Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A green chemistry-based classification model for the synthesis of silver nanoparticles†
Marco
Cinelli
*a,
Stuart R.
Coles
a,
Mallikarjuna N.
Nadagouda
b,
Jerzy
Błaszczyński
c,
Roman
Słowiński
cd,
Rajender S.
Varma
*e and
Kerry
Kirwan
*a
aWMG, International Manufacturing Centre, University of Warwick, University Road, Coventry, CV4 7AL, UK. E-mail: m.cinelli@warwick.ac.uk; kerry.kirwan@warwick.ac.uk; Tel: +44 (0)24 7657 2540 Tel: +44 (0)24 7652 8444
bORD, NRMRL, WSWRD, WQMB, U.S. Environmental Protection Agency, Cincinnati, Ohio 45268, USA
cInstitute of Computing Science, Poznań University of Technology, 60-965 Poznań, Poland
dSystems Research Institute, Polish Academy of Sciences, 01-447 Warsaw, Poland
eSustainable Technology Division, National Risk Management Research Laboratory, U.S. Environmental Protection Agency, Cincinnati, Ohio 45268, USA. E-mail: Varma.rajender@epa.gov; Tel: +1 (513) 487-2701
First published on 9th February 2015
Abstract
The assessment of the implementation of green chemistry principles in the syntheses of nanomaterials is a complex decision-making problem that necessitates the integration of several evaluation criteria. Multiple Criteria Decision Aiding (MCDA) provides support for such a challenge. One of its methods – Dominance-based Rough Set Approach (DRSA) – was used in this research to develop a model for the green chemistry-based classification of silver nanoparticle synthesis protocols into preference-ordered performance classes. DRSA allowed integration of knowledge from both peer-reviewed literature and experts (decision makers, DMs) in the field, resulting in a model composed of decision rules that are logical statements in the form: “if conditions, then decision”. The approach provides the basis for the design of rules for the greener synthesis of silver nanoparticles. Decision rules are supported by synthesis protocols that enforce the principles of green chemistry to various extents, resulting in robust recommendations for the development and assessment of silver nanoparticle synthesis that perform at one of five pre-determined levels. The DRSA-based approach is transparent and structured and can be easily updated. New perspectives and criteria could be added into the model if relevant data were available and domain-specific experts could collaborate through the MCDA procedure.
1. Introduction
The need to steer the development of the synthesis of nanomaterials towards more sustainable practices is a pressing issue for the future of nanotechnology.1–8 Presently, a lot of synthesis protocols for nanomaterials are based on existing industrial processes, which were developed with little consideration for sustainability. Typical conditions include the use of high pressures and temperatures and the use of toxic chemicals.2 Laser ablation, hydrothermal and solvothermal processes and colloidal methods are some popular choices.9,10 A wide variety of techniques have been proposed to produce metal nanoparticles, including chemical reduction,11–13 electrochemical and photochemical reduction,14–16 sonochemistry17 and heat evaporation.18 Chemical reduction has been the most common route due to the convenient operation, simple equipment, cost effectiveness and process control.10,19,201.1 Sustainable development of nanomaterials
There have been calls for the development of nanomaterials on the basis of the principles of green chemistry21 and engineering,22 and in consequence, a variety of studies have emerged.6,21,23–25 Some of the proposed solutions are based on the substitution of reagents with more benign counterparts such as supercritical fluids and solvent-free techniques.5,6 In this regard, a lot of interest has been placed on developing a more environmentally friendly synthesis for silver nanoparticles due to the wide variety of potential applications that these nanomaterials can enhance, including biosensor materials, composites, cosmetics, antimicrobial applications and electronic appliances.26Furthermore, there have been specific calls for the development of lists of sustainability-oriented design practices and standards in order to define products as “green nano”1,5,7,27 and to design rules for new classes of nanomaterials that have desired properties in conjunction with the implementation of the principles of green chemistry.6,7,28
Novel routes have been developed in line with the demands for more sustainable synthesis of nanomaterials to reduce the impacts of nanoparticle synthesis based on bio-inspired reduction.11,29,30 Many “alternative” raw materials have been explored to produce nanomaterials including bacteria, fungi, plants, plant extracts, yeasts and algae.3,9,26,29 These approaches integrate several principles of green chemistry, the use of renewable feedstocks, the prevention of waste production, the use of less hazardous materials, the use of safer solvents, the increase of energy efficiency in the manufacturing and the reduction in the number of synthesis steps.2,6,8,23,30–32
Nanoparticles have been produced using materials that are safe, benign and abundant such as vitamin B2,33,34 sugars,20,35,36 vitamin C,37 tea and coffee extracts,38 ubiquitous antioxidant glutathione,39 beet juice,40,41 glycerol,42 red grape pomace,43 blackberry, blueberry, pomegranate, turmeric extracts,31 orange peel24 and basil leaves;44 a compendium of these studies have been reviewed.11,26,29
An emergent synthesis route that couples the use of the aforementioned biorenewable feedstocks and alternative heating method of microwave (MW) irradiation has seen growing interest in the last decade.2,30,31,45,46 This technique allows the synthesis of nanoparticles of uniform small size within minutes, with desired shapes and improved reaction yields.30,32,47
Critical factors that affect the properties of nanoparticles are the size, shape and monodispersity.48 Consequently, the development of synthesis protocols that enable controlling these parameters is of vital importance.29 This applies to bio-inspired reduction protocols, and it is one of the reasons why researchers have examined different synthesis routes aimed at optimizing the synthesis process including choice of feedstock, pH, reaction time, temperature and pressure, precursor concentrations and MW irradiation/agitation.2,11,48
One of the drawbacks of the use of microorganisms to synthesize nanoparticles is the longer time period required in comparison with conventional techniques.48 Different considerations emerge from the studies using plants and plant extracts, where it has been shown that the reaction times can be as low as a few minutes and hence these processes can be considered as more cost effective, environmentally friendly and the best candidates for scale-up and industrial synthesis of metal nanoparticles.3,11,49
1.2. Use of life cycle assessment to quantify benefits
The successful implementation of these synthesis protocols also requires quantitative analysis of the benefits that they provide and communication of them to the relevant stakeholders. A common method of conducting this is through life cycle assessment (LCA),50 however quantitative analyses of sustainability implications are currently limited by (i) lack of specific inventory data to cover all the life cycle stages,51–58 (ii) deficiency of appropriate characterization of emission entities and pathways,57 and (iii) scarcity of data origin information, transparency and results disaggregation.59 Nonetheless, as far as conventional synthesis protocols for nanomaterials (e.g., arc discharge, laser ablation, chemical vapour deposition and chemical reduction) are concerned, LCA studies have started emerging,55,57,58,60–66 whereas the evaluation of the bio-inspired processes is still unexplored. There is only one study that has been published recently on the LCA of synthesis protocols for metal nanoparticles (gold) that adopt renewable sources;67 the main limitation has been the lack of information about the reducing agents, which leads to their exclusion in most of the LCA calculations. A major impediment to the quantification of impacts of these synthesis protocols is the lack of understanding of the reduction mechanism of silver salt to silver nanoparticles. Several proposals for such a mechanism have been suggested44,68–70 but there is still a level of uncertainty about the crucial role of phenolic compounds in the reaction, which limits the modelling and consequent quantitative assessment of such processes. Another limitation in this regard is the allocation of upstream input to waste material, due to the bio-renewable nature of the feedstocks.Although scientific research will lead to the generation of experimental data to be used in quantitative tools, the available information and expertise can be used and integrated to provide a qualitative evaluation of these synthesis protocols.
Nanosilver is widely used in many applications,2,26,49 which raises concerns about its implications during the life cycle stages. Synthesis is one of those operations and practitioners in this area who are green chemists can reduce its impacts, however small, when compared to other stages. This stage in the life cycle of Ag nanoparticle synthesis was chosen to demonstrate the effectiveness of a method belonging to Multiple Criteria Decision Aiding (MCDA)71 for comparing synthetic approaches and thus quantifying how “green” they are, using as a case study an example that would be understood by most green chemistry practitioners.
1.3 Decision support through Multiple Criteria Decision Aiding
The assessment of how “green” the synthesis methods of nanoparticles truly are requires the consideration of a variety of protocol attributes/criteria for a certain number of alternatives, and this problem can be effectively tackled with MCDA.71,72 MCDA constitutes a framework to support decision makers (DMs) in structuring their decision problems and to offer them tools and methods leading to recommendations about the decisions at stake.71,73 The recommendations are usually based on the comprehensive evaluation of the considered alternatives, by performing some kind of aggregation of evaluations of the alternatives on the criteria used to characterize them. Any MCDA problem is shaped at minimum by two figures, a DM and an analyst.74 The DM is a person who is in charge of making the decision, has intimate knowledge in the field under investigation and does not necessarily need to have competency in MCDA. The analyst is the person referred to as facilitator or researcher who is in charge of the implementation of the decision aiding process by helping the DM to structure the problem, investigate his/her preferences, and select a MCDA model in compliance with the decision context.75 MCDA has several advantages in supporting decision-making:76–78• It does not necessarily require a pre-defined set of data as input;
• It works with very limited and uncertain information;
• It can include experts’ and other stakeholders’ knowledge;
• It provides an adaptable structure that is adequate for the process of identification of criteria and the management thereof.
The definition of MCDA shows the potential to support the assessment of the performance of synthesis protocols that lack quantitative information allowing one to conduct environmental sustainability evaluations.
There is a wide availability of MCDA methods that can be used to integrate information and either classify alternatives into preference-ordered classes or rank them from the best to the worst.72,79 Three main families of MCDA methods can be identified: value/utility theory methods; outranking methods; and rules-based methods.80 Some MCDA approaches require input data transformations that might not be possible in certain contexts, or they might require the definition of quantitative preference models that the DMs can perceive as cognitively difficult to understand and interpret.77 A successful assessment tool should use the same language as the DM and it must be perceived as a “glass box” rather than as a “black box” that provides the DM with some “right” answer that is guaranteed by the analyst's authority.81–84
In order to use MCDA methods for decision-making processes oriented towards sustainability, these instruments must satisfy several requirements as discussed in the literature.76,77,85 The main outcomes of these studies indicate that rules-based approaches are very good candidates for conducting sustainability assessments as they allow (i) enforcing a strong concept of sustainability that does not imply compensation among criteria, (ii) handling qualitative and quantitative information without the need to employ any data transformation, and (iii) making the decision aiding process more transparent and intelligible for the DMs.76
This article proposes a model based on an MCDA method known as Dominance-based Rough Set Approach, for the green chemistry-based classification of synthesis protocols used for nanoparticles into preference-ordered performance classes, by combining the information available in the peer-reviewed literature with the knowledge of experts in the field. Furthermore, the model presented in terms of “if … , then …” decision rules provides the methodological basis for the development of a set of design rules for the synthesis of greener silver nanoparticles.
The main aim of this paper is to show that the MCDA process can make a substantial contribution to supporting decision-making in the governance of silver nanoparticle synthesis. An important point is that the scope of the article is not to provide an exhaustive and quantitative set of assessment criteria, but rather to introduce a decision support procedure for the assessment of synthesis protocols for nanomaterials that can be improved on a regular basis. The MCDA model developed in this paper is a tool for the assessment of synthesis protocols in view of preferences a decision maker could have in favor of green aspects of the protocols. The synthesis of silver nanoparticles was selected as the case study to develop the model. There are three main reasons for that: (i) it was possible to create a database of comparable synthesis protocols using green chemistry-based criteria for this nanomaterial; (ii) experts with knowledge in the area agreed to take part in the decision aiding process; and (iii) a wide range of successful applications are enabled and envisioned by silver nanoparticles, such as electronic products, composite fibers, biosensor materials, cosmetics and antimicrobial products.2,26,49
2. Methodology
Assessing the implementation of green chemistry principles in silver nanoparticle synthesis is a complex decision-making problem that requires measurement and integration of several evaluation criteria. MCDA provides a framework to handle this type of challenge and hence it was adapted for evaluation of these synthesis protocols.In MCDA, the alternatives are the objects (also called actions, scenarios, plans and programs) that need to be assessed during the decision aiding process.71,73 The criteria are built on elementary features of the objects to assess the alternatives.86
2.1 The MCDA process
The MCDA process aims to support the interaction between the DM and the analyst and it comprises four main stages that are linked and interdependent:74• Problem situation representation;
• Problem formulation;
• Evaluation model;
• Final recommendation.
Expert input is pivotal in a field that is characterized by uncertainty and lack of quantitative data, as is the case for nanomaterial synthesis. At this time there is a lack of available knowledge regarding the synthesis of nanomaterials because of either proprietary issues or the lack of research findings.57,66,67,87 The use of expert judgment is considered as a reliable solution when limited data are available and quantitative or historical information is not in place.88–90 Consequently, the inclusion of expert opinions in the context of nanomaterials synthesis assessment is a solution to overcome the existing data gaps.
| Criterion | Criterion values | Preference order of the values | Rationale for the preference order |
|---|---|---|---|
| a Detailed information for preference order selection of criteria values is available in ESI. | |||
| Reducing agenta | Renewable – waste | ↑ | Reducing, capping and solvent are three main areas of opportunity for the implementation of green chemistry in the reduction of metal ion salts in metal nanoparticles.34,38,39,44 It is possible to choose among waste from renewable sources (RW), primary renewable materials (RP), biodegradable polymers (BP) and synthetic chemicals (SC). Preference was defined as: RW > RP > BP > SC. |
| Renewable – primary | |||
| Biodegradable polymer | |||
| Synthetic | |||
| Capping agenta | Not needed | ↑ | Implementable green chemistry principles: 1, 3, 4, 5, 7, 8, 10, 12 |
| Renewable – waste | |||
| Renewable – primary | |||
| Biodegradable polymer | |||
| Synthetic | |||
| Solventa | Renewable – waste | ↑ | |
| Renewable – primary | |||
| Biodegradable polymer | |||
| Synthetic | |||
| Local resources use | Yes | ↑ | This parameter relates to the protocols that employ renewable materials. When local resources are used, this can be considered a benefit in terms of reduction of transportation impacts and costs. |
| No | Implementable green chemistry principles: 7 | ||
| Reaction time | In seconds | ↓ | Reaction speed is important in materials synthesis as the longer the process the higher the amount of energy needed to run the equipment. As a result this criterion has to be minimized. |
| Implementable green chemistry principles: 6, 12 | |||
| Reaction temperature | In Celsius | ↓ | Synthesis processes can be performed at different temperatures depending on the reaction, type of equipment and its setup. From a green chemistry perspective the lower the temperature the better as less energy is required and safer operating conditions are in place. Consequently the criterion has to be minimized. All the protocols used to prepare the nanoparticles operate at reactions temperatures that are equal or higher compared to ambient temperature. As a result, the higher the temperature with respect to the ambient one, the worse the processing condition. |
| Implementable green chemistry principles: 6, 12 | |||
| Equipment typea | Static | ↑ | Several bottom-up approaches are available starting from very simple equipment such as a stirring plate, up to a laboratory microwave oven and oil baths. The preference order was defined from an integrated experts’ judgment of each equipment type in terms of: energy consumption, process safety, waste production, reaction speed and simplicity of operation. |
| Stirring for at most 5 minutes | |||
| Stirring | |||
| Microwave – sealed vessel ≤(300 W) | |||
| Microwave – sealed vessel >(300 W) | |||
| Microwave – open vessel | |||
| Conventional (oil bath, steam bath) | Implementable green chemistry principles: 1, 6, 11, 12 | ||
| Not reported | |||
| Size range | 0 ≤ particle size ≤ 30 nm | ↑ | Synthesis protocols lead to the silver nanoparticles that are normally within a certain size ranges rather than of a unique and particular size. The preference introduced for this criterion is that the smaller the particles the better. This is in accordance with what has been reported for the antimicrobial activity of silver, which has shown size-dependency; the smaller the size the higher the antimicrobial potential.96–99 However, there are no agreed cut-offs values for the sizes of the particles that can induce higher antimicrobial effects and hence five size range classes where introduced. |
| 0 ≤ particle size ≤ 60 nm | |||
| 30 < particle size ≤ 60 nm | |||
| 0 < particle size ≤ 100 nm | |||
| 60 < particle size ≤ 100 nm | |||
Web of Science,‡ which includes more than 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 journals and 30
000 journals and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 books worldwide, was used as the database for the identification of potential studies reporting the synthesis of silver nanoparticles through chemical or biological reduction. The studies selected to be part of the dataset were those reporting information on the criteria selected for the evaluation model (see Table 1 and ESI† for details on rationale for protocols comparability).
000 books worldwide, was used as the database for the identification of potential studies reporting the synthesis of silver nanoparticles through chemical or biological reduction. The studies selected to be part of the dataset were those reporting information on the criteria selected for the evaluation model (see Table 1 and ESI† for details on rationale for protocols comparability).
The final stage of MCDA (i.e., final recommendation) is described in the next section, since it represents the main outcome of the model, namely the decision support tool for classification of new or existing silver nanoparticle synthesis protocols in preference-ordered classes.
2.2 Dominance-based Rough Set Approach for performance classification of silver nanoparticle synthesis protocols
The rules-based approach named Dominance-based Rough Set Approach (DRSA), an MCDA method introduced and characterized in ref. 84,100–102 was selected to tackle this problem and derive a decision model to classify synthesis protocols for silver nanoparticles based on the principles of green chemistry.DRSA handles knowledge about alternatives in the form of an information table (Table 2), whose rows are defined as objects to be evaluated, while the columns are divided into condition attributes (i.e., C), namely the evaluation criteria that are needed to assess the objects and the decision attribute (i.e., D), which represents an overall evaluation of each object in the table.
| Silver nanoparticle synthesis protocol | Condition attributes (criteria) | Decision attribute (Performance class) | ||
|---|---|---|---|---|
| Reducing agent | Temperature (°C) | Reaction time (s) | ||
| I | Renewable-waste | 35 | 120 | High |
| II | Biodegradable polymer | 40 | 2400 | Low |
| … | … | … | … | … |
In this case study, the objects under assessment are the considered nanoparticle synthesis protocols (I, II, etc.), while the criteria are those reported in Table 1, and the decision attribute represents the level of “performance” of each synthesis protocol. This performance can take one of five possible preference-ordered classes, which can be assigned by the DM on the basis of the implementation of green chemistry principles and satisfaction of quality requirements that the criteria of each protocol convey. The preference-ordered classes (i.e., A > B > C > D > E) are:
• A (very high) = a wide set of green chemistry principles is adopted and the process can be seen as a reference for future research aimed to improve the performance of synthesis protocols for silver nanoparticles;
• B (high) = quality requirements are satisfied, a considerable set of green chemistry principles is applied;
• C (medium) = principles of green chemistry are partially implemented and there can be quality improvement possibilities;
• D (low) = the synthesis protocol shows limited implementation of principles of green chemistry and/or satisfaction of quality requirements;
• E (very low) = complete lack of green chemistry perspective and disregard for environmental implications of the synthesis protocol.
In this case study the class for each synthesis protocol was assigned by two experts who participated in the decision aiding process, after a series of sessions devoted to the achievement of a classification agreement. The complete information table with silver nanoparticle synthesis protocols and expert classification is reported in Table S1 in the ESI.† DRSA, implemented with jMAF software,§ was used to analyze Table S1† and derive a set of logical statements in the form of “if … , then …” rules, which explains the classifications made by the DMs.¶
The rules derived from DRSA represent robust knowledge of the DM that participated in classification of protocols. Once these rules are discussed and accepted by the DM they become a decision model that can be used to assess new (unseen) alternatives.82,103,104 In this case study, they could be adopted for the classification of new synthesis protocols for silver nanoparticles with reference to green chemistry principles. More specifically, the model that is derived from the comprehensive use of the decision rules could be employed as a decision support tool for the assignment to performance classes of new or existing synthesis protocols for silver nanoparticles. Two classification schemes, named standard and new,105 were selected and contextualized to this multiple criteria problem.
The net value, ScorenetR(Clt, m), resulting from Score+R(Clt, m) − Score−R(Clt, m), is an indication of the strength of the assignment to class Clt and the final recommendation of a class depends on the one with the highest net score (details about the score calculations can be found in ESI†).
3. Results and discussion
The MCDA procedure was applied with the collaboration of the DMs and jMAF software,106 which implements DRSA, was used in order to answer five questions that this MCDA method can tackle to support decision-making:• Have the DMs been consistent with their judgments?
• Are there any subsets of criteria (reducts) that allow achieving the same quality of approximation as the whole group of criteria?
• What is the classification model based on decision rules derived from the experts judgments?
• What are the most relevant criteria for the classification?
• How can the decision model be used to classify existing or new synthesis protocols for silver nanoparticles?
Firstly, DRSA shows that the assignment of classes to the synthesis protocols by the experts was completely consistent, resulting in a quality of approximation equal to 1. This indicates that there are no ambiguous objects in the information table and the criteria finely discriminate the choice of the classes (Table 3). The DRSA analysis shows full consistency in the assessment, which is a significant prerequisite for acceptance of the results and their credibility. Unitary quality of approximation is an indication of appropriate problem formulation, including criteria choices and database construction. However, full consistency and agreement among experts is not a necessary prerequisite for DRSA, as inconsistent input information and multiple DMs with different judgments can be handled as well.107,108
| At most E | At most D | At least D | At most C | At least C | At most B | At least B | At least A | |
|---|---|---|---|---|---|---|---|---|
| a Difference between lower and upper approximation. b Ratio of the number of protocols in the lower approximation to the number of protocols in the upper approximation. | ||||||||
| Lower approximation | 2 | 14 | 46 | 21 | 34 | 33 | 27 | 15 |
| Upper approximation | 2 | 14 | 46 | 21 | 34 | 33 | 27 | 15 |
| Boundarya | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Accuracyb | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
As far as the reducts are concerned, only one was found composed of all the criteria with the exclusion of parameter “local resources use”. This means that all criteria but one were used to distinguish the assignment of the protocols among the classes. The irrelevance of “local resources use” is an indication that this parameter is not needed to obtain the same quality of classification as with the whole set of criteria. The reason for this is that the DMs did not perceive sufficient information about the origin of the materials to be able to judge how the source location could change the environmental impacts. As a consequence they discarded the information expressed by this criterion.
Thirdly, DRSA conveyed 26 decision rules (Table 4): four for class at least A; three for class at least B; three for class at least C; three for class at least D; two for class at most E; three for class at most D; four for class at most C; and four for class at most B. Each rule is composed of a conditional part that includes the value(s) of the criterion/criteria and a decisional part, which is the assigned class to every process. The rules were shown to the experts using maps obtained with Mindjet software109 in order to aid graphical representation and intelligibility (Fig. S1 and S2 in ESI†). Each rule is represented with the conditions that characterize it, the resulting decision/performance class assignment, its support and coverage factor in percentage (see Table 4 for details). The DMs easily understood the rules and agreed on all of them, which became the decision model for this classification problem. The rules obtained with DRSA highlight the assumptions that the experts made in their choices, which pose the basis for directing future quantitative assessment of green synthesis of silver nanoparticles.
| Rule type | Rule INa | Conditions | Decision/performance class | Supporting protocolsb | Rule coverage factorc |
|---|---|---|---|---|---|
| a Rule Identification Number (IN). b Number of protocols that support the rule. c Percentage of number of protocols that satisfy the conditions of the rule and are assigned to the class or union of classes. | |||||
| At least | 1 | (Solvent >= Renewable primary) & (Equipment >= Stirring under 5 min) & (Size range <= 0_60 nm) | >= A | 9 | 60.00% |
| 2 | (Reducing agent >= Renewable primary) & (Reaction time <= 30 s) | >= A | 3 | 20.00% | |
| 3 | (Reaction time <= 60 s) & (Temperature <= 47 °C) | >= A | 4 | 26.70% | |
| 4 | (Local resources use >= Yes) & (Temperature <= 30 °C) | >= A | 4 | 26.70% | |
| 5 | (Reducing agent >= Renewable primary) & (Capping agent >= Not needed) & (Reaction time <= 30 min) | >= B | 16 | 59.25% | |
| 6 | (Capping agent >= Renewable primary) & (Reaction time <= 60 s) | >= B | 7 | 25.92% | |
| 7 | (Capping agent >= Renewable primary) & (Solvent >= Renewable primary) & (Temperature <= 40 °C) & (Size range <= 0_30 nm) | >= B | 12 | 44.44% | |
| 8 | (Reducing agent >= Renewable primary) & (Capping agent >= Renewable primary) & (Solvent >= Renewable primary) | >= C | 32 | 94.10% | |
| 9 | (Reducing agent >= Renewable primary) & (Reaction time <= 45 s) | >= C | 5 | 14.71% | |
| 10 | (Solvent >= Renewable primary) & (Equipment >= Static) | >= C | 5 | 14.71% | |
| 11 | (Solvent >= Renewable primary) | >= D | 42 | 91.30% | |
| 12 | (Capping agent >= Biodegradable polymer) | >= D | 40 | 86.95% | |
| 13 | (Reaction time <= 60 s) | >= D | 11 | 23.91% | |
| At most | 14 | (Capping agent <= Synthetic) & (Temperature >= 170 °C) | <= E | 1 | 50.00% |
| 15 | (Capping agent <= Synthetic) & (Solvent <= Synthetic) & (Reaction time >= 4 h 15 min) | <= E | 1 | 50.00% | |
| 16 | (Reducing agent <= Synthetic) | <= D | 11 | 78.57% | |
| 17 | (Solvent <= Synthetic) | <= D | 6 | 42.85% | |
| 18 | (Capping agent <= Synthetic) & (Equipment <= Not known) | <= D | 2 | 14.28% | |
| 19 | (Capping agent <= Biodegradable polymer) & (Temperature >= 80 °C) | <= C | 9 | 42.85% | |
| 20 | (Local resources use <= No) & (Size range >= 30_60 nm) | <= C | 3 | 14.28% | |
| 21 | (Reaction time >= 45 min) & (Temperature >= 60 °C) | <= C | 8 | 38.10% | |
| 22 | (Reaction time >= 8 h) & (Equipment <= Stirring) & (Size range >= 0_60 nm) | <= C | 1 | 4.80% | |
| 23 | (Size range >= 30_60 nm) | <= B | 4 | 12.12% | |
| 24 | (Reaction time >= 45 s) & (Temperature >= 55 °C) | <= B | 21 | 63.63% | |
| 25 | (Reaction time >= 10 min) & (Equipment <= Conventional) | <= B | 18 | 54.54% | |
| 26 | (Equipment <= Stirring) & (Size range >= 0_60 nm) | <= B | 7 | 21.21% | |
The decision model can be used to support the development of new and emergent synthesis protocols for silver nanoparticles or for the assessment of current ones. The synthesis protocols that satisfy the conditions of the class A are covered by rule 1 to 4. Rule 1 includes very simple systems that operate with renewable solvents under static conditions or with limited stirring. All the protocols qualifying for class A use renewable reducing agents, which were indicated by the experts as another driving consideration for this choice (i.e. PIN|| 4, 5, 6, 7, 8, 13, 14, 15, 16, 17, 18, 19, 20, 46, 48). Based on experts’ judgments, such protocols can implement several green chemistry principles (GCP) concurrently, including waste prevention, reduction in use of hazardous chemicals and derivatives, adoption of safer solvents and renewable feedstocks, and inherently safer chemistry. A major consideration that emerges from rule 1 is the need for further research on the role that different compounds of renewable materials have in the formation, kinetics and stabilization of the nanomaterials. Such understanding can lead to a more informed selection of those materials that can have the widest potentials for increasing reaction speed and yield, thus posing a strong basis for large scale synthesis.
Other protocols satisfying the assignment to class A are those employing MW technology with reaction times and temperature equal to or lower than 1 min and 47 °C, respectively, together with the use of renewable materials (rules 2 and 3 in Table 4 and Fig. S1†). Microwave (MW)-enhanced protocols have received great attention in the green chemistry literature as they allow remarkable increment of reaction speed, leading to complete salt reductions with very low irradiation power (e.g., 50 W), even in less than one minute.39 MW irradiation has the advantages, when compared to conventional techniques, of providing uniform heating, increasing the kinetics of the reactions by one or two orders of magnitude, improving the kinetics of crystallization and reducing the production of waste.30,32,46,110–113 Several protocols that adopt the MW heating technique (i.e., PIN 2, 4, 5, 6, 7, 8, 39, 42), couple it with the use of renewable resources as substitutes for harsh chemicals (e.g., sodium borohydride), which leads to the fulfillment of many GCP, namely reduction in the hazardousness of chemical synthesis together with the resulting waste, use of safer solvents (e.g., water) and inherently safer chemistry (e.g., closed vessels and low power). Although these protocols under consideration are for small and medium scales reactions, the scale-up of MW technology has been investigated, showing that these systems perform even better at higher scales (i.e., liters) from an energy efficiency perspective,113 which additionally improves the appeal of this equipment.
As far as class B synthesis protocols are concerned, rule 5 focuses on protocols that adopt multifunctional materials, in other words those having both a reducing and capping agent function, which can be an environmental sustainability upside as it allows decreasing materials usage and waste production, together with elimination of synthesis steps. Phenolic compounds can both reduce silver salts to nanoparticles and prevent their aggregation by providing excellent capping function, as reported for example in the case of silver nanoparticle synthesis with basil plant,44 red pomace43 and Lippia citriodora.114 Furthermore, proteins present in the extract can have a premier role in the capping of NPs.44,70,115 This rule indicates that multifunctionality has to be coupled with the use of renewable reducing agent(s) and reaction times as long as 30 minutes. Almost 60% of the protocols in at least the class B satisfy these conditions, showing a strong pattern in the dataset. Plant extracts are the primary candidates for this multifunctional advantage, as has been widely reported in the literature.3,30,34,38,49,70,110 However, the specific reducing and capping mechanisms of these multifunctional materials are not yet well understood,70 and this represents a major area of investigation that could lead to more rational and motivated investments on certain plants types.
Nonetheless, conventional techniques for synthesis protocols are still a major option for producing silver nanoparticles and they can achieve high performance too in cases where renewable materials are used for reactions operating at temperatures up to 40 °C (rule 7). More specifically, these mild reaction temperatures can be coupled with reaction times as low as 10 minutes, which are relatively short for this type of equipment (i.e., PIN 36, 37, 43). Such protocols show the potential high performance even for conventional heating techniques when combined with multifunctional renewable reducing agents (i.e., basil plant44 and Xerophytes – Bryophyllum sp.116).
94.1% of the protocols that were assigned to classes A, B and C utilize at a minimum a renewable-primary reducing and capping agent together with a renewable-primary solvent (rule 8). This is a strong pattern, which shows how the GCP are widely implementable, even when varying the renewable raw materials choice (e.g., sugars,35,36 amino acids,39,117 plant extracts of various sources,38,68,118 vitamins34 and renewable polymers119). These choices lead to prevention of harmful waste, less hazardous chemical synthesis and reduction of derivatives. Some of them even perform both reducing and capping actions using waste materials (i.e., red grape pomace and orange peel extract).24,43 Further consideration is needed to better understand the viability of waste materials as candidate sources for high value green products.30,43,67
Contrasting considerations are obtained with the “at most” rules. Rule 14 (Table 4 and Fig. S2†) includes worst performing protocols, whose conditions are the use of a capping agent that is of synthetic origin and a temperature above or equal to 170 °C. The processes covered by this rule violate several GCP, including the reduction of waste production, the elimination of synthesis steps and the use of renewable and benign reagents, leading to assignment in the lowest class. Similar considerations emerge from rule 15, which states that the use of a synthetic capping agent and solvent for reactions lasting over 4 hours and 15 minutes heavily compromises the GCP, resulting in a very low class due to reduced energy efficiency and process safety.
Analysis of rules 16 and 17 (Table 4 and Fig. S2†) shows over 75% and 40% of the protocols assigned to at most class D used a synthetic reducing agent and solvent, respectively. More specifically, the DMs underlined the fact that most of the chemicals used in these protocols are hazardous (e.g., sodium borohydride, 1-nonanethiol, chloroformic solution, dodecylthiol, toluene, polypropyleneimine, naphthalene, hydrazine), which is in conflict with the need to use renewable feedstocks, reducing waste production and adopting safer chemistry.
Almost 43% of the protocols assigned to at most class C operate at a temperature of at least 80 °C with either a biodegradable polymer or a synthetic capping agent (rule 19). This is an interesting feature of the decision model, which reaffirms how use of a redundant component as the capping agent and a relatively high temperature can relegate protocols to class C at maximum.
Reaction time and temperature have an important role in the assignment to at most class C (rule 21). A reaction period of at least 45 minutes in conjunction with a temperature of at least 60 °C is a trigger for assignment up to medium performance. This kind of information can be seen as indirect extraction through DRSA of combined discriminatory thresholds for reaction length and temperature. The experts stressed how the combination of relatively long reaction times and high temperatures are indicative of the need to develop energy efficient protocols to minimize energy use. With the exclusion of one protocol that employs MW (i.e., PIN 22), all the remaining ones are based on conventional heating. Relatively long times for complete reaction are actually one of the major drawbacks of this equipment when compared to MW technology.32,45,46,120
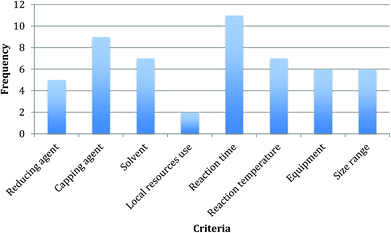
The different relevance that the criteria have in the assessment can be measured by means of their frequency in the rules,121,122 which is shown in Fig. 1. Reaction time and capping agent type are the most recurrent criteria in the decision rules (present 11 times and 9 times respectively), which is confirmed by the unquestionable link that they have with the potentials of reducing environmental impact and improving design for waste prevention and energy efficiency. The remaining parameters are rather equal in terms of appearance in the rules, with the exclusion of the use of local resources, which scores very low (in two rules only), possibly due to the limited discriminatory potentials of its two-categories domain as discussed above.
The last contribution of the decision model is its possible use for allocation to performance classes of new or existing silver nanoparticle synthesis protocols. The assignment procedure follows the methodology proposed in ref. 105, adapted to this decision-making problem (see section 2.2 for details and ESI†).
As an example, five hypothetical test synthesis protocols (Table 5) were prepared and the recommended classes for standard and new classification scheme of DRSA are presented in Table 6. Both classification strategies allocate process t1 to class A, whereas they assign t2 and t5 to class D. Protocols t3 and t4 are appointed to more than one class with the standard classification method, D or C for t3 and C or B for t4, respectively. Such an outcome is due to the interval of classes that result from the intersection of the covering rules. However, a univocal assignment can be obtained with the new classification scheme, which suggests class D for t3 and class B for t4.
| Test protocol | Reducing agent class | Capping agent class | Solvent class | Local resource use class | Reaction time | Temperature (Celsius) | Equipment class | Size class |
|---|---|---|---|---|---|---|---|---|
| t1 | Renewable primary | Not needed | Renewable primary | No | 55 seconds | 42 | Microwave – sealed vessel ≤(300 W) | 0_30 |
| t2 | Renewable primary | Biodegradable polymer | Synthetic | Yes | 43 minutes | 85 | Conventional | 0_60 |
| t3 | Biodegradable polymer | Biodegradable polymer | Renewable primary | No | 10 minutes | 90 | Conventional | 30_60 |
| t4 | Renewable primary | Renewable primary | Renewable primary | No | 70 seconds | 65 | Microwave – sealed vessel ≤(300 W) | 0_30 |
| t5 | Synthetic | Synthetic | Synthetic | No | 8 minutes | 100 | Microwave – open vessel | 0_30 |
| Test protocol | Recommended class by standard scheme | Recommended class by new scheme | Maximum score for new scheme | IN of matching rulesa |
|---|---|---|---|---|
| a Identification number of rules whose conditions match the test protocol. | ||||
| t1 | A | A | 0.33 | 3, 5, 6, 8, 11, 12, 13 |
| t2 | D | D | 0.22 | 12, 17, 19, 24, 25, 26 |
| t3 | D or C | D | 0.26 | 11, 12, 19, 20, 23, 24, 25, 26 |
| t4 | C or B | B | 0.26 | 8, 11, 12, 24 |
| t5 | D | D | 0.33 | 16, 17, 19, 24 |
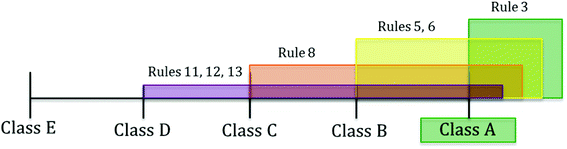
Fig. 2 illustrates the rationale behind the assignment of class with the standard classification scheme for test protocol t1. Seven decision rules (i.e., 3, 5, 6, 8, 11, 12, 13), all of the type “at least”, match the conditions of the test protocol. Rule 3 recommends class at least A (green colour), rules 5 and 6 suggest class at least B (yellow colour), rule 8 advances class at least C (orange colour) and rules 11, 12 and 13 suggest class at least D (purple colour). The recommended class derives from the intersection of the lowest class covered by all the rules, which in this case is A.
This result is reinforced by the new classification scheme, whose highest score (0.33) is also for the class A, indicating that the strongest concordance of the decision rules is on such a class (Table 7). Its value is calculated as follows:
Score−R(Clvery high, t1) is 0 as there are no rules that do not cover class A as a possible recommendation and consequently ScorenetR(Clvery high, t1) is equal to 0.33. Table 7 reports all the scores for each class based on the new classification scheme (see ESI† for detailed score calculations for each class). The Score+ values indicate the strength of the support that the covering rules provide in the assignment to each class. More specifically, for class A, all the covering rules (i.e., 3, 5, 6, 8, 11, 12, 13) include it and they all concur to the calculation of the value. For class B all the covering rules but rule 3 embrace it, which results in a slightly lower value than that for the best class. Conversely, Score– values account for the strength of assignment to a different class from the one under consideration. In the case of class A, there are no rules that do not cover it and consequently Score– is 0, whereas it increases as the classes become worse. Class B has rule 3 that does not include it and consequently it works against its assignment to such class. For class C and D, rules 3, 5, 6 and 3, 5, 6 and 8 exert this role, respectively.
| Class | Score+ | Score– | Scorenet |
|---|---|---|---|
| A | 0.33 | 0.00 | 0.33 |
| B | 0.27 | 0.05 | 0.22 |
| C | 0.15 | 0.44 | −0.29 |
| D | 0.22 | 0.89 | −0.67 |
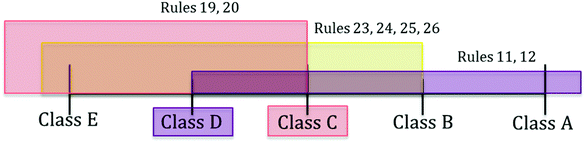
A different case is test protocol t3, where the standard classification method suggests the assignment to class D or C without possibility of refinement (Fig. 3). This is the interval between the recommended class from “at least” rules (i.e., D, rules 11 and 12) and the one from “at most” rules (i.e., C, intersection of classes for rules 19, 20, 23, 24, 25, 26). Nonetheless, the new scheme indicates that the strongest support of the rules is for class D, which results in the assignment to a specific class in this ambiguous case too (Tables 6 and 8). More specifically, the scores for class D are the following:
| Score−R(Cllow, t3) = 0 |
| ScorenetR(Cllow, t3) = 0.26 − 0 = 0.26 |
| Class | Score+ | Score– | Scorenet |
|---|---|---|---|
| A | 0.33 | 0.91 | −0.58 |
| B | 0.27 | 0.31 | −0.04 |
| C | 0.15 | 0.00 | 0.15 |
| D | 0.26 | 0.00 | 0.26 |
| E | 0.02 | 0.98 | −0.96 |
Table 8 indicates that although the strength of the recommendation for class C is much higher than that for class E, B or A, the value for class D is the highest, which triggers the assignment to such a class (see ESI† for detailed score calculations for each class). Class A has a relatively high Score+ (i.e. 0.33) due to the strong support of rules 11 and 12, however rules 19, 20, 23, 24, 25, 26 advance the assignment to a class other than A, resulting in a high Score– (0.91). The difference between these scores is −0.58, which conveys a strong discouragement for the assignment of the protocol to class A. On the other hand, class C and D are supported by all the covering rules and consequently their Score– is 0. Score+ for class C is lower than that for class D as their value depends on the number of protocols in each class, which is higher for class D compared to class C.
This relatively simple example demonstrates the potential use of the rules as a decision-making support for the green chemistry-oriented synthesis of silver nanoparticles. In fact, the DM can define the synthesis protocol that needs to be assessed, obtain the recommended classes with both classification schemes based on DRSA and discuss the results in order to find rational and robust considerations about the decisions at stake.
MCDA has been shown to be very useful in engaging the DMs in selecting the evaluation criteria and the development of the dataset. Additionally, the explanation of experts’ choices through the use of “if … , then …” decision rules is perceived as easily intelligible and the model is seen as supportive for future screening of new silver nanoparticle production protocols. The structured process that MCDA follows provides stakeholders with the possibility of tracking all the evaluation stages and its conclusions, thus supporting more transparent decision-making.
Regarding the criteria selection, the main constraints to their number and possible values have been the limited information reported in many protocols that are part of the dataset. In this regard, the categories for the types of reducing agent, capping agent and solvent were limited to renewable (waste or primary), biodegradable polymers and synthetic types. No considerations about the effective toxicity of most of the renewable materials were taken into account due to the lack of this type of information. Another constraint was the lack of data about the availability of some of the materials that were used, which did not allow accounting for the potential large scale implementation of the synthesis protocols.
Furthermore, the applicability of the rules for the practical synthesis of silver nanoparticles might depend on the location for which the synthesis is planned. The quantitative assessment of the impacts of these choices are out of the scope of this paper and they could become a focus of future research if more detailed information about the implications of material selection becomes available.
Due to the limitations reported above the model that was developed in this case study is not to be seen as a comprehensive tool for evaluating how green silver nanoparticle synthesis protocols are, but rather it is a demonstration that the MCDA process can be of help to better define the complex task of developing synthesis protocols for silver nanoparticles, including the identification of the main parameters and stakeholders that drive this decision-making problem. DRSA has shown how a wide variety of information type and quality can be aggregated through experts’ elicitation, providing the basis for the development of easily intelligible decision support tools for green chemistry-based synthesis of nanomaterials.
This research advances recommendations that can be used to conceive tools for a more detailed assessment of synthesis protocols for silver nanoparticles. Firstly, comparability of processes can be greatly increased if information about how the surface chemistry, sample purity, and particle coating affect their function. Secondly, a thorough investigation of the toxicity of the materials used and produced is needed so that toxicity-based categories for the types of materials can be added, strengthening the approach from a regulatory perspective. Thirdly, investigations about the availability of the raw materials should be conducted in order to consider the potentials for actual exploitation of raw materials for large-scale synthesis. In addition, quantification of the synthesis processes in terms of reactions yield, waste production and energy consumption of the considered (and additional) equipment (e.g., sonication) would greatly benefit quantitative assessments of the implications of each protocol.
The model that was developed is not limited to the use of GCP, and it can be expanded to include other sustainability-related criteria (e.g., LCA, risk assessment, socio-economic data), provided that this additional information is made available and domain-specific experts are involved in the MCDA process. The latter consideration is of paramount importance as each problem is characterized by DMs who have the role of making decisions. The DMs in MCDA are the individuals who are involved in defining the problem, selecting the evaluation criteria and building the decision model. If these persons are not involved in the MCDA procedure there is no real decision aiding, which also will not lead to implementation of assessment criteria in the real value chain of the alternatives (e.g., nanomaterials synthesis protocols, nanomaterials recycling, nanomaterials distribution, etc.) under assessment.
4. Conclusions
The MCDA procedure proposed in this paper can be used as a decision support tool to include stakeholders in the development and assessment of protocols for the synthesis of silver nanoparticles. The approach can be used to structure the decision problem, identify the alternatives and the criteria to be used for comparing them, elicit the preferences of DMs and derive a classification model for existing or new silver nanoparticle synthesis protocols.DRSA was selected as an MCDA method due to its flexibility in handling heterogeneous information, the lack of compensation among the criteria, the intelligibility of its results in the form of “if … , then …” decision rules, and the simplicity of their application. All these factors were well received from the two DMs involved in the decision aiding process, confirming that this methodology can be considered as a “glass box” when compared to conventional MCDA approaches. DMs also provided their expert classification for each synthesis process among a five-class set from very high to very low on the basis of their interpretation of implementation of the principles of green chemistry.
DRSA results show that DMs’ judgments were all consistent, leading to a unitary quality of classification, an indication of relevant problem structuring. The presence of a reduct with all but one of the criteria suggests that almost the whole set plays a discriminatory role in the protocol evaluations.
26 decision rules that explain DMs’ expertise and knowledge for the classification of silver nanoparticle synthesis in preference-ordered classes were derived; 13 for the at least classes and 13 for the at most classes. The best performance (class A) was assigned to the protocols that adopt very simple equipment, renewable resources and low temperatures (≤30 °C).
The use of multifunctional renewable materials is a main driver for high performance classification. Nonetheless, more research should be devoted to the understanding of the reducing and capping mechanisms of such materials, in order to provide a strong basis for the selection and exploitation on a large scale of the optimum resources types. This would require further investigation into the formation, kinetics and stabilization processes for the synthesis of silver nanoparticles mediated by renewable sources. On the other hand, the use of a synthetic material as a capping agent relegates the synthesis process to a low performance class because such a choice results in an increase of waste production, harmful processing and no implementation of raw materials multifunctionality. The use of hazardous synthetic materials is against the need of employing benign feedstocks and reducing solvent-intensive purification steps. As a result, protocols with these features are normally relegated to a low to very low category. Furthermore, thresholds for combinations of reaction times and temperatures were derived for classification of some medium to very low performance protocols, showing the potentials of the MCDA approach as an aid to identifying preference values that would be otherwise difficult to elicit from DMs.
The decision rules represent a decision model that can be utilized as a tool supporting the assignment of new or existing synthesis protocols for silver nanoparticles to performance classes, based on green chemistry principles, showing that as a proof of concept a classification model in this area of research can be devised. Nonetheless, there are still limitations in terms of data availability for the development of an assessment tool inclusive of important parameters that can render the model applicable in experimental settings, such as material toxicity and availability.
Several advantages emerged from the use of DRSA in this case study:
• It does not require direct elicitation of cognitively demanding information (such as criteria weights, assessment of virtual lotteries, pairwise comparisons of criteria and alternatives on an intensity scale, and comparison thresholds) from the DMs, as it is for other MCDA methods (e.g., Multi Attribute Utility Theory, Analytical Hierarchy Process, outranking methods);
• The preference information is obtained by means of comprehensive judgments on exemplary protocols, which can be provided in an easy and comfortable manner from the DMs;
• No transformation of criteria domains from ordinal to cardinal scales is required;
• The approach provides information about the classification ability of the selected criteria and the minimal set of criteria indispensable for the consistent assessment;
• The decision model is composed of decision rules expressed as “if (condition), then (decision)”, which are transparent and easily understandable by the DMs. The rules are related to specific alternatives (e.g., nanoparticle synthesis protocols), which allows tracing and improving the decision process;
• It can deal with the inconsistencies in judgments and handle heterogeneous information;
• DRSA does not need any preliminary or additional information about the data, such as probability distributions in statistics, or grade of membership or the value of possibility in fuzzy set theory.
The whole DRSA-based procedure has been shown to be a good solution to support decision-making for the governance of silver nanoparticle synthesis, introducing several benefits that might not be achievable with traditional approaches. The process is transparent and structured, qualifying as a management tool that can be updated regularly. Stakeholders can be directly involved in the decisional process and additional perspectives and relevant criteria can be included in the problem at hand. An important point is that in order to effectively tackle decision-making problems, inclusion of DMs in the whole MCDA process is mandatory, since there cannot be real decision aiding without actually supporting the persons who make the decisions. DRSA fully satisfies this requirement and it can be used as an indirect preference elicitation tool that can support DMs in better understanding their choices, knowledge and expectations. A model based on DRSA can be proved useful not only to academics and researchers trying to derive robust and transparent recommendations about their choices but also to businesses whose interest is to find justifiable and understandable explanations to the decisions that they have made.
Disclaimer
The U.S. Environmental Protection Agency, through its Office of Research and Development, funded and managed, or partially funded and collaborated in, the research described herein. It has been subjected to the Agency's administrative review and has been approved for external publication. Any opinions expressed in this paper are those of the author(s) and do not necessarily reflect the views of the Agency, therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.Acknowledgements
The authors greatly acknowledge the funding from the UK Engineering and Physical Sciences Research Council, WMG Department of the University of Warwick (UK) and the “CL4W”, Cleaning Land for Wealth, project (grant number EP/K026216/1). The authors would like to thank Dr David E. Meyer, Dr Michael Gonzalez and Dr Gerardo Ruiz-Mercado from U.S. EPA for the thorough and constructive feedback provided during the paper development.References
- R. Luque, Mater. Sci. Nanotechnol., 2013, 1, 1–2 Search PubMed.
- J. Virkutyte and R. S. Varma, in Sustainable Preparation of Metal Nanoparticles: Methods and Applications, The Royal Society of Chemistry, 2013, pp. 7–33, 10.1039/9781849735469-00007.
- J. Virkutyte and R. S. Varma, in Sustainable Nanotechnology and the Environment: Advances and Achievements, American Chemical Society, 2013, ch. 2, vol. 1124, pp. 11–39 Search PubMed.
- B. Karn, J. Ind. Ecol., 2008, 12, 263–266 CrossRef PubMed.
- B. Karn and S. S. Wong, in Sustainable Nanotechnology and the Environment: Advances and Achievements, American Chemical Society, 2013, ch. 1, vol. 1124, pp. 1–10 Search PubMed.
- J. E. Hutchison, ACS Nano, 2008, 2, 395–402 CrossRef CAS PubMed.
- K. J. M. Matus, J. E. Hutchinson, R. Peoples, S. Rung and R. Tanguay, Green Nanotechnology Challenges And Opportunities, American Chemical Society, 2011 Search PubMed.
- L. C. McKenzie and J. E. Hutchison, Chem. Today, 2004, 30–33 CAS.
- S. K. Das and E. Marsili, in Nanomaterials, ed. M. Rahman, InTech, 2011, pp. 253–278 Search PubMed.
- H. Wang, X. Qiao, J. Chen, X. Wang and S. Ding, Mater. Chem. Phys., 2005, 94, 449–453 CrossRef CAS PubMed.
- S. Iravani, Green Chem., 2011, 13, 2638–2650 RSC.
- M. G. Guzmán, J. Dille and S. Godet, Eng. Technol., 2008, 2, 315–322 Search PubMed.
- K.-S. Chou and C.-Y. Ren, Mater. Chem. Phys., 2000, 64, 241–246 CrossRef CAS.
- Y.-C. Liu and L.-H. Lin, Electrochem. Commun., 2004, 6, 1163–1168 CrossRef CAS PubMed.
- G. Sandmann, H. Dietz and W. Plieth, J. Electroanal. Chem., 2000, 491, 78–86 CrossRef CAS.
- K. Mallick, M. J. Witcomb and M. S. Scurrell, Mater. Chem. Phys., 2005, 90, 221–224 CrossRef CAS PubMed.
- J. Zhu, S. Liu, O. Palchik, Y. Koltypin and A. Gedanken, Langmuir, 2000, 16, 6396–6399 CrossRef CAS.
- C. H. Bae, S. H. Nam and S. M. Park, Appl. Surf. Sci., 2002, 197–198, 628–634 CrossRef CAS.
- C. Dong, X. Zhang, H. Cai and C. Cao, J. Mol. Liq., 2014, 196, 135–141 CrossRef CAS PubMed.
- P. Raveendran, J. Fu and S. L. Wallen, Green Chem., 2006, 8, 34–38 RSC.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- P. T. Anastas and J. B. Zimmerman, Environ. Sci. Technol., 2003, 37, 94A–101A CrossRef.
- J. M. Patete, X. Peng, C. Koenigsmann, Y. Xu, B. Karn and S. S. Wong, Green Chem., 2011, 13, 482–519 RSC.
- S. Kaviya, J. Santhanalakshmi, B. Viswanathan, J. Muthumary and K. Srinivasan, Spectrochim. Acta, Part A, 2011, 79, 594–598 CrossRef CAS PubMed.
- M. J. Eckelman, J. B. Zimmerman and P. T. Anastas, J. Ind. Ecol., 2008, 12, 316–328 CrossRef CAS PubMed.
- H. Korbekandi and S. Iravani, in The Delivery of Nanoparticles, ed. A. A. Hashim, InTech, 2012, pp. 3–36 Search PubMed.
- L. L. Bergeson, ACS Sustainable Chem. Eng., 2013, 1, 724–730 CAS.
- J. A. Dahl, B. L. S. Maddux and J. E. Hutchison, Chem. Rev., 2007, 107, 2228–2269 CrossRef CAS PubMed.
- H. Korbekandi, S. Iravani and S. Abbasi, Crit. Rev. Biotechnol., 2009, 29, 279–306 CrossRef CAS PubMed.
- R. S. Varma, Green Chem., 2014, 16, 2027–2041 RSC.
- M. N. Nadagouda, N. Iyanna, J. Lalley, C. Han, D. D. Dionysiou and R. S. Varma, ACS Sustainable Chem. Eng., 2014, 2, 1717–1723 CrossRef CAS.
- M. B. Gawande, S. N. Shelke, R. Zboril and R. S. Varma, Acc. Chem. Res., 2014, 47, 1338–1348 CrossRef CAS PubMed.
- M. N. Nadagouda and R. S. Varma, Green Chem., 2006, 8, 516–518 RSC.
- M. N. Nadagouda and R. S. Varma, J. Nanomater., 2008 Search PubMed , 782358.
- M. N. Nadagouda and R. S. Varma, Cryst. Growth Des., 2007, 7, 686–690 Search PubMed.
- P. Raveendran, J. Fu and S. L. Wallen, J. Am. Chem. Soc., 2003, 125, 13940–13941 CrossRef CAS PubMed.
- M. N. Nadagouda and R. S. Varma, Cryst. Growth Des., 2007, 7, 2582–2587 CAS.
- M. N. Nadagouda and R. S. Varma, Green Chem., 2008, 10, 859–862 RSC.
- B. Baruwati, V. Polshettiwar and R. S. Varma, Green Chem., 2009, 11, 926–930 RSC.
- J. Kou and R. S. Varma, RSC Adv., 2012, 2, 10283 RSC.
- J. Kou and R. S. Varma, ChemSusChem, 2012, 5, 2435–2441 CrossRef CAS PubMed.
- J. Kou and R. S. Varma, Chem. Commun., 2013, 49, 692–694 RSC.
- B. Baruwati and R. S. Varma, ChemSusChem, 2009, 2, 1041–1044 CrossRef CAS PubMed.
- N. Ahmad, S. Sharma, M. K. Alam, V. N. Singh, S. F. Shamsi, B. R. Mehta and A. Fatma, Colloids Surf., B, 2010, 81, 81–86 CrossRef CAS PubMed.
- M. N. Nadagouda, T. F. Speth and R. S. Varma, Acc. Chem. Res., 2011, 44, 469–478 CrossRef CAS PubMed.
- M. Tsuji, M. Hashimoto, Y. Nishizawa, M. Kubokawa and T. Tsuji, Chem. – Eur. J., 2005, 11, 440–452 CrossRef CAS PubMed.
- V. Polshettiwar, B. Baruwati and R. S. Varma, ACS Nano, 2009, 3, 728–736 CrossRef CAS PubMed.
- X. Zhang, S. Yan, R. D. Tyagi and R. Y. Surampalli, Chemosphere, 2011, 82, 489–494 CrossRef CAS PubMed.
- D. Hebbalalu, J. Lalley, M. N. Nadagouda and R. S. Varma, ACS Sustainable Chem. Eng., 2013, 1, 703–712 CAS.
- M. Cinelli, S. R. Coles, A. Jørgensen, A. Zamagni, C. Fernando and K. Kirwan, Int. J. Life Cycle Assess., 2013, 18, 1421–1424 CrossRef.
- S. M. Lloyd, L. B. Lave and H. S. Matthews, Environ. Sci. Technol., 2005, 39, 1384–1392 CrossRef CAS.
- D. E. Meyer, M. A. Curran and M. A. Gonzalez, J. Nanopart. Res., 2010, 13, 147–156 CrossRef.
- A. L. Mergula, V. Khanna and R. B. Bakshi, in Proceedings of the 2010 IEEE International Symposium on Sustainable Systems and Technology, ISSST, Arlington, VA, USA, 17–19 May 2010, pp. 1–6, DOI:10.1109/ISSST.2010.5507724.
- A. Moign, A. Vardelle, N. J. Themelis and J. G. Legoux, Surf. Coat. Technol., 2010, 205, 668–673 CrossRef CAS PubMed.
- S. Gavankar, S. Suh and A. F. Keller, Int. J. Life Cycle Assess., 2012, 17, 295–303 CrossRef.
- D. E. Meyer, M. A. Curran and M. A. Gonzalez, Environ. Sci. Technol., 2009, 43, 1256–1263 CrossRef CAS.
- R. Hischier and T. Walser, Sci. Total Environ., 2012, 425, 271–282 CrossRef CAS PubMed.
- D. E. Meyer and V. K. K. Upadhyayula, Clean Technol. Environ. Policy, 2014, 16, 757–772 CrossRef PubMed.
- H. Şengül, T. L. Theis and S. Ghosh, J. Ind. Ecol., 2008, 12, 329–359 CrossRef PubMed.
- M. C. B. de Figueirêdo, M. d. F. Rosa, C. M. L. Ugaya, M. d. S. M. d. Souza Filho, A. C. C. d. Silva Braid and L. F. L. d. Melo, J. Cleaner Prod., 2012, 35, 130–139 CrossRef PubMed.
- V. K. K. Upadhyayula, D. E. Meyer, M. A. Curran and M. A. Gonzalez, J. Cleaner Prod., 2012, 26, 37–47 CrossRef CAS PubMed.
- D. E. Meyer, M. A. Curran and M. A. Gonzalez, J. Nanopart. Res., 2011, 13, 147–156 CrossRef CAS.
- T. L. Theis, B. R. Bakshi, D. Durham, V. M. Fthenakis, T. G. Gutowski, J. A. Isaacs, T. Seager and M. R. Wiesner, Phys. Status Solidi RRL, 2011, 5, 312–317 CrossRef CAS.
- C. Som, M. Berges, Q. Chaudhry, M. Dusinska, T. F. Fernandes, S. I. Olsen and B. Nowack, Toxicology, 2010, 269, 160–169 CrossRef CAS PubMed.
- A. R. Köhler, C. Som, A. Helland and F. Gottschalk, J. Cleaner Prod., 2008, 16, 927–937 CrossRef PubMed.
- C. Bauer, J. Buchgeister, R. Hischier, W. R. Poganietz, L. Schebek and J. Warsen, J. Cleaner Prod., 2008, 16, 910–926 CrossRef PubMed.
- P. Pati, M. Sean and P. Vikeseland, Environ. Eng. Sci., 2014, 31, 410–420 CrossRef CAS.
- K. B. Narayanan and N. Sakthivel, Mater. Res. Bull., 2011, 46, 1708–1713 CrossRef CAS PubMed.
- V. K. Vidhu, S. A. Aswathy and D. Philip, Spectrochim. Acta, Part A, 2011, 83, 392–397 CrossRef CAS PubMed.
- S. A. O. Santos, R. J. B. Pinto, S. M. Rocha, P. A. A. P. Marques, C. P. Neto, A. J. D. Silvestre and C. S. R. Freire, ChemSusChem, 2014, 7, 2704–2711 CrossRef CAS PubMed.
- V. Belton and T. J. Stewart, Multiple criteria decision analysis; an integrated approach, Kluwer Academic Publisher, 2002 Search PubMed.
- J. Figueira, S. Greco and M. Ehrgott, Multi Criteria Decision Analysis: State of the Art Surveys, Springer, New York, 2005 Search PubMed.
- V. Belton and T. Stewart, in Trends in Multiple Criteria Decision Analysis, ed. M. Ehrgott, J. R. Figueira and S. Greco, Springer, USA, 2010, ch. 8, vol. 142, pp. 209–239 Search PubMed.
- A. Tsoukiàs, Ann. Oper. Res., 2007, 154, 3–27 CrossRef.
- B. Roy and R. Słowiński, EURO J. Decis. Processes, 2013, 1, 69–97 CrossRef PubMed.
- M. Cinelli, S. Coles and K. Kirwan, Ecol. Indic., 2014, 46, 138–148 CrossRef PubMed.
- W. Sadok, F. Angevin, J.-É. Bergez, C. Bockstaller, B. Colomb, L. Guichard, R. Reau and T. Doré, Agron. Sustainable Dev., 2008, 28, 163–174 CrossRef.
- G. Munda, in Multiple Criteria Decision Analysis: State of the Art Surveys, ed. J. Figueira, S. Greco and M. Ehrgott, Springer, New York, 2005, pp. 953–986 Search PubMed.
- M. Herva and E. Roca, J. Cleaner Prod., 2013, 39, 355–371 CrossRef PubMed.
- R. Słowiński, S. Greco and B. Matarazzo, Control Cybern., 2002, 31, 1005–1035 Search PubMed.
- J. B. Dent, G. Edwards-Jones and M. J. McGregor, Agric. Syst., 1995, 49, 337–351 CrossRef.
- M. G. Augeri, R. Colombrita, S. Greco, A. Lo Certo, B. Matarazzo and R. Słowiński, J. Infrastruct. Syst., 2011, 17, 75–85 CrossRef.
- R. Słowiński, S. Greco and B. Matarazzo, Pesquisa Operacional, 2012, 32, 213–270 CrossRef PubMed.
- S. Greco, B. Matarazzo and R. Słowiński, Eur. J. Oper. Res., 2001, 129, 1–47 CrossRef.
- W. Sadok, F. Angevin, J.-E. Bergez, C. Bockstaller, B. Colomb, L. Guichard, R. Reau, A. Messéan and T. Doré, Agron. Sustainable Dev., 2009, 29, 447–461 CrossRef PubMed.
- B. Roy, Multicriteria Methodology for Decision Aiding, Kluwer Academic Publishers, 1996 Search PubMed.
- R. Hischier, Int. J. Life Cycle Assess., 2014, 19, 838–849 CrossRef CAS PubMed.
- M. Amer and T. Daim, in Research and Technology Management in the Electricity Industry, ed. D. Tugrul, O. Terry and K. Jisun, Springer-Verlag, London, 2013, vol. VIII, pp. 31–65 Search PubMed.
- R. R. Hoffman and G. Lintern, in Cambridge handbook of expertise and expert performance, ed. K. A. Ericsson, N. Charness, P. Feltovich and R. Hoffman, Cambridge University Press, New York, 2006, pp. 203–222 Search PubMed.
- R. R. Hoffman, N. R. Shadbolt, A. M. Burton and G. Klein, Organizational Behavior and Human Decision Processes, 1995, 62, 129–158 CrossRef.
- K. S. Kavitha, B. Syed, D. Rakshith, H. U. Kavitha, R. H. C. Yashwantha, B. P. Harini and S. Satish, Int. Res. J. Biol. Sci., 2013, 2, 66–76 Search PubMed.
- J. Kou, C. Bennett-Stamper and R. S. Varma, ACS Sustainable Chem. Eng., 2013, 1, 810–816 CAS.
- H. Changseok, A. Joel, C. P. Suresh, F. Rachel, F. Polycarpos, J. A. Byrne, S. M. D. Patrick, C. Hyeok, J. Wenjun, O. S. Kevin and D. D. Dionysios, in Sustainable Nanotechnology and the Environment: Advances and Achievements, American Chemical Society, 2013, ch. 12, vol. 1124, pp. 201–229 Search PubMed.
- S. P. Dubey, M. Lahtinen and M. Sillanpää, Colloids Surf., A, 2010, 364, 34–41 CrossRef CAS PubMed.
- R. Senjen, Challenges and opportunities to green nanotechnologies, EEB, 2009 Search PubMed.
- A. Panáček, L. Kvítek, R. Prucek, M. Kolář, R. Večeřová, N. Pizúrová, V. K. Sharma, T. j. Nevěčná and R. Zbořil, J. Phys. Chem. B, 2006, 110, 16248–16253 CrossRef PubMed.
- N. Durán, P. D. Marcato, R. De Conti, O. L. Alves, F. T. M. Costa and M. Brocchi, J. Braz. Chem. Soc., 2010, 21, 949–959 CrossRef PubMed.
- Y. M. Mohan, K. Lee, T. Premkumar and K. E. Geckeler, Polymer, 2007, 48, 158–164 CrossRef CAS PubMed.
- G. A. Martínez-Castañón, N. Niño-Martínez, F. Martínez-Gutierrez, J. R. Martínez-Mendoza and F. Ruiz, J. Nanopart. Res., 2008, 10, 1343–1348 CrossRef.
- S. Greco, B. Matarazzo and R. Słowiński, in Multicriteria Decision Making, ed. T. Gal, T. Stewart and T. Hanne, Springer, USA, 1999, ch. 14, vol. 21, pp. 397–455 Search PubMed.
- S. Greco, B. Matarazzo and R. Słowiński, in Operational Tools in the Management of Financial Risks, ed. C. Zopounidis, Springer, USA, 1998, ch. 8, pp. 121–136, DOI:10.1007/978-1-4615-5495-0_8.
- S. Greco, B. Matarazzo and R. Słowiński, Eur. J. Oper. Res., 1999, 117, 63–83 CrossRef.
- R. Słowiński, S. Greco and B. Matarazzo, in Encyclopedia of complexity and systems science, ed. R. Meyers, Springer, New York, 2009, pp. 7753–7786 Search PubMed.
- S. Greco, B. Matarazzo and R. Słowiński, in Multi Criteria Decision Analysis: State of the Art Surveys, ed. J. Figueira, S. Greco and M. Ehrgott, Springer, New York, 2005, pp. 507–555 Search PubMed.
- J. Błaszczyński, S. Greco and R. Słowiński, Eur. J. Oper. Res., 2007, 181, 1030–1044 CrossRef PubMed.
- J. Błaszczyński, S. Greco, B. Matarazzo, R. Słowiński and M. Szeląg, in Rough Sets and Intelligent Systems - Professor Zdzisław Pawlak in Memoriam, ed. A. Skowron and Z. Suraj, Springer, Berlin, Heidelberg, 2013, ch. 5, vol. 42, pp. 185–209 Search PubMed.
- S. Greco, B. Matarazzo and R. Słowiński, in Rough Sets and Current Trends in Computing, ed. S. Greco, Y. Hata, S. Hirano, M. Inuiguchi, S. Miyamoto, H. Nguyen and R. Słowiński, Springer, Berlin, Heidelberg, 2006, ch. 33, vol. 4259, pp. 306–317 Search PubMed.
- S. Chakhar and I. Saad, Decision Support Systems, 2012, 54, 372–380 CrossRef PubMed.
- Mindjet, http://www.mindjet.com, 2014.
- R. S. Varma, in Innovations in Green Chemistry and Green Engineering, ed. P. T. Anastas and J. B. Zimmerman, Springer, New York, 2013, ch. 5, pp. 115–156, DOI:10.1007/978-1-4614-5817-3_5.
- A. Nirmala Grace and K. Pandian, Mater. Chem. Phys., 2007, 104, 191–198 CrossRef PubMed.
- S. Komarneni, D. Li, B. Newalkar, H. Katsuki and A. S. Bhalla, Langmuir, 2002, 18, 5959–5962 CrossRef CAS.
- J. D. Moseley and C. O. Kappe, Green Chem., 2011, 13, 794–806 RSC.
- D. Cruz, P. L. Falé, A. Mourato, P. D. Vaz, M. Luisa Serralheiro and A. R. L. Lino, Colloids Surf., B, 2010, 81, 67–73 CrossRef CAS PubMed.
- G. Singhal, R. Bhavesh, K. Kasariya, A. Sharma and R. Singh, J. Nanopart. Res., 2011, 13, 2981–2988 CrossRef CAS.
- A. K. Jha, K. Prasad, K. Prasad and A. R. Kulkarni, Colloids Surf., B, 2009, 73, 219–223 CrossRef CAS PubMed.
- B. Hu, S.-B. Wang, K. Wang, M. Zhang and S.-H. Yu, J. Phys. Chem. C, 2008, 112, 11169–11174 CAS.
- D. S. Sheny, J. Mathew and D. Philip, Spectrochim. Acta, Part A, 2011, 79, 254–262 CrossRef CAS PubMed.
- X. Gao, L. Wei, H. Yan and B. Xu, Mater. Lett., 2011, 65, 2963–2965 CrossRef CAS PubMed.
- A. Pal, S. Shah and S. Devi, Mater. Chem. Phys., 2009, 114, 530–532 CrossRef CAS PubMed.
- J. J. H. Liou and G.-H. Tzeng, Inf. Sci., 2010, 180, 2230–2238 CrossRef PubMed.
- F. Abastante, M. Bottero, S. Greco and I. Lami, Journal of Multi-Criteria Decision Analysis, 2013, 21, 3–23 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4gc02088j |
| ‡ http://thomsonreuters.com/thomson-reuters-web-of-science/ |
| § http://www.cs.put.poznan.pl/jblaszczynski/Site/jRS.html |
| ¶ Basic notions of DRSA and decision rule extraction are described in detail in ESI.† |
| || Protocol Identification Number (PIN): Identification number for silver nanoparticle synthesis protocols as reported in Table S1 in ESI.† |
| This journal is © The Royal Society of Chemistry 2015 |