table 2. However, the amount of betulin produced is approximately 1.6 times more than RB but with a solvent consumption (liter solvent per gram product) of 4.5 times higher. The value obtained for each cycle could be of interest if a thorough analysis of the economic feasibility for scaling up should be investigated, as it would then be possible to determine if only one or two cycles should be used which would reduce solvent consumption. For instance, using only one extraction cycle in PLE and introducing a precipitation step resulted in the pure product (Fig. S5†) with 1.2 times higher yield and 1.4 times higher solvent consumption compared to RB. However, RB still outperforms PLE in terms of purity of the product, see Table 2. Both additional steps in RB (pre-boiling and precipitation) result in increased purity, with the exception for MS detection (which was mainly used for characterization of impurities as discussed above). None of these additional steps result in a significant loss of betulin so combining this with the data in ESI Table S7,† showing that many impurities are extracted by all three techniques, the potential of increasing the purity of PLE extract is apparent as can be seen in ESI Fig. S5.†
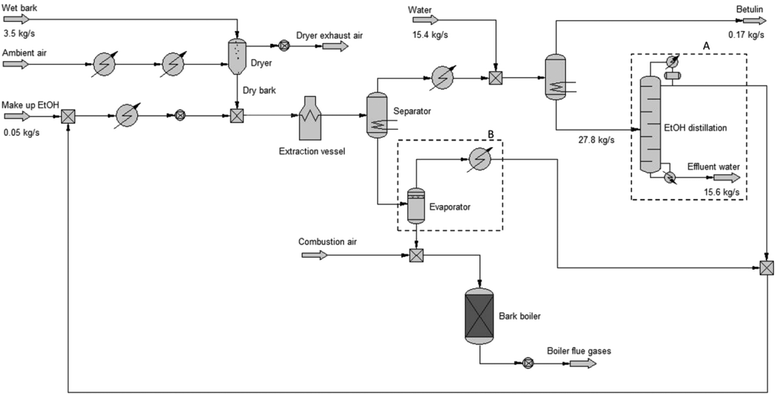
Industrial feasibility of the RB extraction process
The RB extraction process using ethanol was selected for the further modelling of industrial implementation as it performed well in terms of yield and purity and also because the process utilizes both low temperature (ca. 78 °C) and pressure (ambient). The mass and energy balances for RB extraction was established based on experimental data and the process configuration and production data from Gruvöns Mill, Sweden. The process sequences used in the laboratory extraction tests were modelled and applied to the total birch bark flow from the debarking process. After initial rough energy calculations, two additional unit operations were added to the simulation model compared to the laboratory tests in order to lend the simulated case more industrial relevance: (A) ethanol distillation enabling ethanol recirculation and therefore reducing the ethanol consumption by more than 40 tons per h and (B) ethanol evaporation from the bark prior to bark combustion, saving more than 10 tons per h ethanol. Furthermore, the latter adjustment is necessary as the ethanol-saturated bark would otherwise generate approximately 100 MW heat in the bark boiler, which would overload the bark boiler and impede combustion of softwood bark and other solid fuels generated on the site. The resulting model is shown in Fig. 3. For simulation, actual mill data and assumed parameters were used (cf. Table 3). The Wilson thermodynamic model was used as it gives good correlation with actual vapor–liquid equilibrium data for ethanol/water.25| Modelling assumptions | |
|---|---|
| Actual data from Gruvön Mill | |
| Birch bark production (tons per year dry solids) | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Bark dry content (%) | 50 |
| Biomass boiler design capacity (MWth) | 90 |
| Steam turbine | |
| Admission data (bara/°C) | 58/470 |
| Low pressure steam (turbine outlet) (bara) | 4.2 |
| Biomass moisture content (%) | 50 |
| Assumed data | |
| Biomass boiler flue gas temperature (°C) | 170 |
| O2 in boiler flue gases (vol% wet) | 6 |
| Steam turbine | |
| Isentropic efficiency | 0.80 |
| Atmospheric distillation | |
| No of ideal stages | 30 |
| Feed stage | 25 |
| Bottom ethanol concentration (% weight) | 0.1 |
| Calculated results | |
| Betulin production (tons per year) | 2500 |
| Distillation reboiler duty (MW) | 49.4 |
| Distillation condenser duty (MW) | 41.1 |
| Change in mill power production (MW) | 13.3 |
| Change in mill fuel consumption (wet tons per annum) | 259![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
The main results from the Chemcad simulations are shown in Table 3. As can be seen, adding the betulin extraction process to the mill entails significant new mass and energy streams. With the simulated process configuration, the total mill low-pressure steam demand increases to 93 tons per h, corresponding to 56 MW of additional heat usage. The combustion of the dried birch bark in the biomass boiler instead of the present wet bark only generates 4.5 tons per h additional steam. In order to generate the remaining 89 tons per h steam, the biomass consumption is increased by 9.0 kg s−1. The additional steam usage results in an additional power production of approximately 13.3 MW.
From an industrialization perspective, the large-scale betulin production outlined in this study could be considered viable from a mass handling perspective. However, for the actual mill the concept would be troublesome due to the high energy consumption, especially for ethanol recovery. An additional steam production of almost 90 tons per h cannot be provided without very significant investments in new boiler capacity. In order to identify a more feasible process concept the issue of the main heat consumer, the distillation reboiler, should be addressed. When, for example, the ethanol concentration from the distillation column increases from 90 to 95 wt%, the energy requirement increases from approx. 30 MW to 100 MW.
As shown above the major cost in the process is the recycling of ethanol. Extraction with a lower ethanol concentration than close to the azeotropic ethanol water mixture (95 vol%), used in this study would be clearly beneficial from an energy consumption point of view and should be tested in future work. Furthermore, a modified process configuration utilizing evaporation of the extract prior to the precipitation might be a feasible method to reduce the distillation load and should be verified in further laboratory trials. Finally, the potential for heat integration between streams within such a modified betulin process as well as the mill's secondary heat streams should be further investigated.
Experimental
Chemicals
For all extraction methods, 95% ethanol (Solveco, Rosersberg, Sweden) was used whereas in the RB method MilliQ water from a MilliQ plus system was also used (Millipore, Billerica, Massachusetts, USA).For the analysis methods LC-MS grade methanol and acetonitrile from Fisher Scientific (Västra Frölunda, Sweden) and water from Sigma Aldrich Chemie GmbH (Schnelldorf, Germany) were used respectively. Betulin, betulinic acid and progesterone (all ≥98% purity) were obtained from Sigma Aldrich Chemie GmbH and NaNO3 (≥99.5% purity) from Merck (Darmstadt, Germany).
Standard preparation
A stock solution of 0.540 mg per ml betulin was prepared in methanol and diluted to standards ranging from 0.003 to 0.070 mg ml−1. A standard reference sample was prepared containing 0.044 mg per ml betulin, 0.046 mg per ml betulinic acid and 0.032 mg per ml progesterone as the Internal Standard (IS).Sample preparation and handling
The bark from birch (Betula pendula) was collected at Gruvöns Mill (BillerudKorsnäs, Grums, Sweden) directly from the transport conveyor below the debarking machine. The collected bark was dried at room temperature for a week and thereafter chopped twice in a garden compost crusher. Finally, the processed bark pieces were frozen in a freezer at −18 °C followed by a “splash” of liquid nitrogen for making the bark brittle and thereafter directly processed in a food processor to obtain approximately 1 × 1 cm2 pieces.Extraction by classical reflux boiling (RB)
Three different types of experiments were performed to determine: (1) suitable boiling time followed by two alternative ways to increase the purity of the final product, either by (2) combining RB with precipitation by adding water to the extract or (3) pre-boiling the bark in water prior to extraction. All experiments were conducted at least in triplicates and stored in a refrigerator (+8 °C) until analysis; all processed samples were analyzed the same day. For details see ESI.†Pressurized liquid extraction (PLE)
The PLE conditions were based on a previous study, in which a maximized betulin yield was the aim.14 Extractions with 95% ethanol were performed using an ASE 200 (Thermo Fischer, Germering, Germany) equipped with a solvent controller. The extraction was done in triplicate and each extraction was performed in three cycles of 5 min each, with each cycle collected separately. Internal standard was added to monitor solvent losses during storage. The samples were stored at −18 °C until analysis. The procedure is described in more detail in the ESI.†Analysis
An Agilent 1100 series system (Agilent Technologies, Waldbronn, Germany) with a Kromasil C18 column (2.1 × 100 mm, with a nominal particle size of 3.5 μm, AkzoNobel, Bohus, Sweden) was used for separation of the extracts. The mobile phases consisted of LC-MS grade water (A) and acetonitrile (B), and a linear gradient of 5–95% B over 30 min followed by 10 min at 95% B was used. Three different detectors were used. A diode array detector (Agilent Technologies) was used at 210 nm to evaluate betulin content and purity (210–400 nm was recorded). A Corona ultra RS CAD (Dionex, Sunnyvale, CA) was also used for these purposes (gradient compensation was utilized to keep the composition of mobile phase reaching the detector constant). A Q-Trap 3200 mass spectrometer (AB Sciex, Concord, ON, Canada) operating in a positive ion mode with atmospheric pressure chemical ionization (APCI) was used to collect MS and MS/MS data with enhanced mass spectrometry (EMS) and enhanced product ion (EPI) experiments (100–1200 Th were recorded in both experiments).Determination of purity and amount of extracted betulin
Chromatographic purity utilizing the DAD (210 nm) and CAD was determined by taking the area ratio of the betulin peak to the total integrated area (excluding the contribution from IS if used) between 3 and 44 min (area threshold: 5, height threshold: 1 for DAD and area threshold: 2, height threshold: 0.3 for CAD). Since a gradient was employed for separation, blank subtraction was performed for the DAD data. In addition gravimetric purity was determined from the concentration obtained by DAD; the procedure is described in the ESI.† From the MS data, the total ion chromatogram was used to estimate the chromatographic purity and m/z from MS (Extracted Ion Chromatograms, XICs) and MS/MS were used to tentatively identify potential impurities, though most impurities were of very low intensity to yield any MS/MS signals.Calculations of mass and energy balances for industrial feasibility
Mass and energy balances for RB extraction were calculated based on experimental data and production data from Gruvöns Mill where the bark for the experiments were collected. Gruvöns Mill is an integrated paper mill with an annual production of 685 kton based on softwood and hardwood chemical pulping. Around 25% of the used wood is birch. Debarking of birch wood prior to pulping yields approximately 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons of dry substance bark per year. The material and heat balances process was modelled, using Chemcad 6.4.1 (Chemstations Europe GmbH, Berlin, Germany).
000 metric tons of dry substance bark per year. The material and heat balances process was modelled, using Chemcad 6.4.1 (Chemstations Europe GmbH, Berlin, Germany).
Conclusions
In this study we have extracted betulin from birch bark utilizing two different methods: reflux boiling (RB) and pressurized liquid extraction (PLE). The classical RB gave the highest purity of betulin. Our results show that PLE did not give better selectivity towards the product than RB. PLE with three cycles gave up to 1.6 times higher amount of extracted betulin as compared to RB. On the other hand, the solvent consumption per gram product for PLE was around 4.5 times higher as compared to RB. Using just the first cycle in the PLE process results in 1.4 times higher solvent consumption and 1.2 times higher amount of extracted betulin compared to RB. Therefore, PLE is likely a more energy-demanding and expensive process, since a larger amount of solvent is required in the process and more advanced equipment is used. One could recycle the solvent to reduce the cost, however, recycling ethanol is quite an energy-consuming process.26 We also found that pretreatment of birch bark with boiling water prior to the RB step to remove hydrophilic contaminations did remove contaminations in the process fluid (cf.Fig. 2); however, the highest impact on purification was accomplished by the introduction of a precipitation step.The optimized RB process was selected as a model for industrial calculations for 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of birch bark. The RB method is suitable for scale-up and a number of potential process modifications have been identified that would significantly improve the feasibility for large-scale purification. Among others it could be demonstrated that ethanol concentration from the distillation column <90% results in drastically decreasing energy consumption. A further techno-economical study is planned in which process parameters and heat integration will be optimized. Extraction tests will be carried out in order to verify that betulin yield and purity can be maintained. If successful, the proposed extraction and purification process can be used as a valorization process in the forest industry.
000 tons of birch bark. The RB method is suitable for scale-up and a number of potential process modifications have been identified that would significantly improve the feasibility for large-scale purification. Among others it could be demonstrated that ethanol concentration from the distillation column <90% results in drastically decreasing energy consumption. A further techno-economical study is planned in which process parameters and heat integration will be optimized. Extraction tests will be carried out in order to verify that betulin yield and purity can be maintained. If successful, the proposed extraction and purification process can be used as a valorization process in the forest industry.
Acknowledgements
This study was funded by the Swedish research council FORMAS; grant number 229-2009-1527.References
- A. Ekman, M. Campos, S. Lindahl, M. Co, P. Börjesson, E. N. Karlsson and C. Turner, J. Cleaner Prod., 2013, 57, 46 CrossRef CAS.
- M. M. Oconnell, M. D. Bentley, C. S. Campbell and B. J. W. Cole, Phytochemistry, 1988, 27, 2175 CrossRef CAS.
- R. Ekman, Holzforschung, 1983, 37, 205 CrossRef CAS.
- L. Holonec, F. Ranga, D. Crainic, A. Truta and C. Socaciu, Not. Bot. Horti Agrobot. Cluj-Napoca, 2012, 40, 99 CAS.
- W. Lu, J. L. Sibley, C. H. Gilliam, J. S. Bannon and Y. Zhang, J. Environ. Hortic., 2006, 24, 29 Search PubMed.
- J. Patočka, J. Appl. Biomed., 2003, 7 Search PubMed.
- A. Barthel, S. Stark and R. Csuk, Tetrahedron, 2008, 64, 9225 CrossRef CAS.
- V. Zuco, R. Supino, S. C. Righetti, L. Cleris, E. Marchesi, C. Gambacorti-Passerini and F. Formelli, Cancer Lett., 2002, 175, 17 CrossRef CAS PubMed.
- I. Jeremias, H. H. Steiner, A. Benner, K. M. Debatin and C. Herold-Mende, Acta Neurochir., 2004, 146, 721 CrossRef CAS PubMed.
- P. Bernard, T. Scior, B. Didier, M. Hibert and J. Y. Berthon, Phytochemistry, 2001, 58, 865 CrossRef CAS PubMed.
- O. B. Flekhter, N. I. Medvedeva, L. T. Karachurina, L. A. Baltina, F. S. Zarudii, F. Z. Galin and G. A. Tolstikov, Pharm. Chem. J., 2002, 36, 488 CrossRef CAS.
- Y. Kashiwada, J. Chiyo, Y. Ikeshiro, T. Nagao, H. Okabe, L. M. Cosentino, K. Fowke and K. H. Lee, Bioorg. Med. Chem. Lett., 2001, 11, 183 CrossRef CAS PubMed.
- X. O. Yuan, L. Huang, P. Ho, C. Labranche and C. H. Chen, Virology, 2004, 324, 525 CrossRef CAS PubMed.
- M. Co, P. Koskela, P. Eklund-Akergren, K. Srinivas, J. W. King, P. J. R. Sjöberg and C. Turner, Green Chem., 2009, 11, 668 RSC.
- Z. Yu-hong, Y. Tao and W. Yang, J. For. Res., 2003, 14, 202 CrossRef.
- R. Ferreira, H. Garcia, A. F. Sousa, C. S. R. Freire, A. J. D. Silvestre, W. Kunz, L. P. N. Rebelo and C. S. Pereira, RSC Adv., 2013, 3, 21285 RSC.
- F. N. Lugemwa, Molecules, 2012, 17, 9274 CrossRef CAS PubMed.
- J. Azmir, I. S. M. Zaidul, M. M. Rahman, K. M. Sharif, A. Mohamed, F. Sahena, M. H. A. Jahurul, K. Ghafoor, N. A. N. Norulaini and A. K. M. Omar, J. Food Eng., 2013, 117, 426 CrossRef CAS.
- P. A. Krasutsky, Nat. Prod. Rep., 2006, 23, 919 RSC.
- Food and Drug Administration, Federal Food, Drug, and Cosmetic Act, ch. V, 2006 Search PubMed.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients Q7, 2010.
- Fischer Scientific, https://fscimage.fishersci.com/cmsassets/downloads/segment/Scientific/pdf/Chemicals/fisherchem_grades.pdf, Accessed 2014-12-17.
- Sigma-Aldrich, Accessed 2014-12-17.
- D. S. Kosyakov, N. V. Ul'yanovskii and D. I. Falev, J. Anal. Chem., 2014, 69 Search PubMed.
- G. J. Suppes, Selecting Thermodynamic Models for Process Simulation of Organic VLE and LLE Systems, Dept. of Chemical Engineering, University of Missouri-Colombia, 2002 Search PubMed.
- C. Capello, U. Fischer and K. Hungerbuhler, Green Chem., 2007, 9, 927 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5gc00519a |
| This journal is © The Royal Society of Chemistry 2016 |