Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrocarbon liquid production via the bioCRACK process and catalytic hydroprocessing of the product oil
N.
Schwaiger
*ac,
D. C.
Elliott
b,
J.
Ritzberger
c,
H.
Wang
b,
P.
Pucher
c and
M.
Siebenhofer
a
aInstitute of Chemical Engineering and Environmental Technology, NAWI Graz, Central Lab Biobased Products, Graz University of Technology, Inffeldgasse 25/C, 8010 Graz, Austria. E-mail: nikolaus.schwaiger@tugraz.at
bPacific Northwest National Laboratory, PO Box 999, Richland, WA 99352, USA
cBDI-BioEnergy International AG, Parkring 18, 8074 Grambach/Graz, Austria
First published on 13th February 2015
Abstract
Continuous hydroprocessing of liquid phase pyrolysis Bio-oil, provided by BDI-BioEnergy International bioCRACK pilot plant at OMV Refinery in Schwechat/Vienna Austria was investigated. These hydroprocessing tests showed promising results using catalytic hydroprocessing strategies developed for unfractionated Bio-oil. A sulfided base metal catalyst (CoMo on Al2O3) was evaluated. The bed of catalyst was operated at 400 °C in a continuous-flow reactor at a pressure of 12.1 MPa with flowing hydrogen. The condensed liquid products were analyzed and found that the hydrocarbon liquid was significantly hydrotreated so that nitrogen and sulfur were below the level of detection (<0.05), while the residual oxygen ranged from 0.7 to 1.2%. The density of the products varied from 0.71 g mL−1 up to 0.79 g mL−1 with a correlated change of the hydrogen to carbon atomic ratio from 2.1 down to 1.9. The product quality remained high throughout the extended tests suggesting minimal loss of catalyst activity through the test. These tests provided the data needed to assess the quality of liquid fuel products obtained from the bioCRACK process as well as the activity of the catalyst for comparison with products obtained from hydrotreated fast pyrolysis Bio-oils from fluidized-bed operation.
Introduction
Fast pyrolysis of biomass is a viable technology for the direct production of liquid fuels.1 Liquid phase pyrolysis of biomass is an alternative technology to fast pyrolysis. Although product classes, such as liquid phase Bio-oil and biochar, are similar, the differences in operation and product composition are significant. Liquid phase pyrolysis is usually powered with a liquid heat carrier.2 This heat carrier limits the operation temperature to less than 400 °C according to the boiling point and thermal stability. This temperature limit leads to a higher amount of biochar and less liquid phase Bio-oil production with higher water content and acid number. A major advantage of liquid phase pyrolysis over fast pyrolysis in fluidized bed operation is elevated heat transfer in the liquid heat carrier phase. Also, biochar and inorganics are retained in the liquid heat carrier. Liquid phase Bio-oil is not contaminated with solids. Thus, hot vapor filtration3 for dust removal from the vapor phase is not needed.4 However, depending on the heat carrier biomass is partially dissolved in it.The Bio-oil product from fast pyrolysis and liquid phase pyrolysis, however, is not of sufficient quality for direct use as petroleum refinery feedstock. Catalytic hydroprocessing has been developed to convert the highly oxygenated Bio-oil components into hydrocarbons.5 Conventional hydrotreating processes cannot be directly applied for upgrading of fast pyrolysis Bio-oil. Specifically, the necessity of a two-temperature strategy was identified.6
The objective of this research project was to develop a catalytic hydrotreating process for the production of crude petroleum refinery feedstock from biomass, specifically from condensate of the bioCRACK process. From bioCRACK pyrolysis two different fractions of condensate, high aqueous Bio-oil and Dehydrated Bio-oil, are collected. These feedstocks need hydroprocessing to produce a refinery compatible hydrocarbon-like feedstock. Previous hydrodeoxygenation studies have been performed in a batch reactor with the bioCRACK Bio-oil and Dehydrated Bio-oil using precious and base metal catalysts at lower temperature. The process resulted in a partially deoxygenated Bio-oil with some improvements in reduced heavy product compared to conventional fast pyrolysis Bio-oil hydroprocessing.7
Investigations focused on hydrotreating of condensate from liquid phase pyrolysis of spruce wood pellets. The Bio-oils were produced in a bioCRACK reactor located at the OMV refinery complex in Schwechat, Austria. The Bio-oil products were hydrotreated in a bench-scale, continuous-flow, packed-bed catalytic reactor at Pacific Northwest National Laboratory (PNNL).
Experimental
The pyrolysis experiments were performed in the BDI-BioEnergy International AG bioCRACK pilot plant facility at OMV refinery Vienna/Schwechat. Fig. 1 shows an image of the pilot plant facility. Spruce pellets were the feedstock for liquid phase pyrolysis. VGO (vacuum gas oil) was the liquid heat carrier. The biomass feed rate was between 60–100 kg h−1. The ratio of biomass and VGO varied between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. Pyrolysis temperature was between 350–400 °C.
6. Pyrolysis temperature was between 350–400 °C.
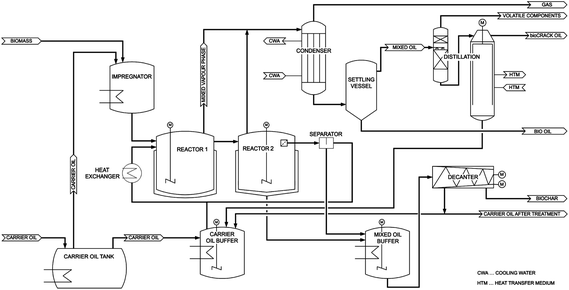
The flow sheet of the bioCRACK pilot plant is shown in Fig. 2. Biomass and liquid heat carrier oil are fed simultaneously into the impregnator. From there a biomass heat carrier slurry is transferred into the reactor 1 and 2 were the biomass is immediately heated to 375 °C. The biogenic and the fossil vapors are cooled in the condenser. The settling vessel separates the condensed vapors into an aqueous Bio-oil fraction and the non-polar bioCRACK oil fraction. In the following distillation step high boiling heat carrier residues are separated from the nonpolar bioCRACK oil fraction. After pyrolysis the heat carrier is separated from biochar.
For further lab scale processing the residual heat carrier is separated from biochar by solid liquid extraction. Biochar can then undergo liquefaction.8–10
bioCRACK bio-oil dehydration
Due to the high water content of aqueous Bio-oil, dehydration was tested to raise the energy content and to lower transport volume. Dehydration of flash pyrolysis Bio-oil was already tested,11,12 but there is no data available for liquid phase pyrolysis Bio-oil.Dehydration was performed by short path distillation. The apparatus had a heat exchanger surface of 0.1 m2. The heat carrier operating temperature was 130 °C and operating pressure was 130 mbar. It has been reported,13 that upgrade of Bio-oil distillate with ethanol may increase economic revenue.
Hydroprocessing
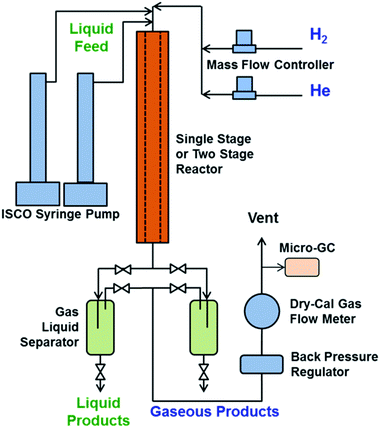
bioCRACK Bio-oil samples of dehydrated Bio-oil and a native Bio-oil were shipped to PNNL. The Bio-oils were hydroprocessed in a mini-hydrotreater (see Fig. 3). The hydrotreater is a single pass, co-current, continuous, down-flow reactor. The system can operate up to 12.4 MPa (1800 psig) with a maximum catalyst temperature of 400 °C. The setup consists of a gas feed and liquid feed system, the reactor and a gas–liquid separation system. The gas feed system consists of a manifold for feeding hydrogen through one mass flow controller and helium through a second mass flow controller. The liquid Bio-oil feedstock is delivered to the pressurized reactor system by two high pressure ISCO syringe pumps. The tubular fixed-bed catalytic hydrotreater is made of 316 stainless steel, 13 mm (1/2′′) internal diameter by 64 cm long with 40 ml capacity for single stage heater or 24 + 24 ml capacity for two-stage hydrotreating. The reactor is heated by a single heating zone. The liquid feedstock and hydrogen gas entered the top of the catalyst bed and passed downward through the bed in a trickle flow. The temperature of the catalyst bed was monitored by thermocouples in a thermocouple well (5 mm (3/16′′) tubing). After exiting the catalytic reactor, the liquid products were separated from the gaseous products in one of two pressurized and cooled traps placed in parallel flow downstream of the reactor system. Periodically liquid samples were collected when switching collection vessels and venting/draining the trap. The recovered liquid products were phase-separated, weighed, and sampled for further analysis. The off-gas passed a back-pressure regulator and was then directed through a DryCal gas meter to monitor the gas flowrate. Periodically gas samples were analyzed by an online Inficon Micro-GC 3000 4-Channels micro gas chromatograph with molecular sieve, Plot U, Alumina, and Stabilwax columns. Prior to each hydrotreating test, the micro GC was calibrated using a calibration gas standard.Campaigns were performed for each feed over the course of a five-day test, and the products and feed were collected to assess performance for each Bio-oil for comparison with the results obtained from processing of fast pyrolysis Bio-oil.
The hydroprocessing tests performed well with CoMo catalyst, sulfided in situ. The reactor tube containing the catalyst was heated to 150 °C in H2 flow, followed by a temperature ramp from 150 °C to 350 °C over 3 h and H2 flow and sulfiding agent (35% di-tertiarybutyl-disulfide (DTBDS) in decane). Then temperature was raised to 400 °C and held constant for 5 h with H2 and sulfiding agent flow.
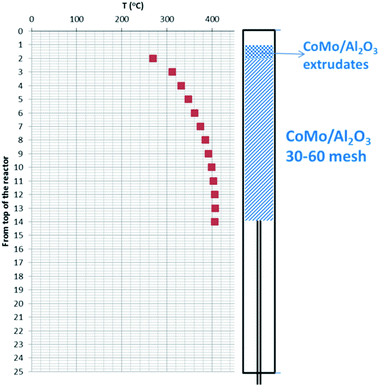
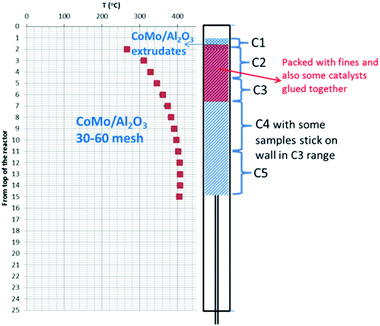
For the hydroprocessing tests the flow ratio of H2/liquid was 2508 L H2 (L Bio-oil)−1. The operating pressure was 12.1 MPa (1750 psi). The Bio-oil feedstock was spiked with DTBDS equaling 150 ppm of sulfur. Fig. 4 shows a schematic of the catalyst bed with a super-imposed temperature profile for the single stage testing mode. The temperatures were monitored at the center line of the catalyst bed by a thermocouple which was adjustable within a full length thermowell. The isothermal part of the catalyst bed is clearly shown and the length of the isothermal part of the catalyst was used to calculate the space velocity.
Analytical methods
The feedstock and Bio-oil products, as produced, were analyzed at BDI-BioEnergy International AG. All liquid and solid products and the feed were characterized by elemental analysis in CHN mode with a Vario macro CHNO-analyzer, from Elementar Analysensysteme. The heat carrier and entrained heat carrier composition and boiling characteristics were determined with a GC-SimDis MXT 2887, 10 m column from Restek and Agilent 7890A GC. Water was measured with GC-TCD. Determination of biomass volatiles was done according to Standard EN 15148. 14C analytics was done by Beta Analytic Limited. For CO and CO2 detection an ABB gas analyser with an uras 26 infrared photometer was used and Oxygen was measured with a Magnos 206 detector.The Bio-oils and hydrotreated products were characterized at PNNL for elemental analysis, including C, H, N, O, & S, Total Acid Number (TAN), water content, metals content, and by GC-MS. Using a DB-5 column over a temperature program, separation of the Bio-oils was performed and mass spectrometric analysis undertaken with a Mass Selective Detector.
Results
Feedstock
Results from the feedstock analyses are shown in Table 1.| Proximate analysis (wt%) | Ash | Ultimate analysis (wt%) | |||||
|---|---|---|---|---|---|---|---|
| Volatiles | Fixed carbon | C | H | N | O by diff. | ||
| Spruce pellets | 84.94 | 14.68 | 0.38 | 50.67 | 6.30 | 0.04 | 42.99 |
Results of liquid phase pyrolysis according to the bioCRACK process
The yield of the major products (oil, char, and gas) of the bioCRACK process is shown in Fig. 5. The figure shows the mass balance based on 14C analysis of an experiment at 375 °C with a biomass feed of 65 kg h−1. The amount of biomass fed, Bio-oil fractions, and char were determined gravimetrically.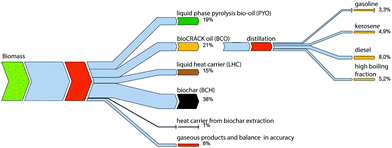 | ||
| Fig. 5 Biogenous carbon mass balance of liquid phase pyrolysis as performed in the bioCRACK pilot plant at OMV Refinery Vienna.14 | ||
During liquid phase pyrolysis in the bioCRACK process biochar (BCH) and gas/vapor is formed from biomass constituents. Table 2 shows the elemental composition of the product streams. Differently to flash pyrolysis three liquid product streams are formed in the bioCRACK process. The first fraction is a high boiling fraction of decomposed biomass, which is dissolved during liquefaction into the heat carrier. 15 (wt%) of the biogenous carbon feed is solved into this fraction and the concentration of biogenous carbon in this fraction is 2.0 (wt%). The second liquid fraction is the so called bioCRACK oil (BCO). This is a non-polar phase of biomass decomposition products and the degraded heat carrier. During pyrolysis 21% of the biogenic carbon from biomass is directly dissolved into this hydrocarbon fraction and the concentration of biogenous carbon is 6.7 (wt%). The bioCRACK oil can be fractionated into a gasoline, kerosene, diesel and high boiling fraction by distillation or further processed to a Diesel like Fuel by catalytic co-hydrodeoxygenation with Bio-oil.15
| Product stream | C (wt%) | H (wt%) | N (wt%) | Residual (wt%) |
|---|---|---|---|---|
| Biochar (BCH) | 80.9% | 5.4% | <1 | 13.5% |
| BioCRACK oil (BCO) | 84.8% | 12.4% | <1 | 2.4% |
| Liquid heat carrier (LHC) | 86.5% | 12.1% | <1 | 0.9% |
This bioCRACK oil is evaporated together with the Bio-oil fraction, which is the third liquid fraction of the bioCRACK process. The dissolution of biogenic compounds into the heat carrier and the bioCRACK oil phase is the major reason for the low carbon content, the high acid content and the high water- and oxygen content of the polar aqueous bioCRACK Bio-oil.
The major gas components are given in Table 3.
| Sample | CO (v/v%) | CO2 (v/v%) | CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO CO |
|---|---|---|---|
| 43.8 | 44.5 | 1.01 |
Bio-oil dehydration
During short path distillation Bio-oil was split in two fractions, 74% of condensate and 22% bottom product, latter being used for hydrodeoxygenation. 4% of the feed were lost as light boiling fraction due to low pressure operation at 130 mbar. Table 4 shows the composition of the feed compared to the dehydration products (Bio-oil).| Sample | C (wt%) | H (wt%) | N (wt%) | O (wt%) | Ash (wt%) | H2O (wt%) | Density (g mL−1) | pH |
|---|---|---|---|---|---|---|---|---|
| Dehydrated Bio-oil | 50.5 | 7.1 | 0.4 | 41.5 | 0.5 | 9.9 | 1.22 | 2.7 |
| Bio-oil | 23.2 | 9.4 | 0.3 | 67.1 | NA | 56.3 | 1.07 | 2.6 |
| Bio-oil condensate | 14.4 | 9.97 | 0.3 | 75.3 | NA | 68.9 | 1.04 | 3.0 |
Bio-oil fraction analysis
The results of ultimate, proximate, and water by Karl–Fisher titration analysis are in Table 4. These analyses are of the bioCRACK Bio-oil fractions as recovered from the pilot plant. The organic O contents in the Bio-oils were calculated from the difference in total O (determined by difference) and O in water.The Bio-oils were analyzed at PNNL. The results are shown in Table 5. The C, H, O composition is calculated from wet oil composition by subtracting the amount of oxygen and hydrogen of the measured moisture content. Detailed trace element analysis of the wet Bio-oils was performed by ICP. The results are shown in Table 6. The Bio-oils are essentially mineral free, but with a significant amount of sulfur. The TAN (total acid number) was also determined by PNNL. Viscosity and density were determined with a Stabinger viscosimeter according to ASTM D7042.
| Sample name | C (wt% dry) | H (wt% dry) | H/C ratio dry basis | O (wt% dry) | Moisture (wt%) | N (wt% wet) | S (wt% wet) | Density (g ml−1 @40 °C) | TAN (mg KOH g−1) | Viscosity (mm2 s−1 @40 °C) |
|---|---|---|---|---|---|---|---|---|---|---|
| Dehydrated Bio-oil | 59.1 | 6.7 | 1.36 | 33.4 | 10.24 | 0.14 | 0.50 | 1.226 | 135 | 105 |
| Bio-oil | 51.1 | 6.2 | 1.45 | 42.6 | 57.43 | <0.05 | 0.03 | 1.097 | 101 | 2.3 |
| S (ppm) | Al (ppm) | Si (ppm) | K (ppm) | Fe (ppm) | Ca (ppm) | Mg (ppm) | P (ppm) | |
|---|---|---|---|---|---|---|---|---|
| Dehydrated bio-oil | 3372 | <15 | <15 | 24 | 39 | 17 | <15 | <15 |
| Bio-oil | 557 | <15 | <15 | <15 | <15 | <15 | <15 | <15 |
Semi-quantitative analysis of the two bioCRACK feedstocks was performed with gas chromatography-mass spectrometry (GC-MS). With the Agilent peak matching program tentative identifications were applied to the components and their relative quantities were determined based on total ion current. The results are presented in Table 7, showing the relative quantities of the identified components. The two bio-oil fractions show some distinct differences in composition. Overwhelmingly they contain typical fast pyrolysis Bio-oil components, a mixture of guaiacols and light oxygenates. The guaiacol (2-methoxyphenol) compounds have the typical alkyl and carbonyl substituents on the 4 position. There is a significant amount of levoglucosan in both Bio-oil fractions, but significantly lower concentration in the whole Bio-oil. The Bio-oil product has a large number of light oxygenates, which were not found in the Dehydrated Bio-oil. These compounds, e.g. acetic acid and acetol (hydroxyacetone), were separated during distillation. On the other hand the Dehydrated Bio-oil has a larger concentration of all the phenolic compounds, with the exception of guaiacol and methyl guaiacol.
| Component | Dehydrated Bio-oil | Bio-oil | ||
|---|---|---|---|---|
| Retention time | Quantity | Retention time | Quantity | |
| a ND = not detected. | ||||
| Methyl acetate | 1.756 | 1.3 | 1.737 | 3 |
| Formic acid | NDa | 1.96 | 0.5 | |
| Acetic acid | 2.37–2.49 | 6.8 | 2.7 | 25.9 |
| Acetol (hydroxyacetone) | 2.79–3.01 | 3.8 | 2.82 | 21.1 |
| Propionic acid | ND | 4.20–4.34 | 1.5 | |
| 1-Hydroxy-2-butanone | ND | 5.10–5.16 | 0.3 | |
| Butanedial | ND | 5.74–5.90 | 0.4 | |
| Methylene cyclopropane | ND | 7.92–7.95 | 0.2 | |
| Cyclopentenones | ND | 8.00–8.10 | 0.2 | |
| Methyl cyclopentenone | ND | 10.86–10.90 | 0.4 | |
| γ-Butyrolactone | 11.64–11.77 | 0.4 | 11.34–11.44 | 1.3 |
| Methyl furfural | ND | 12.51 | 0.5 | |
| 3-Methyl-2,5-dihydrofuran | ND | 13.04 | 0.4 | |
| Corylone (hydroxymethylcyclopentenone) | 13.79–13.91 | 6.1 | 13.71–14.02 | 7.5 |
| Methyl-2,3-dihydrofuran | 13.92–13.96 | 5 | ND | |
| Trans-cyclopentanediol | ND | 14.05 | 1 | |
| Guaiacol | 14.63–14.65 | 1.2 | 14.56 | 2.4 |
| Methyl guaiacol | 16.02–16.08 | 3.8 | 15.99 | 3.8 |
| Catechol | 16.94 | 1.4 | 16.94 | 0.8 |
| Ethyl guaiacol | 17.06 | 3.4 | 17.06 | 2.2 |
| Hydroxy dimethyl cyclopentenone | 17.21 | 0.3 | 17.26 | 1.1 |
| Hydroquinone | 17.76 | 4.1 | 17.81–17.92 | 2.8 |
| Propyl guaiacol | 18.05 | 2.9 | 18.05 | 1.6 |
| Guaiacol formaldehyde (vanillin) | 18.68 | 6.6 | 18.73 | 2.4 |
| Methyl benzaldehyde | 19.03 | 3.6 | ND | |
| Guaiacol ethanone | 19.55 | 4.3 | 19.57 | 1.6 |
| Guaiacol propanone | 19.92 | 8.2 | 19.93 | 3.3 |
| Levoglucosan | 20.15–20.38 | 35.3 | 20.20–20.69 | 13.7 |
| Ethyl homovanillate | 26.54 | 1.4 | 26.58 | 0.3 |
Hydroprocessing results
For both of the reported tests the products and data were collected over the entire period with individual products and data sets collected in operating windows from 6 to 12 h long. The hydrogen consumption has been calculated and the yield of gas and oil products determined.The Dehydrated Bio-oil feedstock was pumped directly into the mini-hydrotreater without pre-processing. The feedstock was assumed to have <0.1% filterable solids content, based on BDI data provided. A fixed bed of pre-sulfided CoMo on alumina catalyst (3.5% CoO and 14% MoO3) from AlfaAesar (#40435) ground to a 30–60 mesh particle size was used at standard conditions of nominally 400 °C, 12.1 mPa, and a liquid hourly space velocity of 0.2. Three oil samples selected to represent the product over the 54 h test were analyzed as reported in Table 8. Elemental contents are normalized to 100%; S and N were <0.02 and <0.05, respectively.
| C content dry basis | H content dry basis | O content dry basis | H/C ratio dry basis | Density, g ml−1 | Moisture content | Total acid number | Mass balance | Carbon balance |
|---|---|---|---|---|---|---|---|---|
| 85.04 | 13.86 | 1.10 | 1.94 | 0.755 | 0.24 | <0.01 | 93.6 | 90.6 |
| 85.55 | 13.24 | 1.21 | 1.84 | 0.784 | 0.26 | <0.01 | 99.2 | 98.5 |
| 85.41 | 13.51 | 1.08 | 1.88 | 0.789 | 0.30 | <0.01 | 92.4 | 88.3 |
Trace element analysis of the feedstock showed only small amounts of a few expected biomass components, 17 ppm Ca and 24 ppm K with 38 ppm Fe and 3320 ppm S. The iron is likely a corrosion product. The high sulfur level is unexpected. The S number for the feedstock was found by inductively coupled plasma-optical emission spectroscopy (ICP-OES) measurement, but it is similar to that by the thermal method (0.50 wt%). Since a sulfided catalyst was used for the processing there was no conflict. In fact, we added di-tertiarybutyl-disulfide to the feedstock to maintain at least 150 ppm of sulfur.
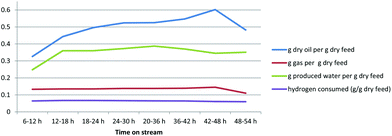
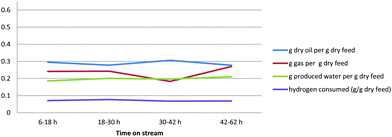
The operating results as shown in Fig. 6 were fairly consistent throughout the test period. The liquid oil yield from the bioCRACK Dehydrated Bio-oil was 0.5 to 0.6 g g−1, with lower but still significant gas and water production. The hydrogen consumption was a bit higher than typically seen with fast pyrolysis Bio-oil.
Gas products were analyzed through the test using gas chromatography. The gas product was composed of carbon oxides (21–26% CO2 and 4–5% CO) and alkane hydrocarbon gases (22–25% CH4, 22–19% C2, 14–12% C3, 6–11% C4, 5% C5) diluted with the excess hydrogen (93–94 vol% of off gas).
The 316 SS tubular reactor is depicted in Fig. 7 and the area of fouled catalyst after the test is shaded in red.
ICP analysis of the spent catalyst bed showed some evidence of deposits in the bed. As might be expected the feed contaminants, iron, calcium, and potassium, were found at levels higher than in the fresh catalyst with exceptionally high levels at the point in the catalyst bed where the reactants exceeded 300 °C. Zinc and manganese (below detection limit in the feed) also followed this trend, as did chromium and nickel, which are likely reactor wall corrosion products.
A similar test was performed with the bioCRACK Bio-oil product. The Bio-oil feedstock was pumped directly into the mini-hydrotreater without pre-processing. Four oil samples selected to represent the product over the 62 h test were analyzed as reported in Table 9. Elemental contents are normalized to 100%; S and N were <0.05 and <0.05, respectively.
| C content dry basis | H content dry basis | O content dry basis | H/C ratio dry basis | Density, g ml−1 | Moisture content | Total acid number | Mass balance | Carbon balance |
|---|---|---|---|---|---|---|---|---|
| 84.30 | 14.96 | 0.74 | 2.11 | 0.712 | 0.36 | <0.01 | 85.6 | 84.9 |
| 83.94 | 15.22 | 0.84 | 2.15 | 0.722 | 0.34 | <0.01 | 85.4 | 81.7 |
| 84.27 | 14.77 | 0.96 | 2.08 | 0.730 | 0.30 | <0.01 | 84.2 | 77.8 |
| 84.41 | 14.91 | 0.68 | 2.10 | 0.726 | 0.44 | <0.01 | 86.5 | 85.2 |
The operating results as shown in Fig. 8 were fairly consistent throughout the four test periods. The liquid oil yield from the bioCRACK Bio-oil was only 0.3 g per g of dry feed with significant gas and water production as well. The yield of dry oil product on a carbon basis is similar to the Dehydrated Bio-oil, at about 50%. The hydrogen consumption was also high at about 7 wt% on a dry feed basis.
Gas products were analyzed through the test using gas chromatography. The gas product was composed of carbon oxides (6–9% CO2 and 0% CO) and alkane hydrocarbon gases (21–17% CH4, 30–35% C2, 25–21% C3, 18–11% C4, 0–5% C5) diluted with the excess hydrogen (95–97 vol% of off gas).
No trace elements were detected in the Bio-oil by ICP (<15 ppm) except sulfur. There were elements found deposited onto the catalyst after the test including Si, Ca, Mg, and Na, which were likely derived from the feedstock. In addition, there were elevated levels of Fe and Cr, which could be attributed to corrosion.
Discussion
The bioCRACK Bio-oil fractions performed well for up to 62 h when using a representative hydrotreating catalyst in a single temperature stage configuration. The light oil phase product was sufficiently hydrotreated so that nitrogen and sulfur were at or below the level of detection, while the residual oxygen content was low, <1%. The density of the products were relatively low compared to literature values for hydrotreated Bio-oil, 0.71 g mL−1 up to 0.79 g mL−1. The lighter products were produced from the Bio-oil fraction which was found to contain lower molecular weight and more saturated components as fed to the hydrotreater. It is no surprise that the product from the higher molecular weight and more aromatic Dehydrated Bio-oil is higher in density. The Dehydrated Bio-oil appears to contain less reactive functional groups, which are less easily deoxygenated as shown by the difference in oxygen analysis, a reduction of 98.1% for the Bio-oil and only 96.6% reduction in the Dehydrated Bio-oil. Since both Bio-oil feedstocks were processed at the same space velocity, the higher oil product yield and lower gas product yield for the dehydrated product is significant. The space velocity of 0.2 used in these tests is also higher than other reports for hydrotreating Bio-oil to similarly high quality hydrocarbon products.The consistency of the operating results and the products over the time of these experiments suggests little loss of catalyst activity through the test. The apparent drop in oil and gas production in the last data window, when feeding the dehydrated Bio-oil, may be better explained as experimental variability in correction of the higher production in the previous data window. The consistency contrasts with most reports in the literature for hydrotreating Bio-oil.16 Similar consistency of operation has only been achieved by a pretreatment of low severity hydroprocessing prior to the actual hydrotreating.17 In addition, a two-temperature stage hydrotreating was used to avoid fouling of the hydrotreating catalyst bed18 or the use of precious metal catalysts.19
Conclusions
With this mini-hydrotreater system we can make a preliminary assessment of the hydrotreating results with the bioCRACK feedstocks. We conclude that these feedstocks can be readily hydrotreated based on high yield of deoxygenated liquid hydrocarbon product. The results contrast with those for fast pyrolysis Bio-oil in that the catalyst bed did not foul in these extended runs and this even when using only a single temperature bed with conventional hydrotreating catalyst and without a precious metal catalyst hydroprocessing pretreatment. The tests do not represent optimized conditions, but only a first proof of principle. The oil products have been highly saturated and the hydrogen consumption could probably be reduced by changes in operating parameters such as lower operating pressure and faster throughput to reduce the residence time in the catalyst bed.Acknowledgements
This research work was supported by BDI-BioEnergy International AG under Work-For-Others contract. The preparation of the publication was performed with support from the U.S. Department of Energy as part of the Bio-oil Stabilization and Commoditization FOA #0686 under Contract No. DE-AC05-76RL01830 at the Pacific Northwest National Laboratory. The authors gratefully acknowledge the support of the Bioenergy Technologies Office and program manager Prasad Gupta. Suh-Jane Lee and Asanga Padmaperuma are acknowledged for their participation in the operations of the minihydrotreater.Notes and references
- A. V. Bridgwater, Biomass Bioenergy, 2012, 38, 68–94 CrossRef CAS PubMed.
- N. Schwaiger, R. Feiner, K. Zahel, A. Pieber, V. Witek, P. Pucher, E. Ahn, P. Wilhelm, B. Chernev, H. Schröttner and M. Siebenhofer, BioEnergy Res., 2011, 4, 294–302 CrossRef PubMed.
- D. Mohan, C. U. Pittman and P. H. Steele, Energy Fuels, 2006, 20, 848–889 CrossRef CAS.
- N. Schwaiger, V. Witek, R. Feiner, H. Pucher, K. Zahel, a. Pieber, P. Pucher, E. Ahn, B. Chernev, H. Schroettner, P. Wilhelm and M. Siebenhofer, Bioresour. Technol., 2012, 124, 90–94 CrossRef CAS PubMed.
- D. C. Elliott, Energy Fuels, 2007, 21, 1792–1815 CrossRef CAS.
- E. Baker and D. Elliott, US Pat.4,795,841, 1989 Search PubMed.
- H. Pucher, N. Schwaiger, R. Feiner, P. Pucher, L. Ellmaier and M. Siebenhofer, Int. J. Energy Res., 2014, 38, 1964–1974 CrossRef CAS.
- R. Feiner, N. Schwaiger and H. Pucher, RSC Adv., 2014, 4, 34955 RSC.
- R. Feiner, N. Schwaiger, H. Pucher, L. Ellmaier, P. Pucher and M. Siebenhofer, RSC Adv., 2013, 43, 1–6 Search PubMed.
- R. Feiner, N. Schwaiger, H. Pucher, L. Ellmaier, A. Reiter, M. Derntl, T. Glatz, P. Pucher and M. Siebenhofer, BioEnergy Res., 2014, 7, 1343–1350 CrossRef CAS.
- S. Wang, Y. Gu, Q. Liu, Y. Yao, Z. Guo, Z. Luo and K. Cen, Fuel Process. Technol., 2009, 90, 738–745 CrossRef CAS PubMed.
- Z. Guo, S. Wang, Y. Gu, G. Xu, X. Li and Z. Luo, Sep. Purif. Technol., 2010, 76, 52–57 CrossRef CAS PubMed.
- S. Wang, Q. Cai, X. Wang, L. Zhang, Y. Wang and Z. Luo, Energy Fuels, 2014, 28, 115–122 CrossRef CAS.
- J. Ritzberger, P. Pucher, N. Schwaiger and M. Siebenhofer, Chem. Eng. Trans., 2014, 39, 1189–1194 Search PubMed.
- H. Pucher, N. Schwaiger, R. Feiner, L. Ellmaier, P. Pucher, B. Chernev and M. Siebenhofer, Green Chem., 2015, 17, 1291–1298 RSC.
- E. Furimsky, Catal. Today, 2013, 217, 13–56 CrossRef CAS PubMed.
- A. H. Zacher, M. V. Olarte, D. M. Santosa, D. C. Elliott and S. B. Jones, Green Chem., 2014, 16, 491 RSC.
- D. C. Elliott, T. R. Hart, G. G. Neuenschwander, L. J. Rotness, M. V. Olarte, A. H. Zacher and Y. Solantausta, Energy Fuels, 2012, 26, 3891–3896 CrossRef CAS.
- J. Wildschut, F. H. Mahfud, R. H. Venderbosch and H. J. Heeres, Ind. Eng. Chem. Res., 2009, 48, 10324–10334 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |