Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Upgrading biogenic furans: blended C10–C12 platform chemicals via lyase-catalyzed carboligations and formation of novel C12 – choline chloride-based deep-eutectic-solvents†
Joseph
Donnelly
ab,
Christoph R.
Müller
a,
Lotte
Wiermans
a,
Christopher J.
Chuck
*c and
Pablo
Domínguez de María‡
*a
aInstitut für Technische und Makromolekulare Chemie (ITMC), RWTH Aachen University, Worringerweg 1, 52074 Aachen, Germany
bDoctoral Training Centre in Sustainable Chemical Technologies, Dept. of Chemical Engineering, University of Bath, Bath, BA2 7AY, UK
cCentre for Sustainable Chemical Technologies, Dept. of Chemical Engineering, University of Bath, Bath, BA2 7AY, UK. E-mail: c.chuck@bath.ac.uk; Tel: +44 (0)1225383537
First published on 9th March 2015
Abstract
Benzaldehyde lyase (BAL) results in an efficient biocatalyst for the umpolung carboligation of furfural, HMF, and mixtures of them, leading to blended C10–C12 platform chemicals. Subsequently, the mixing and gentle heating (<100 °C) of the formed hydroxy-ketone with choline chloride leads to the formation of a novel biomass-derived deep-eutectic-solvent.
A key challenge in the production of cellulose-based chemical intermediates, fuels and novel solvents is the cost-effective transformation of highly functionalized carbohydrate moieties into value-added chemicals. One highly promising route is the production of furan derivatives, such as furfural or 5-hydroxymethylfurfural (HMF), from the acid-catalyzed dehydration of hexoses and pentoses. In particular, HMF is extremely versatile and has the potential to become a key precursor to produce a range of chemicals and fuels in a sustainable biorefinery.1 For example, HMF can be converted into a range of C4 organic acids suitable for the production of biopolymers by selective oxidation reactions, whereas stable gasoline blending agents (such as dimethyl furan) or solvents (THF) can be produced from decarbonylation and hydrogenation. Moreover, key pharmaceutical precursors can also be produced by amidation or esterification reactions, e.g. the etherification of HMF to yield larger intermediates suitable for retroviral drugs or polymer precursors. However, to further broaden the applications, e.g. to access suitable surfactants or precursors to jet and diesel fuels, the carbon number must be increased to the C10–C18 range. This can be achieved chemically through HMF self-condensation – yielding a C12 derivative – or from aldol condensation with additional acetone to access higher carbon range precursors.2–4 Moreover, starting with fractions containing different proportions of furfural and HMF, blended mixtures (C10 to C12) may be achieved. All these compounds can then be either partially hydrogenated to retain functionality for additives or fully hydrogenated to yield hydrocarbon fuels. In this area, a promising but not yet sufficiently explored option for HMF valorization would be its self-condensation in umpolung fashion to afford C12 platform chemicals, both the formed hydroxy-ketone and the subsequently oxidized diketone.
By looking at the high functionalization of those molecules, from a chemical perspective a broad range of options can be anticipated (Scheme 1). HMF is highly reactive, being prone to self-oligomerization, by-product formation and undesired reactions under severe process conditions. Thus, the set-up of HMF-based derivatizations operating under mild and efficient conditions at the same time would become of utmost importance. To this end, for the self-condensation of HMF, organocatalysis – based on NHC carbenes – has been proposed by several research groups, as a mild technology avoiding product degradation.5
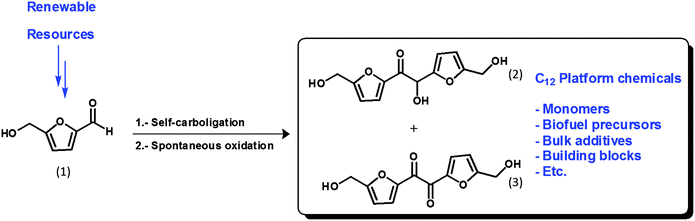 | ||
| Scheme 1 Self-carboligation of HMF in umpolung fashion, produced molecules and some applications thereof. | ||
In this communication, biocatalysis was successfully assessed for the self-carboligation of HMF for the first time. The use of enzymes (both free or immobilised) and whole-cells has gained considerable interest over the past decades, with an ample range of industrial processes already implemented.6 Compared to other catalytic technologies, apart from the well-known high selectivities and mild reaction conditions inherent to enzymes, another important asset is that biocatalysts can be produced at large scale via fermentation of recombinant microorganisms.
Thus, once (bio)catalyst design is performed (e.g. via directed evolution), the requested quantities of the enzyme can be straightforwardly produced under environmental conditions. Last but not least, once the most appropriate biocatalyst has been designed, the use of (immobilized) biocatalysts – either free enzymes or whole-cells – may decrease process costs considerably,7 an aspect that will become obviously crucial in the production of low-added value (bio)commodities, e.g. the aforementioned HMF-based ones.
For the condensation of HMF the use of lyases,8 specifically thiamine-diphosphate dependent lyases (ThDP-lyases), was considered. ThDP-Lyases represent a useful group of enzymes delivering α-hydroxy-ketones by the carboligation of two aldehydes. In this group, Benzaldehyde Lyase (BAL) from Pseudomonas fluorescens is a remarkable case, from which many enantio- and diastereo-selective applications including aromatic and aliphatic aldehydes as substrates have been reported over the last few years.8,9 Furthermore, there are some outstanding examples of lyases in general, and BAL in particular, catalyzing highly efficient processes with excellent productivities by means of whole-cell overexpressing systems.10 Thus, once the proof-of-concept is shown, a subsequent optimization would allow the set-up of a robust biocatalytic process for HMF condensation.
While BAL-catalyzed furoin production (condensation of furfural to afford C10 derivatives) was described years ago,8,9 to our knowledge the use of lyases for (the more challenging) HMF condensation has not been assessed to date. In our concept, BAL would operate under mild aqueous conditions – using a cosolvent for substrate solubility – at room temperature leading to the formation of the hydroxy-ketone 2.§ It may be expected that some spontaneous oxidation of 2 to afford the diketone 3 will be observed. Kinetic results of the BAL-catalyzed process using HMF (20 mM) for proof-of-principle experiments are depicted in Fig. 1. Gratifyingly, BAL is able to accept HMF as substrate to afford the hydroxy-ketone 1. Moreover, under these proof-of-concept and non-optimized conditions an initial rate of ∼7 g L−1 h−1 of hydroxy-ketone 2 was observed. The reaction proceeded until a conversion of around 70% was reached. This is a slightly reduced yield in comparison to the NHC carbene catalysts that have been reported to produce yields in excess of 90% on heating over an hour.5 Presumably BAL – herein used as free enzyme (less stable than within a whole cell or when immobilized) – becomes deactivated at that time. Furthermore, the oxidized diketone 3 is also observed after some time, formed at the cost of 2. This spontaneous oxidation is not observed in the carbene catalysed processes.5 From a process development viewpoint, the establishment of immobilized BAL-containing whole-cells should enable the stable production of 2 under continuous processing, thus performing rapidly the downstream processing and avoiding further oxidation to the diketone. Nevertheless, it must be noted that both products, 2 and 3, may encounter potential applications in many fields.
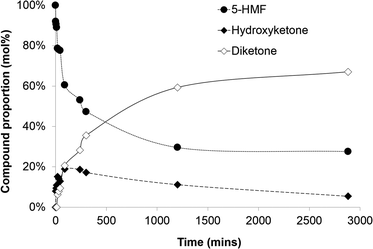 | ||
| Fig. 1 BAL-catalyzed condensation of HMF. Conditions: 20 mM HMF, 1 mg mL−1 BAL, 40 mM ThDP, potassium phosphate buffer (pH 8) with 20 vol% DMSO co-solvent at room temperature. | ||
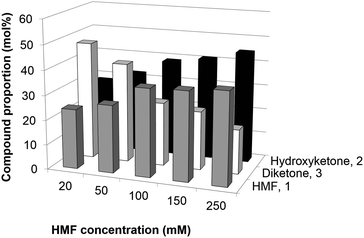
Once BAL-catalyzed proof-of-concept was successfully shown, further experiments with regard to HMF concentration (20–250 mM) and oxidation patterns were conducted. Reactions were run for 18 h, and then analyzed (Fig. 2). Interestingly, BAL is able to perform the carboligation even at higher HMF loadings of up to 250 mM, leading to a remarkable accumulation of ∼35 g L−1 of hydroxy-ketone 2, though the conversion of HMF was reduced slightly from 75% to 63%. The presence of the oxidized diketone 3 is detected at the higher loadings of HMF yet at significantly lower proportions than in the previous experiment. For example, the diketone made up only 18% of the product mixture at an HMF loading of 250 mM compared with near 60% at 30 mM HMF loading. Presumably, the spontaneous (non-enzymatic) oxidation rate remains constant in all reaction conditions, whereas the enzymatic process undergoes faster at higher concentrations (before the free enzyme gets deactivated), thus accumulating 2 in the reaction system.
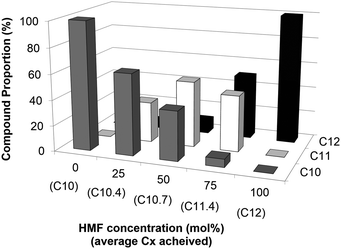
Encouraged by these results, subsequent alternative aqueous mixtures of furfural and HMF were assessed (as would be produced from actual biorefineries whereby pretreatment has been conducted). Depending on the initial proportion of both furans, different blended mixtures C10–C12 may be expected. This may open a novel way of valorizing such mixtures, especially when mixtures with a Cx average range are needed. Results are depicted in Fig. 3. Gratifyingly, BAL is able to also form mixtures of furfural-HMF, thus leading to C11 derivatives. Depending on the initial concentration of the furans, mixtures with Cx averages from C10 to C12 were achieved.
This was achieved with the BAL retaining higher activity than on conversion of the HMF alone. Given the broad substrate range acceptance of BAL – also using aliphatic aldehydes such as acetaldehyde or butyraldehyde, among others, with the concept herein provided, numerous possibilities for bio-based product upgrading can be envisaged.
Apart from HMF upgrading to C12 derivatives, within biorefineries another important trend is the identification of novel biomass-derived neoteric solvents that may be further used for varied applications. For instance, several deep-eutectic-solvents (DES) formed by the combination of a hydrogen-bond donor (HBDs, e.g. alcohols, carboxylic acids) and quaternary ammonium salts, such as choline chloride, have been recently reported.11 Herein, the obtained HMF-based hydroxy-ketone 2 resulted to be a yellowish solid powder. However, bearing three –OH groups in its structure, it might become a promising HBD to form DES. If successful, this approach might lead to the provision of a series of novel neoteric solvents based on HMF-C12 derivatives. Thus, the formation of a deep-eutectic-solvent (DES) between 2 as HBD and choline chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol
1 mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) was assessed. Successful results are depicted in Fig. 4, where it is seen that the combination and gentle mixing (<100 °C) of two solids leads to the formation of a stable viscous liquid at room temperature.
mol) was assessed. Successful results are depicted in Fig. 4, where it is seen that the combination and gentle mixing (<100 °C) of two solids leads to the formation of a stable viscous liquid at room temperature.
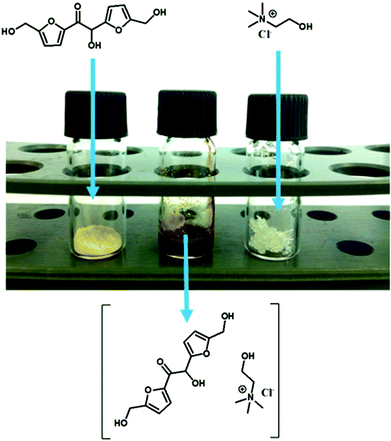 | ||
| Fig. 4 Formation of a DES composed of hydroxy-ketone 2 (1 mol) and choline chloride (1 mol), to afford a liquid viscous solution at room temperature. | ||
Conclusions
In summary, this communication reports successfully for the first time the use of lyases as biocatalysts for the umpolung carboligation to upgrade HMF to C12 platform chemicals. Under non-optimized conditions initial rates of ∼7 g hydroxy-ketone 2 L−1 h−1 have been observed, with accumulation of the product up to 35 g L−1. Moreover, aqueous mixtures of furfural and HMF can be valorized, leading to blended C10–C12 composition, promising for further hydrogenation to deliver tailored blends. For these synthetic approaches, the further choice of a better cosolvent – rather than challenging DMSO –, together with biocatalyst design and process set-up (e.g. use of immobilized BAL or immobilised whole-cells overexpressing BAL) may certainly deliver robust reaction conditions for the valorization of biogenic furans, furfural and HMF. The intrinsic reactivity makes the biocatalytic approach highly appealing for further research and development. Furthermore, the formation of novel DES may lead to novel exciting applications of them as biomaterials and/or solvents.We would like to thank Dr Catherine Lyall (Bath, UK) for her outstanding assistance in the NMR analysis, and gratefully acknowledge financial support from DFG training group 1166 “BioNoCo” (“Biocatalysis in Non-conventional Media”). The authors would also like to extend their thanks to the EPSRC for partially funding this work through the Doctoral Training Centre in Sustainable Chemical Technologies (EP/G03768X/1) and to Roger and Sue Whorrod for their kind endowment to the University resulting in the Whorrod Fellowship in Sustainable Chemical Technologies held by Dr C. Chuck.
Notes and references
- Biorefineries – Industrial processes and products. Status quo and future directions, ed. B. Kamm, P. R. Gruber and M. Kamm, Wiley-VCH, Weinheim, 2010 Search PubMed.
- (a) R. J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499 CrossRef CAS PubMed; (b) S. Lima, M. M. Antunes, M. Pillinger and A. A. Valente, ChemCatChem, 2011, 3, 1686 CrossRef CAS; (c) A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754 RSC; (d) M. E. Zakrzewska, E. Bogel-Lukasik and R. Bogel-Lukasik, Chem. Rev., 2011, 111, 397 CrossRef CAS PubMed.
- Recent examples on HMF synthesis: (a) P. M. Grande, C. Bergs and P. Domínguez de María, ChemSusChem, 2012, 5, 1203 CrossRef CAS PubMed; (b) L. Lai and Y. Zhang, ChemSusChem, 2011, 4, 1745 CrossRef CAS PubMed; (c) R. Huang, W. Qi, R. Su and Z. He, Chem. Commun., 2010, 46, 1115 RSC; (d) B. Kim, J. Jeong, S. Shin, D. Lee, S. Kim, H. J. Yoon and J. K. Cho, ChemSusChem, 2010, 3, 1273 CrossRef CAS PubMed; (e) S. Hu, Z. Zhang, Y. Zhou, J. Song, H. Fan and B. Han, Green Chem., 2009, 11, 873 RSC.
- See, for instance: (a) M. Krystof, M. Pérez-Sánchez and P. Domínguez de María, ChemSusChem, 2013, 6, 630 CrossRef CAS PubMed; (b) M. Krystof, M. Pérez-Sánchez and P. Domínguez de María, ChemSusChem, 2013, 6, 826 CrossRef CAS PubMed.
- (a) B. L. Wegenhart, L. Yang, S. C. Kwan, R. Harris, H. I. Kenttärmaa and M. M. Abu-Omar, ChemSusChem, 2014, 9, 2742 CrossRef PubMed; (b) E. Y.-X. Chen and D. Liu, US20140007497 A1, 2014 Search PubMed; (c) D. Liu and E. Y.-X. Chen, Green Chem., 2014, 16, 964 RSC; (d) D. Liu and E. Y.-X. Chen, ACS Catal., 2014, 4, 1302 CrossRef CAS; (e) D. Liu and E. Y.-X. Chen, ChemSusChem, 2013, 6, 2236 CrossRef CAS PubMed; (f) D. Liu, Y. Zhang and E. Y.-X. Chen, Green Chem., 2012, 14, 2738 RSC.
- See, for instance, (a) U. T. Bornscheuer, G. Huisman, R. J. Kazlauskas, S. Lutz, J. Moore and K. Robins, Nature, 2012, 485, 185 CrossRef CAS PubMed; (b) A. Liese, K. Seelbach and C. Wandrey, Industrial Biotransformations, Wiley-VCH, 2nd edn, 2006 Search PubMed.
- (a) A. Jakoblinnert, R. Mladenov, A. Paul, F. Sibilla, U. Schwaneberg, M. B. Ansorge-Schumacher and P. Domínguez de María, Chem. Commun., 2011, 47, 12230 RSC; (b) R. L. Hanson, B. L. Davis, Y. Chen, S. L. Goldberg, W. L. Parker, T. P. Tully, M. A. Montana and R. N. Patel, Adv. Synth. Catal., 2008, 350, 1367 CrossRef CAS; (c) A. Panova, L. Mersinger, Q. Liu, T. Foo, D. Roe, W. Spillan, A. Sigmund, A. Ben-Bassat, L. Wagner, D. O'Keefe, S. Wu, K. Petrillo, M. Payne, S. Breske, F. Gallagher and R. DiCossimo, Adv. Synth. Catal., 2007, 349, 1462 CrossRef CAS.
- (a) M. Brovetto, D. Gamenara, P. Saenz Méndez and G. A. Seoane, Chem. Rev., 2011, 111, 4346 CrossRef CAS PubMed; (b) P. Hoyos, J. V. Sinisterra, F. Molinari, A. R. Alcántara and P. Domínguez de María, Acc. Chem. Res., 2010, 43, 288 CrossRef CAS PubMed.
- (a) C. R. Müller, M. Pérez-Sánchez and P. Domínguez de María, Org. Biomol. Chem., 2013, 11, 2000 RSC; (b) P. Ayhan and A. S. Demir, Adv. Synth. Catal., 2011, 353, 624 CrossRef CAS; (c) S. Shanmuganathan, D. Natalia, A. Van den Wittenboer, C. Kohlmann, L. Greiner and P. Domínguez de María, Green Chem., 2010, 12, 2240 RSC; (d) P. Domínguez de María, M. Pohl, D. Gocke, H. Gröger, H. Trauthwein, T. Stillger, L. Walter and M. Müller, Eur. J. Org. Chem., 2007, 2940 CrossRef; (e) P. Domínguez de María, T. Stillger, M. Pohl, S. Wallert, K. H. Drauz, H. Gröger, H. Trauthwein and A. Liese, J. Mol. Catal. B: Enzym., 2006, 38, 43 CrossRef PubMed.
- (a) A. Jakoblinnert and D. Rother, Green Chem., 2014, 16, 3472 RSC; (b) M. Oslaj, J. Cluzeau, D. Orkic, G. Kopitar, P. Mrak and Z. Casar, PLoS One, 2013, 8, e62250 CAS; (c) P. Domínguez de María, T. Stillger, M. Pohl, M. Kiesel, A. Liese, H. Gröger and H. Trauthwein, Adv. Synth. Catal., 2008, 350, 165 CrossRef.
- (a) Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108 RSC; (b) D. Carriazo, M. C. Serrano, M. C. Gutiérrez, M. L. Ferrer and F. del Monte, Chem. Soc. Rev., 2012, 41, 4996 RSC; (c) Z. Maugeri and P. Domínguez de María, RSC Adv., 2012, 2, 421 RSC; (d) P. Domínguez de María and Z. Maugeri, Curr. Opin. Chem. Biol., 2011, 15, 220 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5gc00342c |
| ‡ Present address: Sustainable Momentum, SL. Ap. Correos 3517. 35004, Las Palmas de Gran Canaria, Canary Islands, Spain. Tel.: +34 609565237; E-mail: dominguez@itmc.rwth-aachen.de, dominguez@sustainable-momentum.net |
§ Benzaldehyde lyase from Pseudomonas fluorescens was cloned and overexpressed in E. coli cells, and produced by fermentation.9 After fermentation, BAL was lyophilized and stored at −20 °C until use. BAL characterization was performed using benzaldehyde as substrate, and benzoin formation as reaction test, as reported elsewhere.9Standard reaction with BAL for HMF carboligation. To a mixture of 40 mL potassium phosphate buffer (pH 8) with DMSO (20 vol%) was added ThDP (2 mg, 4.03 × 10−3 mmol) and BAL (40 mg). HMF (100.89 mg, 0.8 mmol) was then added to the reaction and the vessel stoppered. The reaction was allowed to stir for 18 h at room temperature. The reaction was quenched by addition of 80 mL EtOAc and the product extracted into the same. EtOAc was removed under reduced pressure and the product dried under high vacuum. Characterisation was effected through NMR spectroscopy. Standard reaction with BAL with aqueous mixtures of furfural and HMF. To mixtures of 20 mL potassium phosphate buffer (pH 8) with DMSO (20 vol%) was added ThDP (1 mg, 2.02 × 10−3 mmol) and BAL (20 mg). HMF and furfural were then added in varying molar proportions (75/25, 50/50, 25/75) to make up 0.4 mmol total substrate and the reaction vessels stoppered. The reactions were allowed to stir for 18 h at room temperature. The reactions were quenched by addition of 40 mL EtOAc and the products extracted into the same. EtOAc was removed under reduced pressure and the products dried under high vacuum. Characterisation was effected through NMR spectroscopy and mass spectrometry. DES formation. 100 mg (0.41 mmol) HMF hydroxyl-ketone 2 and 59.0 mg choline chloride (0.41 mmol) (both solids) were stirred in a GC vial in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 60–70 °C for ∼1 hour. When cooled down, the viscous liquid remained. 1 at 60–70 °C for ∼1 hour. When cooled down, the viscous liquid remained. |
| This journal is © The Royal Society of Chemistry 2015 |