Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rare-earth recycling using a functionalized ionic liquid for the selective dissolution and revalorization of Y2O3:Eu3+ from lamp phosphor waste†
David
Dupont
and
Koen
Binnemans
*
KU Leuven, Department of Chemistry, Celestijnenlaan 200F – P.O. Box 2404, B-3001 Heverlee, Belgium. E-mail: Koen.Binnemans@chem.kuleuven.be
First published on 28th November 2014
Abstract
The supply risk for certain rare-earth elements (REEs) has sparked the development of recycling schemes for end-of-life products like fluorescent lamps. In this paper a new recycling process for lamp phosphor waste is proposed based on the use of the functionalized ionic liquid betainium bis(trifluoromethylsulfonyl)imide, [Hbet][Tf2N]. This innovative method allows the selective dissolution of the valuable red phosphor Y2O3:Eu3+ (YOX) without leaching the other constituents of the waste powder (other phosphors, glass particles and alumina). A selective dissolution of YOX is useful because this phosphor contains 80 wt% of the REEs although it only represents 20 wt% of the lamp phosphor waste. The proposed recycling process is a major improvement compared to currently used hydrometallurgical processes where the non-valuable halophosphate (HALO) phosphor (Sr,Ca)10(PO4)6(Cl,F)2:Sb3+,Mn2+ is inevitably leached when attempting to dissolve YOX. Since the HALO phosphor can make up as much as 50 wt% of the lamp phosphor waste powder, this consumes significant amounts of acid and complicates the further processing steps (e.g. solvent extraction). The dissolved yttrium and europium can be recovered by a single stripping step using a stoichiometric amount of solid oxalic acid or by contacting the ionic liquid with a hydrochloric acid solution. Both approaches regenerate the ionic liquid, but precipitation stripping with oxalic acid has the additional advantage that there is no loss of ionic liquid to the water phase and that the yttrium/europium oxalate can be calcined as such to reform the red Y2O3:Eu3+ phosphor (purity >99.9 wt%), effectively closing the loop after only three process steps. The red phosphor prepared from the recycled yttrium and europium showed excellent luminescent properties. The resulting recycling process for lamp phosphor waste consumes only oxalic acid and features a selective leaching, a fast stripping and an immediate revalorization step. Combined with the mild conditions, the reusability of the ionic liquid and the fact that no additional waste water is generated, this process is a very green and efficient alternative to traditional mineral acid leaching.
Introduction
Rare-earth elements (REEs) include the 15 lanthanides plus yttrium and scandium. They are used in many high-tech applications, including wind turbines, electric vehicles, NiMH batteries, hard disk drives and fluorescent lamps.1 The demand for these elements is expected to grow by more than 8% per year until 2020.2 China dominates the REE supply from primary mining (>90% of the global production in 2013), and the worldwide recycling rate is still very low (<1%).2 Since the tightening of the Chinese export quota for rare earths in 2010, rare-earth elements have been labeled as critical raw materials by the European Commission and the U.S. Department of Energy.1–4 Opening or reopening mines outside China requires time and large financial investments. Therefore recycling of REEs from end-user products like magnets and fluorescent lamps, which together represent over 70% of the rare-earth market in terms of value (38% for magnets; 32% for lamp phosphors), could help to secure the supply of these critical elements.3 Efficient recycling of these elements could help to create a closed-loop system and to solve the balance problem that is caused by the unwanted co-production of other rare-earth elements during primary mining.3,5 This unwanted co-production has led to the accumulation of large stocks of certain elements (La, Ce) while facing shortages of others (Y, Eu, Tb, Nd, Dy).3–7 New recycling technologies are becoming increasingly selective, efficient and sustainable and they are opening up new possibilities for urban mining and rare-earth waste revalorization.1,3,6–13Originally, the collection of used fluorescent light lamps was put in operation to ensure the safe disposal of the mercury contained in the lamps; however, the lamp phosphors were discarded or stockpiled. It is estimated that by 2020 the stockpiled lamp phosphor waste will contain around 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of rare earths.3 In recent years, the recovery of the REEs contained in these powders has received a lot of attention due to the increasing supply risk for some of these elements.3,9 Lamp phosphor waste powder contains around 20 wt% of REEs including critical (Y, Eu, Tb) and less critical (La, Ce, Gd) REEs.3,5,14 These powders are used in fluorescent light lamps to convert ultraviolet radiation into visible light. They consist of fine particles (1–10 μm) coated on the interior surface of the glass. A blend of red Y2O3:Eu3+ (YOX), blue BaMgAl10O17:Eu2+ (BAM) and green LaPO4:Ce3+, Tb3+ (LAP), (Ce,Tb)MgAl11O19 (CAT) or (Gd,Mg)B5O10: Ce3+, Tb3+ (CBT) phosphors is used to obtain the desired color rendering index (Table 1). In addition to rare-earth phosphors, lamp phosphor waste also often contains large amounts (40–50 wt%) of non-valuable halophosphate (HALO), which emits cold white light and does not contain any REEs.3 The recycling value of the different phosphor components varies greatly. The phosphor with the highest economic value is the red Y2O3:Eu3+ (YOX) phosphor. This phosphor consists almost entirely of the two critical rare earths yttrium and europium as opposed to the other phosphors, which are only doped with small amounts of critical REEs. This explains why YOX holds 80 wt% of the rare earths present in the lamp phosphor waste powder even though YOX only accounts for 20 wt% of the lamp phosphor waste (Table 1).3,14
000 tons of rare earths.3 In recent years, the recovery of the REEs contained in these powders has received a lot of attention due to the increasing supply risk for some of these elements.3,9 Lamp phosphor waste powder contains around 20 wt% of REEs including critical (Y, Eu, Tb) and less critical (La, Ce, Gd) REEs.3,5,14 These powders are used in fluorescent light lamps to convert ultraviolet radiation into visible light. They consist of fine particles (1–10 μm) coated on the interior surface of the glass. A blend of red Y2O3:Eu3+ (YOX), blue BaMgAl10O17:Eu2+ (BAM) and green LaPO4:Ce3+, Tb3+ (LAP), (Ce,Tb)MgAl11O19 (CAT) or (Gd,Mg)B5O10: Ce3+, Tb3+ (CBT) phosphors is used to obtain the desired color rendering index (Table 1). In addition to rare-earth phosphors, lamp phosphor waste also often contains large amounts (40–50 wt%) of non-valuable halophosphate (HALO), which emits cold white light and does not contain any REEs.3 The recycling value of the different phosphor components varies greatly. The phosphor with the highest economic value is the red Y2O3:Eu3+ (YOX) phosphor. This phosphor consists almost entirely of the two critical rare earths yttrium and europium as opposed to the other phosphors, which are only doped with small amounts of critical REEs. This explains why YOX holds 80 wt% of the rare earths present in the lamp phosphor waste powder even though YOX only accounts for 20 wt% of the lamp phosphor waste (Table 1).3,14
| Name | Formula | Waste fractiona (wt%) | Value |
|---|---|---|---|
| a Approximate fraction found in lamp phosphor waste; the remaining consists of SiO2 (as fine glass particles), Al2O3, and small quantities of other phosphors like CAT which behaves similarly to BAM, and CBT. | |||
| HALO | (Sr,Ca)10(PO4)6(Cl,F)2:Sb3+,Mn2+ | 40–50 | Low |
| YOX | Y2O3:Eu3+ | 20 | High |
| BAM | BaMgAl10O17:Eu2+ | 5 | Low |
| LAP | LaPO4:Ce3+,Tb3+ | 6–7 | High |
Many approaches have been proposed for the recycling of lamp phosphors. Physical separation methods like magnetic separation, flotation and centrifugation could allow the direct reuse of the phosphors, but so far they are not used industrially due to the high purity requirements and the deterioration of the phosphor powders during their lifetimes.3,15–17 Chemical methods can be used to dissolve the phosphors completely or selectively based on the increasing difficulty to dissolve some of the phosphors: HALO < YOX ≪ LAP/BAM/CAT.3,9,14,18–21 The halophosphate (HALO) phosphor is easily dissolved in dilute hydrochloric acid solutions at room temperature. The dissolution of Y2O3:Eu3+ (YOX) requires more acidic conditions (e.g. 1 M HCl, 60–90 °C).3,22 LAP requires the use of very strong acidic conditions (e.g. 18 M H2SO4, 120–230 °C),3,22 while the aluminate phosphors BAM and CAT are best dissolved under strongly alkaline conditions (35 wt% NaOH, 150 °C) in an autoclave or by molten alkali (e.g. Na2CO3, 1000 °C).3,14 Ionic liquids have been proposed for the selective extraction of previously dissolved rare earths, but so far they have not been used as a tool for the dissolution of phosphors.22,23 New recycling schemes often focus on the recovery of yttrium and europium from the red phosphor Y2O3:Eu3+ (YOX), because of its high value and the relative ease of dissolving this phosphor compared to BAM, CAT and LAP.18,19,22–24 The main problem with these processes is that the halophosphate phosphor (HALO) is often ignored as they focus mostly on the so-called tri-band phosphors which all contain rare earths (YOX, BAM, LAP, CAT). However, real lamp phosphor waste contains up to 50 wt% of HALO and therefore has to be considered when trying to develop an industrially applicable recycling method.3,9,14,25 Unfortunately, halophosphate (Sr,Ca)10(PO4)6(Cl,F)2:Sb3+,Mn2+ (HALO) is very easily dissolved in dilute acids even at room temperature.3 HALO contains no rare earths and has a very low intrinsic value; therefore dissolving this phosphor leads to considerable pollution of the leachate and introduces a large amount of unwanted exogens (Sr, Ca, F, Sb, Mn, Cl) in the waste water, greatly complicating the further processing (eqn (1)).3
| (Sr,Ca)10(PO4)6(Cl,F)2:Sb3+,Mn2+ + 18H+ → 10(Sr,Ca)2+ + 6H3PO4 + 2(Cl,F)− + Sb3+ + Mn2+ | (1) |
The dissolution of HALO also consumes considerable amounts of acid (18 protons per formula unit of HALO) and leads to the formation of large amounts of H3PO4 (6 molecules per formula unit of HALO) which forms very insoluble YPO4 and EuPO4 precipitates with the dissolved Y3+ and Eu3+ ions from the YOX. The design of a selective dissolution method for YOX without dissolving HALO would therefore greatly increase the efficiency and profitability of a recycling process for lamp phosphor waste, but, to the best of our knowledge, such a process has not been described so far.
At present, only one example is known of an industrially applied recycling process for lamp phosphor waste. This process was successfully implemented by Solvay in 2012 and treats more than 2000 tons of phosphor waste powder per year.8 According to the patent literature, multiple consecutive acidic (HCl, HNO3) and alkaline (NaOH) attacks are required, including an alkaline fusion with Na2CO3 at 1000 °C, to fully disintegrate all the phosphors. The individual rare earths are then separated and recovered using solvent extraction, in order to manufacture new phosphors.14 The main drawbacks of this process are the large consumption of chemicals, the large production of waste water and the large number of process steps required to fully recycle the lamp phosphor waste.3
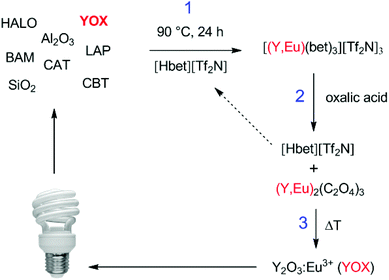
The process described in this paper aims to solve all these issues by proposing a functionalized ionic liquid as an alternative to selectively dissolve and regenerate the valuable red phosphor Y2O3:Eu3+ (YOX). This three-step process (no need for solvent extraction) is very efficient, consumes only oxalic acid and generates zero additional waste (except CO2). The ionic liquid is also automatically regenerated during the stripping step and can be reused. This type of leaching system can be described as ionometallurgy, which is the analogue of hydrometallurgy in ionic liquids and has shown an interesting new behavior in various cases.26–30 Depending on the application, ionic liquids are considered to be green solvents because of their negligible vapor pressure, low flammability and reduced toxicity compared to organic solvents and have led to several important improvements in various fields such as metallurgy, extraction, electrochemistry, organic synthesis and catalysis.10,12,30–35 Here, the functionalized ionic liquid betainium bis(trifluoromethylsulfonyl)imide, [Hbet][Tf2N], is proposed for the selective dissolution of Y2O3:Eu3+ without dissolving the other components in the lamp phosphor waste (HALO, BAM, LAP, CAT, SiO2, Al2O3). [Hbet][Tf2N] is a Brønsted acidic functionalized ionic liquid which has the ability to dissolve rare-earth oxides and many transition metal oxides (Fig. 1).36,37 The dissolution of metal oxides in this ionic liquid has been studied extensively by Nockemann et al.36–38 Silica and alumina cannot be dissolved in [Hbet][Tf2N] which is highly relevant to the recycling of lamp phosphor waste since these powders can contain significant amounts of silica (as fine glass particles) and alumina.3,9 The dissolution is driven by the reactivity of the carboxylic acid group located on the cation of the ionic liquid. In the resulting complex the rare-earth ions are coordinated by the betaine ligands, which are zwitterionic compounds when deprotonated.38 The anions in [Hbet][Tf2N] simply act as spectator anions and do not participate in the complex formation. However, the Tf2N− anion is required to obtain a hydrophobic ionic liquid ([Hbet]Cl is a water-soluble salt). The Tf2N− anion is also very stable and well-suited for high-temperature applications.39
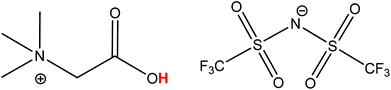 | ||
| Fig. 1 Structure of the ionic liquid betainium bis(trifluoromethylsulfonyl)imide, [Hbet][Tf2N]. The acidic proton of the betaine group is highlighted in red. | ||
A blend of YOX, LAP, BAM and HALO was used to simulate the composition of the lamp phosphor waste and to study the selective dissolution of YOX in [Hbet][Tf2N]. The dissolution of CAT was not investigated in this work because this aluminate phosphor behaves similarly to BAM, meaning that it will not dissolve in the ionic liquid and thus ends up in the same remaining fraction as BAM and LAP.
Experimental
Chemicals
The phosphors BaMgAl10O17:Eu2+ (BAM), Y2O3:Eu3+ (YOX), LaPO4:Ce3+,Tb3+ (LAP), and (Sr,Ca)10(PO4)6(Cl,F)2:Sb3+,Mn2+ (HALO) were purchased from Nichia (Japan) with 99% purity. Betaine chloride (HbetCl) (99%), methyl red sodium salt (pure) and 1,4-dioxane (99.9%) were obtained from Acros Organics (Geel, Belgium). Lithium bis(trifluoromethylsulfonyl)imide (LiTf2N) (99%) was obtained from IoLiTec (Germany) and oxalic acid dihydrate (>99.5%) from J.T. Baker. Heavy water (D2O) (99.9 atom% D) was purchased from Sigma–Aldrich (Belgium). Absolute ethanol and hydrogen chloride (HCl) were obtained from VWR (Belgium). The silicone solution in isopropanol was purchased from SERVA Electrophoresis GmbH (Germany) and the gallium standard solution (1000 ppm) from Merck (Belgium). All chemicals were used as received without further purification.Equipment and characterization
1H NMR and 13C NMR spectra were recorded on a Bruker Avance 300 spectrometer, operating at a frequency of 300 MHz for 1H and 75 MHz for 13C, respectively. The samples were prepared by dissolving a small amount of product in heavy water (D2O). An elemental analysis (carbon, hydrogen, nitrogen) was performed on a CE Instruments EA-1110 element analyzer. A thermogravimetric analysis (TGA) was done on a TA Instruments T500 thermogravimeter (heating rate: 2 °C min−1 from room temperature to 350 °C, under a nitrogen atmosphere). The viscosity of the ionic liquid was measured using an automatic Brookfield plate cone viscometer, Model LVDV-II CP (Brookfield Engineering Laboratories, USA). Photoluminescence spectra and luminescence lifetime measurements were recorded on an Edinburgh Instruments FS920 spectrofluorimeter. This instrument is equipped with a xenon arc lamp (450 W), a microsecond xenon flash lamp (60 W) and a red-sensitive photomultiplier (Hamamatsu R-2658). The morphology and the size distribution were determined by scanning electron microscopy (SEM) using a Philips XL 30 FEG device. Powder X-ray diffraction (XRD) was carried out on an Agilent SuperNova X-ray diffractometer, using Mo Kα radiation (λ = 0.71073 Å) and a CCD detector. ICP-MS measurements were carried out on a Thermo X-Series PlasmaQuad (PQ) 2 device. Microwave irradiation experiments were carried out on a CEM Discover monomode microwave apparatus (2.45 GHz, 100 W) with an integrated IR sensor, using 10 mL glass tube containers, sealed with snap caps. A Heraeus Megafuge 1.0 centrifuge was used to separate the undissolved phosphor particles from the ionic liquid after the leaching experiments. Total reflection X-ray fluorescence spectroscopy (TXRF) was performed with a Bruker S2 Picofox TXRF spectrometer equipped with a molybdenum source. For the sample preparation, plastic microtubes were filled with a small amount of ionic liquid sample (200 mg), ethanol (700 μL) and 100 μL of a gallium standard solution (1000 ppm). Gallium was chosen because this element has a high sensitivity and does not interfere with the lanthanide signals. The microtubes were then vigorously shaken on a vibrating plate (IKA MS 3 basic). Finally, a 1 μL drop of this solution was put on a quartz plate, previously treated with a silicone/isopropanol solution (Serva®) to avoid spreading of the sample droplet on the quartz plate. The quartz plates were then dried for 30 min at 60 °C prior to analysis. Each sample was measured for 10 min.Synthesis of [Hbet][Tf2N]
The ionic liquid [Hbet][Tf2N] was synthesized according to a literature method.36 HbetCl (0.390 mol, 59.91 g) and LiTf2N (0.390 mol, 111.97 g) were dissolved in water (50 mL) and stirred for 2 h at room temperature. The mixture was then allowed to phase-separate and the water phase containing LiCl was removed. [Hbet][Tf2N] was then washed several times with ice water (10 mL) to remove chloride impurities until the AgNO3 test was negative in the water phase after the washing step. The remaining water was removed using a rotary evaporator under reduced pressure. The yield was 79% (0.310 mol, 123.47 g) with a chloride content below detection limit for the TXRF analysis (0.1–0.2 ppm).40 NMR characterization with chemical shifts (δ) given in ppm and J values given in Hz resulted in δH (300 MHz; DMSO; Me4Si) 3.22 (9 H, s, 3 × Me) 4.30 (2 H, s, CH2) and δC (75 MHz; DMSO; Me4Si) 166.34 (s, COO) 119.45 (q, 2 × CF3, J = 312.75), 62.60 (s, N-CH2), 52.88 (3 × CH3). A CHN elemental analysis found (%): C, 21.17; H, 3.96; N, 6.76. Calc. (%) for C7H12N2O6F6S2: C, 21.11; H, 3.04; N, 7.03.Leaching experiments
Small glass vials (4 mL) were filled with a fixed amount of [Hbet][Tf2N] (2 g), a 10 mg sample of each of the four phosphors per g of ionic liquid (4 × 10 mg g−1) and also a small amount of water during some experiments (1–5 wt%). Note that all the quantities are expressed per gram of ionic liquid (mg g−1). A magnetic stirring bar was then added to each of the vials and they were closed using a plastic screw cap. The dissolution experiments were carried out using a heating plate with a silicone oil bath, an integrated magnetic stirrer and a temperature sensor. The vials were placed in the silicone oil bath at the appropriate temperature and stirred for a certain amount of time. The vials were then placed in a centrifuge (5300 rpm, 20 min) to precipitate the undissolved phosphor powders and to obtain a clear ionic liquid. The metal content dissolved in the ionic liquid was determined using total reflection X-ray fluorescence spectroscopy (TXRF).Stripping experiments
Two different stripping methods were investigated. Method 1 involved the use of an aqueous HCl phase to extract the rare-earth ions from the ionic liquid to the water phase, method 2 consisted of adding pure (solid) oxalic acid directly to the ionic liquid to precipitate the rare-earth ions as rare-earth oxalates. For every stripping experiment, Y2O3:Eu3+ (10 mg g−1) was first fully dissolved in a large batch of ionic liquid (5 wt% H2O). Small glass vials (5 mL) were then filled with a fixed amount of this REE-containing ionic liquid (1 g). For method 1, an HCl solution (1 g) was then added to the rare-earth containing ionic liquid. This lowly viscous biphasic mixture was shaken (1500 rpm) using a mechanical shaker at a set temperature (25, 70 and 80 °C). The samples were then centrifuged (1 min, 5300 rpm) to speed up phase separation, and analyzed by TXRF to determine the remaining metal content in the ionic liquid phase. For method 2, pure (solid) oxalic acid was added directly to the ionic liquid phase. These samples were more viscous and consisted of only one phase, so they were stirred using a magnetic stirring bar and a stirring plate/oil bath set-up to control the temperature (25, 50 and 70 °C). The samples were then centrifuged (5300 rpm, 20 min) to remove the oxalate precipitate and the ionic liquid phase was analyzed with TXRF to determine the remaining metal content.Optimized full recycling process
The retained recycling process consisted of three steps. First a synthetic mixture of HALO, YOX, BAM and LAP phosphors was added to the ionic liquid [Hbet][Tf2N] containing 5 wt% of water. The system was optimized for 10 mg of YOX per g of ionic liquid, which corresponds to approximately 40–50 mg of real lamp phosphor waste. A stirring bar was added and the samples were stirred for 24–48 h at 90 °C to obtain a selective and 100% leaching of YOX. The remaining solid lamp phosphor waste was separated from the ionic liquid by centrifugation (5300 rpm, 20 min). A stoichiometric amount of pure oxalic acid was then added to the ionic liquid loaded with yttrium and europium to precipitate the rare-earth ions as oxalates and regenerate the ionic liquid. The samples were stirred for 10 min at 70 °C to obtain 100% stripping. The oxalate precipitate was separated from the ionic liquid by filtration and washed with water (lowers the viscosity). The water can be removed by evaporation later on. An alternative could be to filter at 70 °C at which point the viscosity of the [Hbet][Tf2N] (5 wt% H2O) becomes very low. The mixed (yttrium, europium) oxalate was dried in a vacuum oven at 50 °C and then calcined at 950 °C (5 h) in an oven to obtain new Y2O3:Eu3+ (YOX) with a purity >99.9 wt%.Results and discussion
Selective dissolution of YOX in [Hbet][Tf2N]
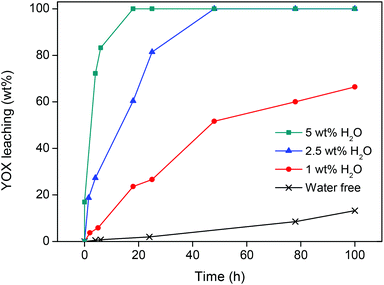
The protonated functionalized ionic liquid betainium bis(trifluoromethylsulfonyl)imide, [Hbet][Tf2N], has the ability to dissolve certain metal oxides, including rare-earth oxides like Y2O3 and Eu2O3.36,37 Different parameters (water content, temperature, etc.) were investigated to find which leaching conditions were required to selectively dissolve the red Y2O3:Eu3+ (YOX) phosphor within a reasonable period of time.In the first experiments, the influence of water addition was investigated. Water-free ionic liquid was used and increasing amounts of water were then added (0, 1, 2.5 and 5 wt%). Care was taken never to exceed the solubility of water in the ionic liquid (13 wt%), because it was important to maintain one homogeneous phase during the leaching process to avoid the loss of metal ions to the water phase.41 A mix of YOX, HALO, BAM and LAP was added to the ionic liquid (4 × 10 mg g−1) and stirred (600 rpm) at 90 °C. The water-free ionic liquid was compared with water-containing ionic liquid (1, 2.5 and 5 wt% water added). Closed vessels with screw caps were used to contain the small amounts of water at elevated temperatures. The results show that the addition of water has a positive influence on the leaching of YOX due to the lower viscosity and better ion diffusion. With a water content of 5 wt% (50 μL g−1) in the ionic liquid, full dissolution of YOX was observed after 24 h (Fig. 2). More importantly, under these conditions very little dissolution of HALO was observed (<0.05 wt%) (Fig. 3) and no leaching of BAM and LAP could be detected. Higher water contents are not advisable since this will lead to increasing dissolution of HALO (Fig. 3). It was also confirmed in a separate experiment that SiO2 and Al2O3 do not dissolve in the [Hbet][Tf2N] ionic liquid.37,42
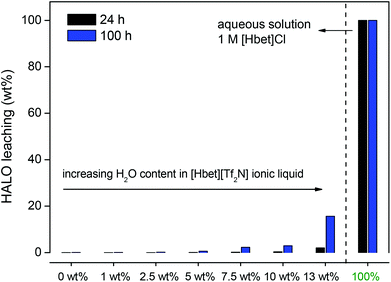 | ||
| Fig. 3 Leaching (wt%) of HALO by [Hbet][Tf2N] at 90 °C (600 rpm) after 24 h and 100 h with increasing amounts of water in the ionic liquid: 0, 1, 2.5, 5, 7.5, 10 and 13 wt% (= water-saturated). Leaching is also shown in an aqueous solution containing [Hbet]Cl (1 M) which has a pKa of 1.83.43 | ||
It is clear from Fig. 2 and 3 that under water-poor conditions (<5 wt% H2O in the ionic liquid) Y2O3:Eu3+ dissolves much better than HALO. This is surprising considering that in dilute acidic aqueous solutions (e.g. 1 M [Hbet]Cl), HALO dissolves much more easily than Y2O3:Eu3+. To explain this unusual behaviour a hypothesis is proposed based on the difficult solvation of anions in [Hbet][Tf2N]. While metal cations (e.g. rare-earth ions) can be coordinated efficiently in [Hbet][Tf2N] by the carboxylate groups of the deprotonated betaine, this is not the case for anions.36–38 It was previously observed by Nockemann et al. that the solubility of oxides in [Hbet][Tf2N] was much higher than that of chloride salts, which is in agreement with our hypothesis.36 This hypothesis was supported by the observation that NaCl, EuCl3 and YCl3 could not be dissolved in the pure ionic liquid, while it was possible to dissolve their corresponding oxides. It was also observed that when [Hbet][Tf2N] was brought in contact with an aqueous YCl3 solution some yttrium was extracted to the ionic liquid phase but no chloride was extracted. The extraction of metal ions is based on the release of protons by [Hbet]+ and the coordination of the metal ion by zwitterionic betaine groups, but there is no suitable mechanism for the coordination or solvation of anions in the ionic liquid. Since the dissolution of metal oxides does not create anions, they easily dissolve in this ionic liquid (eqn (2)) while metal salts like rare-earth chlorides (eqn (3)) (or even NaCl) do not, due to the formation of anions. This behaviour is the opposite of what is found in aqueous solutions, where chlorides dissolve much easier than oxides which require the presence of acid. Only when sufficient amounts of water are added to the ionic liquid, it will dissolve (some) chloride salts because water can solvate anions (eqn (3)). The same is true for HALO, where many phosphate and chloride ions need to be solvated in order for HALO to dissolve (eqn (1)). HALO dissolves very fast in dilute acidic aqueous solutions, but very little dissolution (<0.05 wt%) is observed in [Hbet][Tf2N] when less than 5 wt% of water is present. That is why such a drastic change in the dissolution behavior of HALO is observed when the water content is increased (Fig. 3). The leaching of metal oxides like YOX is also accelerated in the presence of water but this is mainly due to the lower viscosity and faster diffusion of protons in the ionic liquid.36,37 YOX forms, upon dissolution, only water and cations, which are efficiently solvated by the betaine groups, and does not form anions (eqn (2)). The water content is therefore the most crucial parameter as the control of the water content in the ionic liquid allows selective dissolution of YOX, without dissolving HALO.
| Ln2O3 + 6H+ → 2Ln3+ + 3H2O | (2) |
| LnCl3 → Ln3+ + 3Cl− | (3) |
It is important to keep the dissolution of HALO to an absolute minimum, not only from an economic point of view but also from a chemical point of view as it constitutes up to 50 wt% of the waste. The dissolution of HALO consumes a large amount of acid and releases unwanted metal ions (eqn (1)), which means that purification methods (e.g. solvent extraction) are required to retrieve pure rare earths. The dissolution of HALO also leads to the formation of phosphate ions (eqn (1)) which can form very insoluble YPO4 and EuPO4 with the dissolved Y3+ and Eu3+ from the YOX. This hampers further processing because rare-earth phosphates are very difficult to dissolve (like LAP and monazite). The optimal leaching system is therefore a compromise between the fast dissolution of YOX and keeping the leaching of HALO as low as possible. [Hbet][Tf2N] with 5 wt% of H2O (90 °C, 24 h) seems to be the best compromise between speed and selectivity. Under these conditions, the leaching of YOX and HALO is 100 wt% and 0.04 wt% respectively, and BAM and LAP leaching below the detection limit (Fig. 2 and 3).
The influence of temperature on the leaching of YOX was also investigated for this optimized ionic liquid system (5 wt% H2O) and compared with water-free ionic liquid (Fig. 4). In both cases a noticeable increase in leaching speed was observed with increasing temperature (Fig. 4). A temperature of 90 °C with 5 wt% (50 μL g−1) of H2O in the ionic liquid was chosen as the optimal system because of its high leaching efficiency and the fact that the water pressure is still manageable.
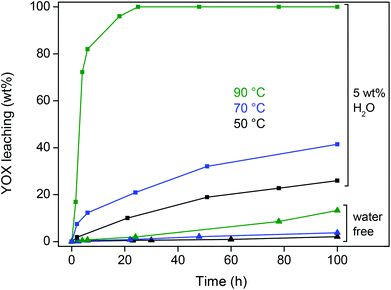 | ||
| Fig. 4 Dissolution (wt%) of YOX in water-free [Hbet][Tf2N] and water-containing (5 wt% H2O) [Hbet][Tf2N] as a function of time and temperature. | ||
A higher temperature is favorable because it accelerates the dissolution reaction and lowers the viscosity of the ionic liquid, which improves the diffusion. This is important because the leaching rate of this solid material is diffusion-controlled. The viscosity was measured as a function of the water content in the ionic liquid and the temperature (Table 2). It is clear that higher water content and temperature drastically diminish the viscosity. The viscosity of the ionic liquid with 5 wt% water at 80 °C is only 25 cP. The viscosity at 90 °C could not be measured with our equipment but it will be even lower. Of course, the viscosity of the leaching system used in this work (ionic liquid with 5 wt% H2O at 90 °C) is still higher than that of water (0.3 cP at 90 °C) but it is sufficiently low in order not to be an issue.
The thermal stability of [Hbet][Tf2N] was tested by thermogravimetric analysis (TGA) and 13C NMR. The thermal stability is an important characteristic since the ionic liquid will be used for leaching at temperatures up to 90 °C for prolonged amounts of time. The Tf2N− anion is known to have a high relative stability compared to other common ionic liquid anions.39 For TGA, the ionic liquid was first dried under reduced pressure at 80 °C until all the remaining traces of water were removed and the mass of the ionic liquid was stable. The ionic liquid was then heated (2 °C min−1) under a nitrogen atmosphere, from room temperature to 350 °C. The results of the dynamic TGA show that the ionic liquid only starts to degrade at temperatures around 200 °C, which proves that it is definitely stable at 90 °C (Fig. 5). A static measurement at 90 °C for a period of 24 h showed negligible (<0.01%) degradation of the ionic liquid. 13C NMR was used to investigate the degradation of water-containing ionic liquid under experimental conditions (90 °C, 5 wt% H2O, 600 rpm, 48 h). A 13C NMR spectrum was taken before and after the experiment to confirm the stability of the ionic liquid under these conditions.
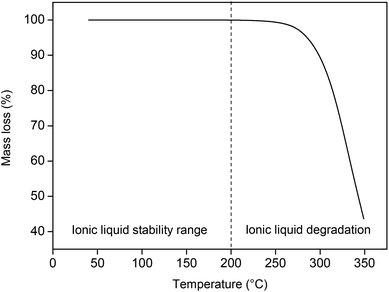 | ||
| Fig. 5 Thermogravimetric analysis of [Hbet][Tf2N], measured from 40 to 350 °C (2 °C min−1) under a nitrogen atmosphere. | ||
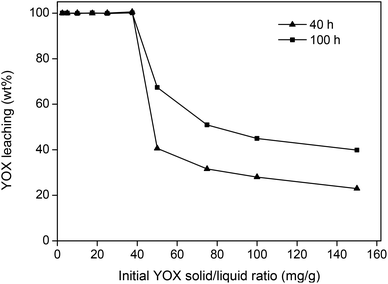
The loading capacity, meaning the amount of YOX that can be dissolved in the ionic liquid, was also investigated. The theoretical maximal stoichiometric solubility of Y2O3 in the ionic liquid amounts to 95 mg g−1. However, this requires the addition of more water under reflux conditions in order to be sufficiently fast.36 This would undermine the selectivity of this system since under these conditions the HALO would easily dissolve in the water phase. Higher loadings also resulted in higher viscosities due to the highly charged rare-earth ions in the ionic liquid, making the system much more difficult to handle. It was shown that under the previously mentioned optimized conditions (90 °C, 5 wt% H2O), up to 40 mg g−1 of YOX could be fully dissolved in the ionic liquid after 40 h (Fig. 6). The parameter optimization in this work was done for a YOX loading of 10 mg g−1 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm), but it is possible to work under more concentrated conditions (solid/liquid ratio) without significant modifications. As shown in Fig. 6, a dissolution of 40 mg g−1 of YOX in the ionic liquid would correspond to a solid/liquid ratio of 200 mg g−1 (200 g kg−1) of waste in the ionic liquid, since fluorescent lamp waste contains around 20 wt% of YOX.
000 ppm), but it is possible to work under more concentrated conditions (solid/liquid ratio) without significant modifications. As shown in Fig. 6, a dissolution of 40 mg g−1 of YOX in the ionic liquid would correspond to a solid/liquid ratio of 200 mg g−1 (200 g kg−1) of waste in the ionic liquid, since fluorescent lamp waste contains around 20 wt% of YOX.
The dissolved Y3+ and Eu3+ (YOX) in [Hbet][Tf2N] was recovered by a stripping step. This was achieved either by bringing the loaded ionic liquid in contact with an acidic water phase to extract the rare-earth ions (method 1) or by directly precipitating the rare earths from the ionic liquid using pure (solid) oxalic acid (method 2). Stripping method 1 has important limitations due to the loss of ionic liquid to the water phase. Stripping with pure oxalic acid (method 2) is clearly a better alternative since no ionic liquid is lost and the YOX phosphor can be immediately regenerated from the yttrium/europium oxalate salt in a simple calcination step. However, it is still interesting to also investigate the stripping with HCl and quantify the loss of ionic liquid to the water phase even if it is not the best method. Both stripping systems are discussed separately and compared.
Stripping method 1: recovery of yttrium and europium using an acidic aqueous phase
The first stripping system involves the use of an acidic aqueous phase, which is brought in contact with the loaded ionic phase. In this process the rare-earth ions (Ln) are transferred to the water phase as soluble compounds (e.g. chlorides). The acidic protons protonate the betaine ligands and regenerate the ionic liquid (eqn (4)).| [Ln(bet)3][Tf2N]3 + 3HCl → LnCl3 + 3[Hbet][Tf2N] | (4) |
The fact that a two-phase system is now used means that it is necessary to look at possible losses of ionic liquid to the water phase. The loss of ionic liquid depends on the phase ratio and the amount and type of dissolved ions.44–46 Ions can be classified as salting-in (e.g. NO3−) and salting-out (e.g. Cl−, SO42−) agents as described by the Hofmeister series.45,47 The former class of ions tends to lead to an improved mixing of ionic liquid/water, while the latter leads to a better separation of ionic liquid/water and therefore a smaller loss of ionic liquid to the water phase. The loss of ionic liquid to the water phase (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 phase ratio by mass) was quantified using 1H NMR spectroscopy with 1,4-dioxane as an internal standard and D2O instead of H2O as the water phase. The loss of [Hbet][Tf2N] to a (pure) water phase was determined to be 13 wt%. Lowering the pH increases the loss of ionic liquid to the water phase even more to 15.1%, 15.4% and 18.1% for 1 M H2SO4, HCl and HNO3 respectively. This is in agreement with the sequence found in the literature.45 Although stripping with H2SO4 resulted in the lowest loss of ionic liquid, stripping with HCl is still the better choice because the solubility of rare-earth sulfates in water is much lower than the corresponding rare-earth chlorides. The loss of ionic liquid is very significant and it is cumbersome to recover the ionic liquid from the water phase, even though various techniques exist to recover ionic liquids at a later stage using for example strong salting-out agents, adsorbents, special membranes, electrodialysis or nanofiltration.48–52 That is why the oxalic acid precipitation route (method 2) is the preferred method.
1 phase ratio by mass) was quantified using 1H NMR spectroscopy with 1,4-dioxane as an internal standard and D2O instead of H2O as the water phase. The loss of [Hbet][Tf2N] to a (pure) water phase was determined to be 13 wt%. Lowering the pH increases the loss of ionic liquid to the water phase even more to 15.1%, 15.4% and 18.1% for 1 M H2SO4, HCl and HNO3 respectively. This is in agreement with the sequence found in the literature.45 Although stripping with H2SO4 resulted in the lowest loss of ionic liquid, stripping with HCl is still the better choice because the solubility of rare-earth sulfates in water is much lower than the corresponding rare-earth chlorides. The loss of ionic liquid is very significant and it is cumbersome to recover the ionic liquid from the water phase, even though various techniques exist to recover ionic liquids at a later stage using for example strong salting-out agents, adsorbents, special membranes, electrodialysis or nanofiltration.48–52 That is why the oxalic acid precipitation route (method 2) is the preferred method.
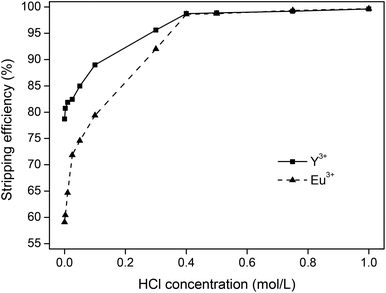
The different stripping parameters were then investigated for stripping with HCl, starting with the influence of acid concentration. First, 10 mg g−1 of YOX was dissolved in [Hbet][Tf2N] (5 wt% H2O). Then, the loaded ionic liquid was contacted with aqueous solutions containing varying concentrations of HCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 phase ratio by mass) and shaken (1500 rpm) for 1 h at 25 °C. The remaining metal content in the ionic liquid was then analyzed using TXRF. The results showed that bringing the ionic liquid in contact with 1 M HCl resulted in 99.6% and 99.7% stripping of Y3+ and Eu3+ respectively (Fig. 7). A sufficient amount of HCl is needed to protonate the betaine and to form the water-soluble YCl3 and EuCl3 compounds. In this set-up, the stoichiometric HCl concentration is 0.3 M in the water phase, but an excess of HCl is required to obtain full stripping (1 M) (Fig. 7). An interesting observation is the fact that under dilute acidic conditions with an excess of rare-earth ions compared to HCl, Y3+ is easier to strip than Eu3+. This is in agreement with previous reports where yttrium(III) had a slightly lower affinity for betaine ligands compared to europium(III).44 For HCl concentrations >0.4 M no difference was observed and the stripping process became very efficient and reproducible. An HCl concentration of 1 M was retained as the optimal stripping concentration because of the fast and reliable stripping.
1 phase ratio by mass) and shaken (1500 rpm) for 1 h at 25 °C. The remaining metal content in the ionic liquid was then analyzed using TXRF. The results showed that bringing the ionic liquid in contact with 1 M HCl resulted in 99.6% and 99.7% stripping of Y3+ and Eu3+ respectively (Fig. 7). A sufficient amount of HCl is needed to protonate the betaine and to form the water-soluble YCl3 and EuCl3 compounds. In this set-up, the stoichiometric HCl concentration is 0.3 M in the water phase, but an excess of HCl is required to obtain full stripping (1 M) (Fig. 7). An interesting observation is the fact that under dilute acidic conditions with an excess of rare-earth ions compared to HCl, Y3+ is easier to strip than Eu3+. This is in agreement with previous reports where yttrium(III) had a slightly lower affinity for betaine ligands compared to europium(III).44 For HCl concentrations >0.4 M no difference was observed and the stripping process became very efficient and reproducible. An HCl concentration of 1 M was retained as the optimal stripping concentration because of the fast and reliable stripping.
Secondly, the stripping kinetics was investigated. When dealing with a biphasic system, the process kinetics is controlled by the extent of the interface since the exchange of ions can only happen at this interface. The area of the interface is usually increased by intense stirring or shaking; however, a special feature of the [Hbet][Tf2N]/H2O system is that it shows thermomorphic behavior with an upper critical solution temperature.44 This means that the biphasic system will become one homogeneous phase above a certain temperature called the cloud point temperature. This is very interesting since the process kinetics will be much faster as they are then no longer limited by the diffusion across an interface. In order to obtain phase separation, the mixture has to be heated above the cloud point and shaken briefly (1 s) to overcome the metastable state, but the homogeneous state then remains stable as long as the temperature is above the cloud point. Below the cloud point, the phase separation automatically occurs again (Fig. 8).
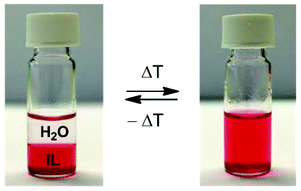 | ||
Fig. 8 Reversible formation of a homogeneous phase when increasing the temperature above the cloud point (55 °C for a pure water/ionic liquid system in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio).44 A coloring agent (methyl red sodium salt) was dissolved in the transparent ionic liquid phase to help the visualization. The density of the [Hbet][Tf2N] liquid is higher than that of water. 1 ratio).44 A coloring agent (methyl red sodium salt) was dissolved in the transparent ionic liquid phase to help the visualization. The density of the [Hbet][Tf2N] liquid is higher than that of water. | ||
The cloud point is dependent not only on the amount and type of ions present in the water phase, but also on the loading of the ionic liquid and the water to ionic liquid ratio.41 The cloud point was determined for this stripping system consisting of one phase with 1 g of ionic liquid in which Y2O3:Eu3+ was dissolved (10 mg g−1), brought in contact with another phase consisting of a 1 g HCl solution (1 M) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 phase ratio. The cloud point was found to be 74 °C by heating the sample to 80 °C and then slowly cooling it down, keeping it steady at every degree for 5 min to see whether it would become cloudy when gently shaken which indicates that the mixture starts to phase separate.53 This cloud point is higher than that for a pure H2O/[Hbet][Tf2N] system, because of the H+, Cl−, Y3+ and Eu3+ ions dissolved in it.41
1 phase ratio. The cloud point was found to be 74 °C by heating the sample to 80 °C and then slowly cooling it down, keeping it steady at every degree for 5 min to see whether it would become cloudy when gently shaken which indicates that the mixture starts to phase separate.53 This cloud point is higher than that for a pure H2O/[Hbet][Tf2N] system, because of the H+, Cl−, Y3+ and Eu3+ ions dissolved in it.41
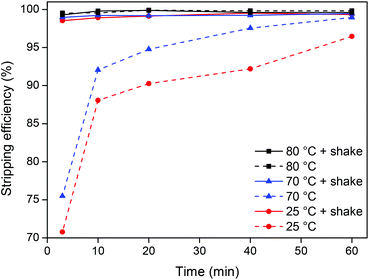
The stripping kinetics was then investigated at room temperature (25 °C) and just below (70 °C) and above (80 °C) the cloud point temperature (74 °C) both with and without shaking (1500 rpm) (Fig. 9).
Raising the temperature from 25 °C to 70 °C only slightly increases the stripping speed. However, when the temperature is raised to 80 °C, which is above the cloud point temperature, the stripping is much faster due to the disappearance of the interface between the ionic liquid and the water phase and the formation of one homogeneous phase. It is also clear that shaking has a major influence when working at temperatures below the cloud point temperature because the system is then a biphasic mixture. Shaking the samples at 80 °C (above the cloud point) has no influence since the system is then one homogeneous phase. It is clear from this experiment (Fig. 9) that from an energy-saving point of view, the best choice for stripping is either to work with a biphasic mixture at 25 °C while shaking or to work at 80 °C without shaking thanks to the formation of a homogeneous phase. Other combinations require more energy and are less efficient.
Stripping method 2: recovery of yttrium and europium using pure oxalic acid
The problem of the loss of ionic liquid to the water phase can be avoided by stripping with solid oxalic acid, directly in the ionic liquid phase. This acid forms insoluble oxalate complexes with the rare-earth ions (eqn (5)) and precipitates them while regenerating the ionic liquid. This stripping precipitation method is very efficient for rare-earth ions. The rare-earth oxalate precipitate can then be separated from the ionic liquid and thermally decomposed in an oven at high temperature to form the corresponding rare-earth oxides.54 By carefully choosing the conditions it is also possible to directly resynthesize the Y2O3:Eu3+ phosphor by calcination of the mixed yttrium/europium oxalate (eqn (6)), as described by the Solvay patent.55 The advantage is that the yttrium and europium are already present in the right ratio to manufacture the YOX phosphor, since they have been selectively leached from the used lamp phosphors in the first place. This stripping system is therefore definitely the preferred choice compared to the stripping system using an acidic water phase. The oxalic acid stripping is much more convenient and can be carried out directly in the ionic liquid, meaning that there is no loss of ionic liquid to the water phase.| 2(Y3+,Eu3+) + 3H2C2O4 → (Y,Eu)2(C2O4)3(s) + 6H+ | (5) |
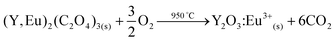 | (6) |
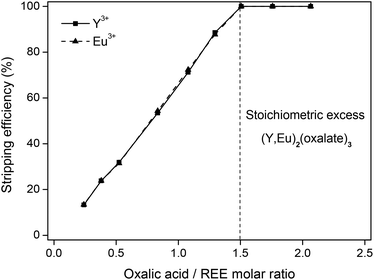
The different parameters of this stripping method were investigated. The results show that a stoichiometric amount (eqn (5)) of oxalic acid compared to the amount of rare-earth ions (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) is sufficient to obtain a stripping efficiency of 100% (Fig. 10). Oxalic acid does not show individual selectivity for yttrium or europium under these conditions.
2) is sufficient to obtain a stripping efficiency of 100% (Fig. 10). Oxalic acid does not show individual selectivity for yttrium or europium under these conditions.
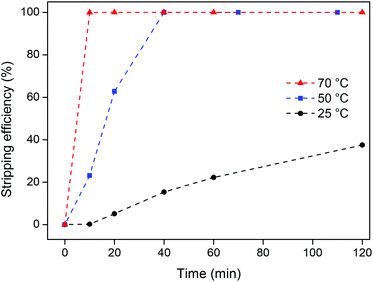
The influence of temperature on the stripping kinetics of this heterogeneous stripping process was investigated. The viscosity of this pure ionic liquid is obviously higher than that for a biphasic water–ionic liquid mixture, but it is still very manageable especially at higher temperatures (Table 2). An ionic liquid containing 10 mg g−1 of YOX and 5 wt% H2O was reused, and a stoichiometric amount of oxalic acid was added compared to the amount of rare-earth ions (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The samples were then stirred (600 rpm) at different temperatures and for increasing amounts of time. The results show a drastic increase in the stripping efficiency when raising the temperature from 25 °C to 50 °C and 70 °C (Fig. 11). This increase is attributed to the lower viscosity (Table 2) and the improved diffusion of the oxalic acid in the ionic liquid. At 70 °C, a stripping efficiency of 100% was obtained after only 10 min, which is a very satisfactory result.
2). The samples were then stirred (600 rpm) at different temperatures and for increasing amounts of time. The results show a drastic increase in the stripping efficiency when raising the temperature from 25 °C to 50 °C and 70 °C (Fig. 11). This increase is attributed to the lower viscosity (Table 2) and the improved diffusion of the oxalic acid in the ionic liquid. At 70 °C, a stripping efficiency of 100% was obtained after only 10 min, which is a very satisfactory result.
It can be concluded that this oxalate stripping process is very efficient since stoichiometric amounts of oxalic acid suffice to get full stripping of the rare-earth ions from the ionic liquid. The formation of the rare-earth oxalate precipitate is also very convenient since the oxalate salt can be transformed into a new YOX phosphor Y2O3:Eu3+ by a simple calcination step. The major advantage of this stripping system compared to the biphasic system (method 1) is the fact that no ionic liquid is lost. The drawback is that this stripping system is kinetically slower and therefore requires heating at 70 °C for 10 min (Fig. 11). The biphasic stripping system using HCl could be carried out efficiently by shaking at room temperature for 10 min (Fig. 9). All things considered, the oxalic acid stripping method was retained for the final recycling process.
Regenerating the Y2O3:Eu3+ phosphor
The (Y,Eu)2(C2O4)3 precipitate was separated from the ionic liquid by filtration with a Büchner funnel and washed with water to lower the viscosity of the ionic liquid. Afterwards the water can be removed by evaporation. An alternative could be to filter at 70 °C at which point the viscosity of the [Hbet][Tf2N] (5 wt% H2O) becomes very low. The mixed oxalate was then dried in a vacuum oven at 50 °C for 12 h. The dry (Y,Eu)2(C2O4)3 was then placed in an oven at 950 °C for 5 h to obtain the Y2O3:Eu3+ phosphor. The YOX particles were characterized using ICP-MS, SEM imaging, luminescence spectroscopy and powder XRD, and compared with the purchased YOX. The purity of the recycled YOX was determined to be >99.9 wt% by TXRF and ICP-MS with only small traces of calcium (<0.1 wt%). The purchased YOX had a certified purity of >99 wt%. SEM images can be found in the ESI (Fig. S1†). They showed that the particles had a size of 4.11 ± 1.41 μm which is in range with the commercial YOX phosphor (3.61 ± 1.52 μm). However, the recycled YOX phosphor had a rougher surface than the purchased phosphor (Fig. S1†). Powder XRD was carried out to compare the commercial YOX with the recycled YOX. The diffractograms were essentially identical, which confirmed the successful synthesis of Y2O3:Eu3+ (Fig. S2†). The luminescence of this material was also compared with the commercially obtained Y2O3:Eu3+ phosphor (Fig. 12). When the spectra are scaled to have the same intensity of the 5D0→7F1 transition, it is interesting to compare the intensity of the 5D0→7F2 transition (around 612 nm) since this is a so-called hypersensitive transition, meaning its intensity is very dependent on its environment.56–58 It can be seen that the maximum intensity peak (5D0→7F2) has the same intensity for the recycled phosphor as for the commercial phosphor. The luminescence lifetime was also measured by studying the decay of the 5D0 emitting state of Eu3+. The samples were excited with 254 nm light and the emission light was collected at 612 nm. The decay curves (Fig. S3†) were fitted as a mono-exponential decay, resulting in a luminescence lifetime of 0.989 ms (R2 = 0.998) and 1.003 ms (R2 = 0.997) for the recycled and purchased YOX phosphor respectively. The very good luminescence and luminescence lifetime of the recycled YOX confirms that the recycling process yields a sufficiently pure yttrium/europium oxalate product to allow direct resynthesis of the YOX phosphor (>99.9 wt% purity). This process is only possible because of the highly selective dissolution of YOX in the ionic liquid, because any dissolution of HALO, BAM or LAP would lead to an impure product and a significant decrease in luminescence. Optimization of the calcination process (flux agents, temperature, time, etc.) could further improve the luminescence properties and particle morphology to obtain an optimal red phosphor, but this is outside the scope of this work and has been studied by others.55,59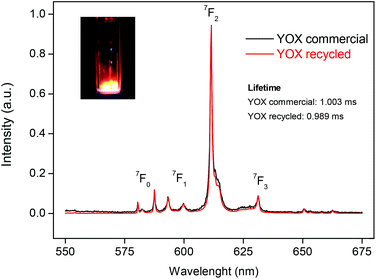 | ||
| Fig. 12 Luminescence spectra (λexc = 254 nm) of the commercial red Y2O3:Eu3+ (YOX) phosphor, compared with the spectrum of the recycled YOX phosphor after calcination of the (Y,Eu)2(C2O4)3 precipitate. The spectra were scaled to have the same intensity for the 5D0→7F1 transition in order to compare the intensity of the hypersensitive 5D0→7F2 transition. Picture: recycled YOX phosphor in a quartz container irradiated with 254 nm light. The luminescence lifetimes are also shown (Fig. S3†). | ||
An overview of the proposed recycling process is shown in Fig. 13. This process selectively recovers the YOX which represents 80 wt% of the critical rare-earths in the lamp phosphor waste powder and approximately 70% of the value.14 Only oxalic acid is consumed (cheap chemical) and it creates no waste besides some CO2 (the ionic liquid is entirely reusable). This process is a sustainable alternative compared to mineral acid leaching and could be applied on industrial scale as an on-site valorization method for recyclers since no complex solvent extraction installations are needed.
This process was optimized using a synthetic mix of phosphors (HALO, YOX, BAM, LAP). However, it is also applicable to real lamp phosphor waste because the other components (CAT, CBT, SiO2, Al2O3) do not interfere with this process as they do not dissolve in [Hbet][Tf2N].37,42 The influence of mercury on this process was not investigated, but many techniques exist to remove mercury from the phosphor powders.3,9,21,60–62 At the end of the recycling scheme, no additional waste has been created. The rest of the lamp phosphor waste (SiO2, Al2O3, HALO, BAM, LAP, CAT, CBT) can be discarded or further processed, for example by doing a rough separation of the HALO with physical separation methods.3,63 This would result in a terbium concentrate (≈8 wt% Tb) held in LAP, CAT and CBT. The terbium content in this concentrate is much higher than that in any commercially exploited ore from primary mining (<1.3 wt% Tb).4 The high demand for terbium could therefore make it worthwhile to dissolve the LAP, CAT and CBT phosphors in a final stage, but these phosphors are much more difficult to dissolve and require a lot of energy input.14
Microwave heating
In most of our experiments, the ionic liquid was heated by a conventional heating source. However, since the leaching of YOX in the ionic liquid requires high temperatures over prolonged periods of time, it is interesting to note that ionic liquids can be heated in a very energy-efficient way by microwave irradiation. Ionic liquids consist entirely of ions as opposed to water or organic solvents which explains the high adsorption of microwave radiation.64 Microwaves do not interfere with the dissolution process and provide a highly economical way to heat an ionic liquid in an industrial context.64,65 The immediate on/off behavior of microwave irradiation is also useful for process control. A programmable microwave oven with temperature and pressure control was used to test this. At 100 W, the ionic liquid [Hbet][Tf2N] was heated to a temperature of 100 °C in less than 15 s (Fig. 14). The temperature can be kept constant at a low cost.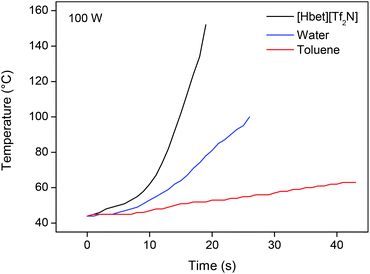 | ||
| Fig. 14 Heating of different solvents in a microwave oven (100 W), equipped with an infrared temperature probe. | ||
Conclusion
The ionic liquid leaching system described in this work is a major improvement compared to mineral acid leaching, since it allows the selective dissolution of Y2O3:Eu3+ (YOX) without dissolving the other phosphors (HALO, BAM, LAP). An interesting feature of the process is that the (Sr,Ca)10(PO4)6(Cl,F)2:Sb3+,Mn2+ phosphor was not leached by the ionic liquid (<0.05 wt%). This non-valuable broad-band white phosphor known as “halophosphate” (HALO) can make up as much as 50 wt% of the lamp phosphor waste fraction. Dissolution of HALO is unavoidable when using dilute mineral acids, especially under the conditions required for the dissolution of Y2O3:Eu3+. The unusual selectivity of the ionic liquid was attributed to the fact that the [Hbet][Tf2N] is unable to solvate anions efficiently, which explains why it is only able to dissolve metal oxides efficiently and not metal salts like (LnCl3, NaCl). The yttrium and europium dissolved in the ionic liquid [Hbet][Tf2N] were then stripped with an HCl solution or by adding pure (solid) oxalic acid directly to the ionic liquid. The oxalic acid route precipitates the rare earths as a mixed (yttrium, europium) oxalate salt, which can be transformed into a new Y2O3:Eu3+ phosphor by a simple calcination step at 950 °C. The ionic liquid is automatically regenerated during the stripping step and can be reused. This innovative approach offers selectivity, mild conditions and reusability, which make this a promising green technology for the targeted recovery of Y2O3:Eu3+ and the valorization of lamp phosphor waste. 70% of the value and 80% of the critical rare earths in the lamp phosphor waste are recovered this way. Further processing of the terbium-rich rest fraction can be done but requires much harsher conditions. No additional waste is generated during this process and there is no need for additional solvent extraction steps since the YOX is dissolved selectively and immediately regenerated. This opens the way to a more simple, economical and energy-efficient industrial process for the revalorization of lamp phosphor waste.Acknowledgements
The authors wish to thank the KU Leuven (projects GOA/13/008 and IOF-KP RARE3) and the FWO Flanders (PhD fellowship to DD) for financial support. The authors would like to thank Dorien Baeten for the help with TGA measurements, Prof. Erik van der Eycken and Geert Hooyberghs for access to microwave oven facilities and Michèle Vanroelen for the ICP-MS measurements. Jeroen Sniekers is also thanked for helping with the SEM images and Neil Brooks for powder XRD characterization.Notes and references
- D. Schüler, M. Buchert, R. Liu, G. S. Dittrich and C. Merz, Study on rare earths and their recycling, Öko-Institut, Darmstadt, 2011 Search PubMed.
- Ad-Hoc Working Group on Defining Critical Raw Materials, Report on Critical Raw Materials for the EU, European Commission, DG Enterprise & Industry, Brussels, 2014 Search PubMed.
- K. Binnemans, P. T. Jones, B. Blanpain, T. Van Gerven, Y. Yang, A. Walton and M. Buchert, J. Cleaner Prod., 2013, 51, 1–22 CrossRef CAS.
- D. Sandalow, D. Bauer, D. Diamond, J. Li, M. McKittrick and P. Telleen, Critical Materials Strategy, U.S. Department of Energy, USA, 2010 Search PubMed.
- K. Binnemans, P. T. Jones, K. Acker, B. Blanpain, B. Mishra and D. Apelian, JOM, 2013, 65, 846–848 CrossRef.
- M. Tanaka, T. Oki, K. Koyama, H. Narita and T. Oishi, in Handbook on the Physics and Chemistry of Rare Earths, ed. J.-C. G. Bünzli and V. K. Pecharsky, Elsevier-Amsterdam, 2013, ch. 255, vol. 43, pp. 159–212 Search PubMed.
- Y. Wu, X. Yin, Q. Zhang, W. Wang and X. Mu, Resour., Conserv., Recycl., 2014, 88, 21–31 CrossRef.
- Solvay, Press release, http://www.solvay.com, 2012.
- K. Binnemans and P. T. Jones, J. Rare Earth, 2014, 32, 195–200 CrossRef CAS.
- T. Vander Hoogerstraete, S. Wellens, K. Verachtert and K. Binnemans, Green Chem., 2013, 15, 919–927 RSC.
- S. Wellens, R. Goovaerts, C. Moller, J. Luyten, B. Thijs and K. Binnemans, Green Chem., 2013, 15, 3160–3164 RSC.
- T. Vander Hoogerstraete and K. Binnemans, Green Chem., 2014, 16, 1594–1606 RSC.
- K. Larsson, C. Ekberg and A. Ødegaard-Jensen, Waste Manage., 2013, 33, 689–698 CrossRef CAS PubMed.
- J. J. Braconnier and A. Rollat, Solvay, European Patent, EP 2419377 A1, 2012 Search PubMed.
- T. Hirajima, A. Bissombolo, K. Sasaki, K. Nakayama, H. Hirai and M. Tsunekawa, Int. J. Miner. Process., 2005, 77, 187–198 CrossRef CAS.
- T. Hirajima, K. Sasaki, A. Bissombolo, H. Hirai, M. Hamada and M. Tsunekawa, Sep. Purif. Technol., 2005, 44, 197–204 CrossRef CAS.
- G. Mei, P. Rao, M. Mitsuaki and F. Toyohisa, J. Wuhan. Univ. Technol., Mater. Sci. Ed., 2009, 24, 418–423 CrossRef CAS.
- V. Innocenzi, I. De Michelis, B. Kopacek and F. Vegliò, Waste Manage., 2014, 34, 1237–1250 CrossRef CAS PubMed.
- H. Liu, S. Zhang, D. Pan, J. Tian, M. Yang, M. Wu and A. A. Volinsky, J. Hazard. Mater., 2014, 272, 96–101 CrossRef CAS PubMed.
- M. A. Rabah, Waste Manage., 2008, 28, 318–325 CrossRef CAS PubMed.
- C. Tunsu, C. Ekberg and T. Retegan, Hydrometallurgy, 2014, 144–145, 91–98 CrossRef CAS.
- F. Yang, F. Kubota, Y. Baba, N. Kamiya and M. Goto, J. Hazard. Mater., 2013, 254–255, 79–88 CrossRef CAS PubMed.
- H. L. Yang, W. Wang, H. M. Cui, D. L. Zhang, Y. Liu and J. Chen, J. Chem. Technol. Biotechnol., 2012, 87, 198–205 CrossRef CAS.
- G. Mei, P. Rao, M. Mitsuaki and F. Toyohisa, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2009, 24 Search PubMed.
- R. Otto and A. Wojtalewicz, US patent, 0027651 A1, 2012 Search PubMed.
- A. P. Abbott, G. Frisch, S. J. Gurman, A. R. Hillman, J. Hartley, F. Holyoak and K. S. Ryder, Chem. Commun., 2011, 47, 10031–10033 RSC.
- S. Wellens, T. Vander Hoogerstraete, C. Möller, B. Thijs, J. Luyten and K. Binnemans, Hydrometallurgy, 2014, 144–145, 27–33 CrossRef CAS.
- A. Rout, S. Wellens and K. Binnemans, RSC Adv., 2014, 4, 5753–5758 RSC.
- S. Wellens, B. Thijs, C. Moller and K. Binnemans, Phys. Chem. Chem. Phys., 2013, 15, 9663–9669 RSC.
- A. P. Abbott, G. Frisch, J. Hartley and K. S. Ryder, Green Chem., 2011, 13, 471–481 RSC.
- S. Wellens, B. Thijs and K. Binnemans, Green Chem., 2012, 14, 1657–1665 RSC.
- Y. Gu, Green Chem., 2012, 14, 2091–2128 RSC.
- Q. Zhang, S. Zhang and Y. Deng, Green Chem., 2011, 13, 2619–2637 RSC.
- T. Welton, Green Chem., 2011, 13, 225–225 RSC.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- P. Nockemann, B. Thijs, S. Pittois, J. Thoen, C. Glorieux, K. Van Hecke, L. Van Meervelt, B. Kirchner and K. Binnemans, J. Phys. Chem. B, 2006, 110, 20978–20992 CrossRef CAS PubMed.
- P. Nockemann, B. Thijs, T. N. Parac-Vogt, K. Van Hecke, L. Van Meervelt, B. Tinant, I. Hartenbach, T. Schleid, V. T. Ngan, M. T. Nguyen and K. Binnemans, Inorg. Chem., 2008, 47, 9987–9999 CrossRef CAS PubMed.
- P. Nockemann, B. Thijs, K. Lunstroot, T. N. Parac-Vogt, C. Görller-Walrand, K. Binnemans, K. Van Hecke, L. Van Meervelt, S. Nikitenko, J. Daniels, C. Hennig and R. Van Deun, Chem. – Eur. J., 2009, 15, 1449–1461 CrossRef CAS PubMed.
- R. Reddy, J. Phase Equilib. Diffus., 2006, 27, 210–211 CrossRef CAS.
- T. Vander Hoogerstraete, S. Jamar, S. Wellens and K. Binnemans, Anal. Chem., 2014, 86, 3931–3938 CrossRef CAS PubMed.
- T. Vander Hoogerstraete, B. Onghena and K. Binnemans, Int. J. Mol. Sci., 2013, 14, 21353–21377 CrossRef PubMed.
- B. Thijs, PhD thesis, KU Leuven – University of Leuven, Belgium, 2007.
- R. M. C. Dawson, Data for Biochemical Research, Oxford University Press, New York, 3rd edn, 1986, pp. 8–9 Search PubMed.
- T. Vander Hoogerstraete, B. Onghena and K. Binnemans, J. Phys. Chem. Lett., 2013, 4, 1659–1663 CrossRef PubMed.
- M. G. Freire, P. J. Carvalho, A. M. S. Silva, L. M. N. B. F. Santos, L. P. N. Rebelo, I. M. Marrucho and J. A. P. Coutinho, J. Phys. Chem. B, 2008, 113, 202–211 CrossRef PubMed.
- M. G. Freire, C. M. S. S. Neves, P. J. Carvalho, R. L. Gardas, A. M. Fernandes, I. M. Marrucho, L. M. N. B. F. Santos and J. A. P. Coutinho, J. Phys. Chem. B, 2007, 111, 13082–13089 CrossRef CAS PubMed.
- Y. Zhang and P. S. Cremer, Curr. Opin. Chem. Biol., 2006, 10, 658–663 CrossRef CAS PubMed.
- L. Bai, X. Wang, Y. Nie, H. Dong, X. Zhang and S. Zhang, Sci. China: Chem., 2013, 56, 1811–1816 CrossRef CAS.
- C. M. S. S. Neves, M. G. Freire and J. A. P. Coutinho, RSC Adv., 2012, 2, 10882–10890 RSC.
- C. Abels, C. Redepenning, A. Moll, T. Melin and M. Wessling, J. Membr. Sci., 2012, 405–406, 1–10 CrossRef CAS.
- J. F. Fernández, D. Waterkamp and J. Thöming, Desalination, 2008, 224, 52–56 CrossRef.
- J. Lemus, J. Palomar, F. Heras, M. A. Gilarranz and J. J. Rodriguez, Sep. Purif. Technol., 2012, 97, 11–19 CrossRef CAS.
- B. Onghena, J. Jacobs, L. Van Meervelt and K. Binnemans, Dalton Trans., 2014, 43, 11566–11578 RSC.
- G. Adachi, N. Imanaka and Z. C. Kang, Binary Rare Earth Oxides, Kluwer Academic Publishers, Dordrecht, 2006 Search PubMed.
- X. Wan, Solvay, China Patent, EP 2176375 A1, 2010 Search PubMed.
- C. R. Ronda, T. Jüstel and H. Nikol, J. Alloys Compd., 1998, 275–277, 669–676 CrossRef CAS.
- N. C. Chang, J. Appl. Phys., 1963, 34, 3500–3504 CrossRef CAS.
- K. Binnemans and C. Gorller-Walrand, J. Rare Earth, 1996, 173–180 Search PubMed.
- T. Hirai, T. Hirano and I. Komasawa, J. Mater. Chem., 2000, 10, 2306–2310 RSC.
- M. Jang, S. M. Hong and J. K. Park, Waste Manage., 2005, 25, 5–14 CrossRef CAS PubMed.
- W. A. Durão Jr., C. A. de Castro and C. C. Windmöller, Waste Manage., 2008, 28, 2311–2319 CrossRef PubMed.
- C. Raposo, C. C. Windmöller and W. A. Durão Júnior, Waste Manage., 2003, 23, 879–886 CrossRef CAS PubMed.
- T. Horikawa and K. Machida, Mater. Integr., 2011, 24, 37–43 CAS.
- J. Hoffmann, M. Nuchter, B. Ondruschka and P. Wasserscheid, Green Chem., 2003, 5, 296–299 RSC.
- A. Arfan and J. P. Bazureau, Org. Process Res. Dev., 2005, 9, 743–748 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of the synthesized Y2O3:Eu3+ phosphor, including SEM images, XRD diffractograms and luminescence decay curves. See DOI: 10.1039/c4gc02107j |
| This journal is © The Royal Society of Chemistry 2015 |