Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rice straw hydrolysate to fuel and volatile fatty acid conversion by Clostridium sporogenes BE01: bio-electrochemical analysis of the electron transport mediators involved
Lalitha Devi
Gottumukkala
*ac,
Rajeev Kumar
Sukumaran
a,
S.
Venkata Mohan
b,
Sajna
Kuttuvan Valappil
a,
Omprakash
Sarkar
b and
Ashok
Pandey
a
aCentre for Biofuels, Biotechnology Division, National Institute for Interdisciplinary Science and Technology – CSIR, Industrial Estate PO, Thiruvananthapuram 695019, India. E-mail: lalithaniist@gmail.com; lalithadevi@sun.ac.za; Fax: +27 808 2059; Tel: +27 21 8089485
bBioengineering and Environmental Centre (BEEC), Indian Institute of Chemical Technology – CSIR, Hyderabad 500 007, India
cDept. of Process Engineering, Stellenbosch University, Private Bag X1, Matieland, 7602, South Africa
First published on 18th March 2015
Abstract
Clostridium sporogenes BE01, a non-acetone forming butanol producer, can produce hydrogen and volatile fatty acids (VFAs) during butanol fermentation from rice straw hydrolysate. Bio-electrochemical analysis revealed the changes that occurred in the redox microenvironment and electron transport mediators during fermentation at different pH and CaCO3 concentrations. CaCO3 played a very important role in enhancing the production of hydrogen, volatile fatty acids and solvents by stimulating the changes in the electron transport system. The electron transport system mediated by NAD/NADH, flavins, Fe–S clusters, protein bound FAD, and cytochrome complex in C. sporogenes BE01 was analysed by cyclic voltammetry (CV). Electrokinetic analysis revealed that the favorability for redox reactions increased with an increase in pH, and the polarization resistance reduced significantly with CaCO3 supplementation.
1. Introduction
Biobutanol has gained significant attention for its properties as a liquid transportation fuel, but its production from lignocellulosic biomass is facing challenges both technically and economically.1 VFAs and hydrogen produced during butanol fermentation can be considered as an added advantage, when an efficient process is in place. The formation of various industrial products from a single substrate in a single run can be considered beneficial for lignocellulosic biorefinery processes. The fluctuation in the redox environment leads to change in the bacterial growth pattern, glucose utilization, and products formed,2 and hence it is essential to understand the redox microenvironment of the bioreactors operated with the desired biomass and microbes. The redox balance in a bioreactor is one among the many key components that controls carbon flux and changes in the metabolic activity of the organism.3 In order to assess the bio-electric potential of a bioreactor, it is essential to perform an analysis of the bio-electro catalytic efficiency of the microbial catalyst. The electrochemical characterization of microbial bioreactors will help in understanding the redox active species participating in the electron transfer reactions.4The metabolic pathway of glucose to butanol conversion is complex and several enzymes are involved in diverting the pathway towards VFAs (acetic acid and butyric acid) and for the assimilation of these VFAs to solvents. It is a highly interlinked chain of redox reactions with many electron transporters involved. The formation of hydrogen and volatile fatty acids is an intrinsic part of the biochemical pathway for butanol fermentation by Clostridia.5 Conversion of glucose to acetyl CoA through pyruvate route generates hydrogen, and acetyl CoA is the precursor for VFAs and solvent production.6,7 Hydrogen generated by Clostridial species is directly related to volatile fatty acid production. The conversion of acetyl CoA to acetate yields hydrogen in twice the yield than the conversion to butyrate8 and the ratio of acetic acid and butyric acid has a tremendous effect on the ratio of the solvents formed.9 Hydrogen produced during the process is an energy rich gaseous fuel and VFAs can be used as precursors for polyhydroxyalkanoates.10 The solvents are the main metabolic products and can be used as biofuels.
This study presents the efficiency of C. sporogenes BE01, a novel non-acetone producing bacteria, to convert fermentable sugars generated from the hydrolysis of lignocellulosic biomass to solvents, VFAs and hydrogen. The study also focuses on understanding the possible electron transport mediators and redox reactions involved during the process. This is the first report on hydrogen production from rice straw hydrolysate using a pure strain of C. sporogenes and also the first report on demonstrating the electron transporter mediated redox activity of Clostridium species during biobutanol fermentation from lignocellulosic biomass.
2. Results and discussion
2.1. Glucose utilization
The dilute acid pretreatment and enzymatic hydrolysis of rice straw generated 45 g L−1 of glucose. However, heat sterilizing the hydrolysate at 121 °C for 10 min resulted in 15 g L−1 sugar loss and the final glucose obtained was 30 g L−1. Pentoses were found in negligible concentrations in the hydrolysate, as the hemicellulosic fraction was efficiently removed during dilute acid pretreatment. The redox microenvironment of C. sporogenes BE01 driven biobutanol fermentation in enzymatic rice straw hydrolysate was studied at various initial pH (5.8, 6.2, 6.4 and 6.8) and with different CaCO3 concentrations (0 g L−1, 5 g L−1 and 10 g L−1). pH plays a very significant role in the microbial catalysis and growth of the bacteria. Especially in butanol fermentation, pH has direct control over the formation and assimilation of acids.11C. sporogenes BE01 exhibited a change in the pattern of glucose utilization with changes in the microenvironment of the bioreactor. pH of the medium in the range of 5.8 to 6.4 had a very low effect on sugar utilization, but the utilization rate enhanced with pH approaching near neutral. This signifies the increased metabolic activity and growth at a near neutral pH (Fig. 1a).Supplementation with CaCO3 in the medium enhanced glucose utilization and it increased with an increasing concentration of CaCO3 (0, 5 and 10 g L−1) (Fig. 1b). Increased glucose consumption and ABE productivity in the presence of ≥4 g L−1 CaCO3 was reported with C. beijerinkii grown in semi defined P2 medium.12 Calcium ions have various effects on a cellular level, which can contribute to increased growth though pH buffering effects might also contribute to stimulatory effects on butanol fermentation. It was reported by Richmond et al. that the presence of CaCO3 increased protein synthesis in Clostridium species and this increase was proportional to the amount of CaCO3 in the medium. It was also stated that these upregulated proteins might be involved in glucose uptake and utilization.13 Similar glucose utilization patterns were observed in our results with C. sporogenes BE01 (Fig. 1b).
2.2. Hydrogen and volatile fatty acids
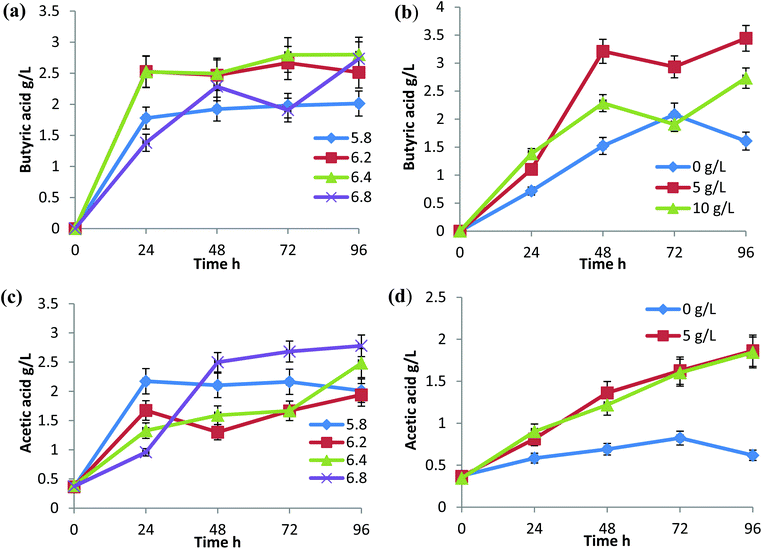
During butanol fermentation, butyric acid production was relatively higher than the acetic acid production under all the conditions tested (Fig. 2). C. sporogenes is a known producer of butyric acid and has been reported for cheese fermentation in combination with C. butyricum and C. beijerinckii.14,15 In C. tyrobutyricum fermentation studies, butyrate at 15 g L−1 showed an inhibitory effect on acetate formation.16 In this study, when overall VFAs production was considered, near neutral pH 6.4 and 6.8 were found to be favourable with a production of 5.2 g L−1 and 5.5 g L−1, respectively. The reactor with a CaCO3 concentration of 5 g L−1 performed considerably well for the conversion of sugars to acids (Fig. 2b and d).C. propionicum, an organic acid producer, showed the highest growth and organic acid production at pH 7.17 Acid forming enzymes are highly pH dependent. The activity of the enzymes can be inhibited or enhanced with an increase or decrease in pH.16 The acidogenic process was highly active in the first 24 h and became stable throughout the fermentation period, except at pH 6.8, VFAs production increased until 48 h, sharply decreased at 72 h and increased again at 96 h (Fig. 2a and c). The same was observed with 5 g L−1 and 10 g L−1 CaCO3 supplementation, which could be attributed to efficient assimilation of acids in the solvents at 72 h. Hydrogen production was in accordance with acid production and the highest percentage of hydrogen (20%) of the total gas produced was at pH 6.4 and 6.8 (Fig. 3a). Although there was no considerable difference in the percentage of hydrogen produced, there was a notable difference in the total gas produced at different pH and showed marked variation when represented in terms of cumulative hydrogen (Fig. 3c and d). The total hydrogen production increased with an increase in pH to 6.4 but reduced at pH 6.8. The total hydrogen production at pH 6.4 continued for 96 h and there was little reduction in the gas production with respect to time (Fig. 3c).
The optimal pH for hydrogen production varies with each species and strains. For C. beijerinkii DSM 1820, the optimum pH reported was 6.7, for C. pasteurianum it was 5.4 and for C. butyricum it was 5.1.18 Hydrogen production varies with a change in glucose concentration. In C. acetobutylicum ATCC824 glucose fermentation, the hydrogen production rate ranged from 680 to 1270 ml g−1 glucose per liter of reactor.19 There was an increase in total gas production and percentage hydrogen production when the CaCO3 concentration was reduced to 5 g L−1 (1260 ml L−1), but in the absence of CaCO3 and with an increase in CaCO3 to 10 g L−1 the total hydrogen produced was 591 ml and 698 ml, respectively (Fig. 3b and d). The long acidogenic phase and higher acid production can be correlated with the increased hydrogen production found with the supplementation of 5 g L−1 CaCO3. The favorability of 10 g L−1 CaCO3 was towards solvents assimilation rather than hydrogen production.
2.3. Solvents production
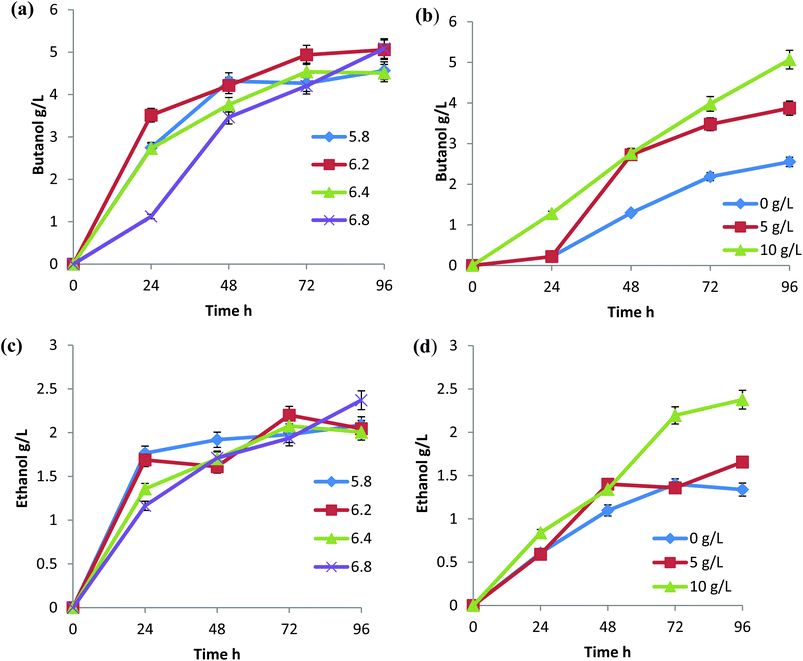
C. sporogenes BE01 was reported in our previous studies for its ability to produce solvents from rice straw hydrolysate.20 Butanol and ethanol were the two solvents produced by C. sporogenes BE01 without forming acetone and their ratio varies with the change in the process parameters. Acetic acid and butyric acid, produced in the acidogenic phase of the culture, were assimilated at the later solventogenic phase of the culture.21C. sporogenes BE01 was able to form solvents at pH as low as 5.8, but solvent production increased when pH approached near neutral (Fig. 4a and c). At 5.8 pH, though the rate of butanol and ethanol formation was high for the first 48 h and 24 h, respectively, solvent formation ceased later and the total solvent produced was comparatively less (Fig. 4a and c). This could be due to the lowered pH with VFAs production and inefficient assimilation of the VFAs. At pH 6.2 and 6.4, a relatively high solvent production was observed for the first 24 h and 72 h, respectively. Further accumulation of solvents was not found from 72 h to 96 h. The efficient conversion of sugars and acids to solvents was achieved in a different mode by C. sporogenes BE01 at pH 6.8. Solvent formation in the first 24 h was considerably low compared to rest of the pH ranges, which signifies that the acid accumulation did not lead to a decrease in the pH to a level wherein assimilation starts for solvent production, but the overall high solvent production 7.3 g L−1 was achieved with a constant increase in solvent accumulation until 96 h without attaining saturation (Fig. 4a and c).CaCO3 was used to maintain the pH in the range by neutralizing the organic acids formed during fermentation. A preliminary study on the effect of CaCO3 on butanol fermentation was mentioned in our previous report,20 and in the current study, we tried to correlate it with the initial pH and solvent formation. Solvent production increased with an increase in the concentration of CaCO3 in the medium (Fig. 4b and d). This could be due to the effect of calcium ions and its effective buffering action, which resulted in efficient VFA assimilation. Without CaCO3 supplementation, the total yield of solvents was just 3.8 g L−1, which could be due to the low glucose utilization rate and low VFA formation. The supplementation of 10 g L−1 CaCO3 increased the total solvent production to 7.4 g L−1. A reduction in CaCO3 concentration from 10 g L−1 to 5 g L−1 lead to a 26% decrease in the total solvents formed (Fig. 4d).
The final pH of all the experiments varied with the changes in the CaCO3 concentration in the medium. In the absence of CaCO3 and with the supplementation of 5 g L−1 CaCO3, the pH dropped from 6.8 (initial pH) to 5.9 (final pH) in 96 h, indicating the formation of acids and their improper assimilation into the solvents; whereas, with 10 g L−1 CaCO3, the pH dropped to 6.3 in 48 h and then gradually increased to 6.5 in 96 h. The CaCO3 concentration was observed to affect the solvent production more than the initial pH of the medium.
2.4. Electrochemical analysis
The variations in the voltammogram peaks obtained during cyclic voltammetry (CV) analysis and the change with respect to time were due to the differences in the initial pH and CaCO3 concentrations in the rice straw hydrolysate medium. The redox currents were found to be high with sterile rice straw hydrolysate alone, which signifies it is potential substrate for bio-electro catalytic reactions. The redox currents did not differ predominantly and were mostly in the range from RC: −18 ± 3 μA to OC: 18 ± 4 μA. With the alterations in the initial pH and various CaCO3 concentrations in the medium, the difference observed was marginal (Fig. 5). The observations indicate that the catalytic activity was more or less the same in all the conditions provided, but the difference was with the shift in metabolic activity and the mediators/electron carriers involved. | ||
| Fig. 5 Cyclic voltammogram profiles recorded at various pH and CaCO3 concentrations: 0% – No CaCO3, 0.5% – 5 g L−1 CaCO3, 1% – 10 g L−1 CaCO3. | ||
Distinct peaks were observed with the change in CaCO3 concentration in the medium. The peaks for cytochrome, quinone and simple Fe–S clusters were commonly observed throughout the fermentation. The major difference was with the frequency of peaks for NADH, protein bound FAD, flavoproteins and 4Fe–4S clusters. Peaks were observed for all the mediators mentioned above with 10 g L−1 CaCO3 containing medium. The presence of many electron transporters implies that the redox activity occurred efficiently and this was also supported by high butanol production. While in the absence of CaCO3 peaks for the common mediators, such as cytochrome bc1 and 4Fe–4S, clusters were observed. Rubredoxins, like Fe–S clusters in bacteria, are low potential electron transporters and this could be the reason for the comparatively lower acid and solvent production. With 5 g L−1 CaCO3, the peaks for cytochrome bc1, NAD/NADH, and flavoproteins were found but protein bound FAD peaks were absent.
In an electron transport system, electrons always flow from a negative to positive redox potential. Based on the CV peaks, during solvent formation, the electrons flow from NADH (−0.32 V) to an enzyme or protein that contains FAD (0.0 to 0.1 V) via flavins (FMN/FAD) (−0.2 to 0.29 V) and the 4Fe–4S clusters (−0.15). Quinones and cytochromes (0.1 to 0.25 V) stabilize the FAD bound enzyme in the reduced state. However, for fermentation conditions wherein the solvent formation was low and VFAs formation was high, the peaks for FAD and 4Fe–4S clusters were less prominent, while peaks for simple Fe–S clusters were dominant. This indicates that there was a shift or bifurcation in electron transportation during solventogenesis.
There are reports on a Clostridial electron transport bifurcation system, wherein the cytoplasmic complex of butyryl CoA dehydrogenase and electron transferring flavoprotein (BCD/ETF) catalyses a key reaction (crotonyl CoA to butyryl CoA) in the butanol pathway and is coupled to a membrane associated proton translocating NADH-ferredoxin reductase complex (Rnf A-G or Rnf A-E).30,31 Signals obtained for the 4Fe–4S clusters, protein bound FAD/FMN, flavoproteins and NAD/NADH are characteristic of the electron bifurcation system, involving the Rnf complex, and have been reported in few Clostridium species.30 Quinones and cytochrome were reported for maintaining the enzymes in their reduced active state.32 The NADH-ferredoxin reductase (RnfA-G) complex that couples ferredoxin oxidation by NAD+ with proton/Na+ translocation is a membrane associated enzyme complex, which contains 4Fe–4S clusters, covalently bound FMN, non-covalently bound FAD and 2Fe–2S clusters. The electrons are proposed to flow from NADH to the Rnf complex, resulting in the translocation of Na+ or H+.30,33 RnfA-G was reported in C. tetanomorphum, but whether Rnf is a real Na+ pump or H+ pump that translocates Na+ during proton gradient formation has to be still established.30 In C. ljungdahlii and C. kluvyeri, proton translocating Rnf has been reported.30,33,34
During butanol fermentation, glucose is converted to pyruvate, which is further oxidized to acetyl CoA and acetate. Hydrogenases linked to pyruvate:ferredoxin oxidoreductase produce hydrogen using proton as a terminal acceptor. However, hydrogenases coupled to NADH: ferredoxin reductases produce hydrogen using NADH as an electron donor and proton as an electron acceptor.35 Rnf/Nqr (NADH:quinone oxidoreductase) was reported in the genome of all C. botulinum group 1, but absent from the group II genomes36 and it is a known fact that C. sporogenes is considered as a non-toxigenic equivalent of the C. botulinum group I.37 This strengthens the idea of Rnf based electron transportation in C. sporogenes during glucose fermentation.
There are no detailed reports on C. sporogenes butanol fermentation and the metabolic pathway involved. A correlation of the CV analyses with the pattern of products formed during fermentation broadly suggest the possible stimulation of the electron transport chain associated with Rnf and butyryl CoA dehydrogenase in the presence of CaCO3. Calcium ions were also reported to stabilize membrane bound proteins.13 The increased production of butanol and butyric acid with the supplementation of CaCO3 can be attributed to stimulation and stabilization of membrane proteins. However, detailed research is necessary to support this hypothesis.
2.5. Bio-electro kinetic analysis
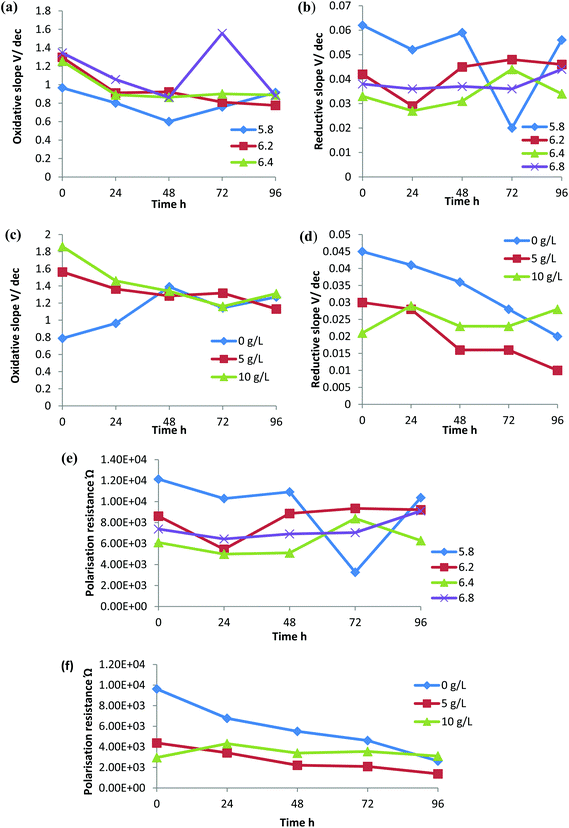
The rate of electron transfer to the electrode by the oxidized and reduced species can be interpreted by a Tafel plot. According to the Tafel equation, when the over potential is large the reverse reaction rate is negligible. The slope of the Tafel plot reveals the value of the electron transfer coefficient.38 A low Tafel slope indicates the high current obtained at low over potential. Therefore, the higher the slope, the higher the activation energy required, which indicates the less favourability of the oxidation/reduction reaction.39 The efficiency of the bioreactor towards reduction or oxidation reactions and the favourability for the membrane associated biochemical redox reactions can be analyzed by the redox slopes obtained under each condition with respect to time. Oxidative Tafel slopes and reductive Tafel slopes were derived from the Tafel plot obtained (Fig. 6). | ||
| Fig. 6 Electrokinetic analysis of butanol fermentation by C. sporogenes BE01 in rice straw hydrolysate medium at various pH and CaCO3 concentrations based on the Tafel plot. | ||
Although there was variation in the Tafel slopes obtained with respect to changes in the pH and CaCO3 concentrations, in all the experimental conditions the oxidative slope was higher than the reductive slope. This indicates that the rice straw hydrolysate bioreactor with C. sporogenes BE01 was more favourable towards the reduction metabolism, i.e. solvent production. In relation to the different initial pH experiments, the oxidative slope increased with increase in pH and the reductive slope decreased with increase in pH (Fig. 7a and b). This indicates that the reduction metabolism was favourable at pH 6.4 and 6.8 compared to that at a lower pH.
In the absence of CaCO3, the oxidation slope was low but increased with an increase in CaCO3 concentration in the medium (Fig. 7c). This suggests that the addition of CaCO3 was not favourable for the oxidation reactions and in the case of the reduction slopes, 5 g L−1 CaCO3 showed a comparatively lower reduction potential followed by 10 g L−1 CaCO3 and no CaCO3 supplementation (Fig. 7d). Reduction in the activation energy required for the redox reactions in the presence of CaCO3 could be responsible for the increased production of hydrogen, acids and solvents.
Polarization resistance, Rp, refers to the resistivity of the electrolytes around the electrode. This could be due to resistance of the electron transfer by the microbe or the insulation effect of the products formed on the electrodes surface. For a reactor to be active in an electron transfer and product formation, the polarization resistance should be low.39 Fermentation with an initial pH of 6.4 showed less Rp compared with the other pH ranges (Fig. 7e). Polarization resistance was high in the reactor without CaCO3 supplementation and decreased with a supplementation of 5 g L−1 CaCO3, but the resistance increased slightly with increased CaCO3 supplementation (Fig. 7f) and as stated before it could be either due to the resistance or insulation effect.
3. Conclusions
Clostridium sporogenes BE01 is capable of producing 7 g L−1 of VFAs and 1.2 L L−1 of hydrogen during butanol fermentation in rice straw hydrolysate with 30 g L−1 of glucose. 7.3 g L−1 of total solvents were produced, of which 5 g L−1 was butanol. Butanol and ethanol production was in a ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Fe–S clusters, Cyt bc1 and quinones were the common electron transporters involved during butanol fermentation. CaCO3 supplementation resulted in high solvent formation by stimulating the electron transport system mediated by protein bound FAD, 4Fe–4S, NADH and flavoproteins. The presence of peak for protein bound FAD was found until 96 h at pH 6.4 and pH 6.8 with 10 g L−1 CaCO3 supplemented medium. The involvement of 4Fe–4S clusters, NAD/NADH, protein bound FAD and flavoproteins during active butanol fermentation presents the possibility of an electron bifurcation system mediated by a membrane bound complex, probably Rnf. The Tafel plot revealed that rice straw hydrolysate supplemented with CaCO3 had a low polarisation resistance, Rp, and required less activation energy for the reduction metabolism making it more favourable for solvent production during fermentation by C. sporogenes BE01.
3. Fe–S clusters, Cyt bc1 and quinones were the common electron transporters involved during butanol fermentation. CaCO3 supplementation resulted in high solvent formation by stimulating the electron transport system mediated by protein bound FAD, 4Fe–4S, NADH and flavoproteins. The presence of peak for protein bound FAD was found until 96 h at pH 6.4 and pH 6.8 with 10 g L−1 CaCO3 supplemented medium. The involvement of 4Fe–4S clusters, NAD/NADH, protein bound FAD and flavoproteins during active butanol fermentation presents the possibility of an electron bifurcation system mediated by a membrane bound complex, probably Rnf. The Tafel plot revealed that rice straw hydrolysate supplemented with CaCO3 had a low polarisation resistance, Rp, and required less activation energy for the reduction metabolism making it more favourable for solvent production during fermentation by C. sporogenes BE01.
4. Experimental methods
4.1. Pretreatment and hydrolysis of rice straw
Dilute acid (0.4% w/w H2SO4) was used to pretreat the rice straw obtained from a local vendor. Rice straw was knife milled to reduce the size and the pretreatment was carried out for 1 h at 120 °C with a solid loading of 15% (w/w). The pentose fraction obtained (liquid stream) was separated from the solids by filtration and centrifugation. Pretreated rice straw was dried at room temperature till the excess moisture was removed. Commercial cellulase (Zytex India ltd, Mumbai) with an enzyme activity of 80 FPU mL−1 was used for the enzymatic hydrolysis of the pretreated rice straw at 50 °C. 30 FPU per gds was used for the hydrolysis and the run was continued for 48 h. The suspended and unhydrolyzed solid mass was separated by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min.
000 rpm for 20 min.
4.2. Fermentation
C. sporogenes BE01 was maintained in its spore form at 4 °C. The spores were heat shocked at 80 °C for 2 min and the temperature was immediately brought down by placing in an ice-water bath. The heat shocked spores were cultured in a TGY medium to develop the preinoculum. Actively growing cells were inoculated into a fresh TGY medium for inoculum generation. The highly motile 12 h old culture was inoculated into rice straw hydrolysate. Rice straw hydrolysate was made anaerobic by cooling under an nitrogen atmosphere after heat sterilization at 120 °C for 10 min. Bottles with loosened caps were placed inside the anaerobic chamber for 12 h before inoculation.Fermentation was carried out for 96 h in 250 mL bottles containing 200 mL of rice straw hydrolysate medium with 10% (v/v) inoculum. As described in the previous reports, CaCO3 and yeast extract (Himedia, India) were the only supplementation and cysteine HCl (Himedia, India) was added as a reducing agent.20 The initial pH of the hydrolysate was 4.8, but the addition of yeast extract and CaCO3 increased the pH of the medium to 6, which was further adjusted to the required pH using 1 N NaOH and 1 N HCl. The initial pH of 5.8, 6.2, 6.4 and 6.8 with 10 g L−1 CaCO3 was chosen to study the effect of the initial pH on the bioprocess. For analysing the effect of pH on glucose utilisation and fermentation, rice straw hydrolysate was supplemented with 10 g L−1 of CaCO3 and the pH was adjusted to 5.8, 5.2, 6.4 and 6.8 and three different concentrations of CaCO3 (0 g L−1, 5 g L−1 and 10 g L−1) were investigated to understand the role and effect of CaCO3 on the redox microenvironment of rice straw hydrolysate inoculated with C. sporogenes BE01. The pH change of the fermentation medium was monitored at 24 h intervals and samples were collected for analysis.
4.3. Analytical methods
Percentage hydrogen analysis of the total gas produced was carried out by gas chromatography (Nucon) equipped with a thermal conductivity detector (TCD). A 2.1/8′′ × 2 m SS column with a molecular sieve stationary phase of size 60/80 mesh was used for gas separation at a temperature of 60 °C. The carrier gas used was nitrogen at a flow rate of 20 mL min−1 under 1 kg cm−2 pressure. The detector and injector were operated at 80 and 50 °C, respectively.
Electrokinetic analysis was performed by plotting the Tafel slope using autolab software. The natural log of the anodic current (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I) was plotted against the applied range (E/V) for presenting the Tafel plot. The oxidation slope and reduction slope for every voltammogram was recorded and plotted against time to understand the fluctuations in the redox environment with respect to time.39 The current versus voltage curve approximates a straight line and the slope obtained from this is the polarisation resistance, Rp.40
I) was plotted against the applied range (E/V) for presenting the Tafel plot. The oxidation slope and reduction slope for every voltammogram was recorded and plotted against time to understand the fluctuations in the redox environment with respect to time.39 The current versus voltage curve approximates a straight line and the slope obtained from this is the polarisation resistance, Rp.40
Acknowledgements
LDG would like to acknowledge the Council of Scientific and Industrial Research (CSIR) for providing a senior research fellowship for her PhD work.Notes and references
- V. Garcia, J. Pakkila, H. Ojamo, E. Muurinen and R. L. Keiski, Renewable Sustainable Energy Rev., 2011, 15, 964–980 CrossRef CAS PubMed.
- E. M. Barnes and M. Ingram, J. Appl. Bacteriol., 1956, 19, 117–128 CrossRef CAS PubMed.
- G. Rao and R. Mutharasan, Appl. Environ. Microbiol., 1987, 53, 1232–1235 CAS.
- E. Marsili, J. B. Rollefson, D. B. Baron, R. M. Hozalski and D. R. Bond, Appl. Environ. Microbiol., 2008, 74, 7329–7337 CrossRef CAS PubMed.
- R. Geshlagi, J. M. Scharer, M. Moo-Young and C. P. Chao, Biotechnol. Adv., 2009, 764–781 CrossRef PubMed.
- M. Juanita and W. Guangyi, Int. J. Hydrogen Energy, 2009, 34, 7404–7416 CrossRef PubMed.
- KEGG PATHWAY: Pyruvate metabolism – Clostridium acetobutylicum. Genome.jp. Retrieved 2010-07-01.
- Y. Tao, Y. Chen, Y. Wu, Y. He and Z. Zhou, Int. J. Hydrogen Energy, 2007, 32, 200–206 CrossRef CAS PubMed.
- G. Matta-el-Ammouri, R. Janati-Idrissi, A. M. Junelles, H. Petitdemange and R. Gay, Biochimie, 1987, 69, 109–115 CrossRef CAS.
- W. S. Lee, A. S. May Chua, H. K. Yeoh and G. C. Ngoh, J. Chem. Technol. Biotechnol., 2014, 89, 1038–1043 CrossRef CAS.
- X. Yang, M. Tu, R. Xie, S. Adhikari and Z. Tong, AMB Express., 2013, 3, 3, DOI:10.1186/2191-0855-3-3.
- B. Han, V. Ujor, L. B. Lai, V. Gopalan and T. C. Ezeji, Appl. Environ. Microbiol., 2013, 79, 282–293 CrossRef CAS PubMed.
- C. Richmond, B. Han and T. C. Ezeji, Cont. J. Microbiol., 2011, 5, 18–28 Search PubMed.
- A. G. Le Bourhis, J. Doré, J. P. Carlier, J. F. Chamba, M. R. Popoff and J. L. Tholozan, Int. J. Food Microbiol., 2007, 25(113), 154–163 CrossRef PubMed.
- T. J. Montville, N. Parris and L. K. Conway, Appl. Environ. Microbiol., 1985, 49, 733–736 CAS.
- Z. Ying and Y. Shang-Tian, J. Biotechnol., 2004, 110, 143–157 CrossRef PubMed.
- E. E. Stinson and K. A. Naftulin, J. Ind. Microbiol., 1991, 8, 59–63 CrossRef CAS.
- J. Masset, M. Calusinska, C. Hamilton, S. Hiligsmann, B. Joris, A. Wilmotte and P. Thonart, Biotechnol. Biofuels, 2012, 5, 35, DOI:10.1186/1754-6834-5-35.
- H. Zhang, M. A. Bruns and B. E. Logan, Water Res., 2006, 40, 728–734 CrossRef CAS PubMed.
- L. D. Gottumukkala, B. Parameswaran, S. K. Valappil, K. Mathiyazhakan, A. Pandey and R. K. Sukumaran, Bioresour. Technol., 2013, 145, 182–187 CrossRef CAS PubMed.
- S. Y. Lee, J. H. Park, S. H. Jang, L. K. Nielsen, J. Kim and K. S. Jung, Biotechnol. Bioeng., 2008, 101, 209–227 CrossRef CAS PubMed.
- G. Karp, in Cell and Molecular Biology: Concepts and experiments, John Wiley & Sons, 6th edn, 2009, ch. 4, pp. 173–205 Search PubMed.
- S. G. Mayhew, in Flavoprotein protocols, ed. S. K. Chapman and G. A. Reid, Springer, 1999, vol. 131, ch. 4, pp. 49–60 Search PubMed.
- R. Klaus-Heinrich, in eLS, John Wiley & Sons Ltd, Chichester, 2001, http://www.els.net, DOI:10.1038/npg.els.0001373.
- T. Ohnishi, Biochim. Biophys. Acta, 1998, 1364, 186–206 CrossRef CAS.
- I.-J. Lin, E. B. Gebel, T. E. Machonkin, W. M. Westler and J. L. Markley, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 14581–14586 CrossRef CAS PubMed.
- P. Berto, M. Di Valentin, L. Cendron, F. Vallese, M. Albertini, E. Salvadori, G. M. Giacometti, D. Carbonera and P. Costantini, Biochim. Biophys. Acta, 2012, 1817, 2149–2157 CrossRef CAS PubMed.
- Y. Zhang and T. C. Ezeji, Biotechnol. Biofuels, 2013, 6, 66, DOI:10.1186/1754-6834-6-66.
- K. Brzoska, S. Meczynska and M. Kruszewski, Acta Biochim. Pol., 2006, 53, 685–691 CAS.
- W. Buckel and R. K. Thauer, Biochim. Biophys. Acta, 2013, 1827, 94–113 CrossRef CAS PubMed.
- B. Ward, in Molecular medical microbiology: Bacterial energy metabolism, ed. Y.-W. Tang, M. Sussman, D. Liu, I. Poxton and J. Schwatrzman, Academic press, 2nd edn, 2014, vol. 1, ch. 11, pp. 201–234, 2002 Search PubMed.
- M. Calusinska, T. Happe, B. Joris and A. Wilmotte, Microbiology, 2010, 156, 1575–88 CrossRef CAS PubMed.
- H. Seederf, W. F. Fricke, B. Veith, H. Bruggemann, H. Liesegang, A. Strittmatter, M. Miethke, W. Buckel, J. Hinderberger, F. Li, C. Hagemeier, R. K. Thauer and G. Gottschalk, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 2128–2133 CrossRef PubMed.
- H. Nagarajan, M. Sahin, J. Nogales, H. Latif, D. R. Lovley, A. Ebrahim and K. zengler, Microb. Cell Fact., 2013, 12, 118 CrossRef PubMed.
- M. Calusinska, T. Happe, B. Joris and A. Wilmotte, Microbiology, 2010, 156, 1575–1588 CrossRef CAS PubMed.
- H. Bruggemann, A. Wollherr, C. Mazuet and M. R. Popoff, in Genomes of foodborne and waterborne pathogens: Clostridium botulinum, ed. P. Fratamico, Y. Liu and S. Kathariou, ASM press, 2010, ch. 13 Search PubMed.
- A. T. Carter and M. W. Peck, Res. Microbiol., 2014 DOI:10.1016/j.resmic.2014.10.010.
- A. J. Bard and L. R. Faulkner, in Electrochemical Methods: Fundamentals and Applications, Wiley, 2nd edn, 2000, ch. 3, pp. 105–134 Search PubMed.
- S. V. Mohan, C. N. Reddy, A. N. Kumar and J. A. Modestra, Bioresour. Technol., 2013, 147, 424–433 CrossRef PubMed.
- Metrohm Autolab B.V, http://www.ecochemie.nl/download/Applicationnotes/Autolab_Application_Note_COR03.pdf (accessed February 2015).
| This journal is © The Royal Society of Chemistry 2015 |