Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The effect of changing the components of an ionic liquid upon the solubility of lignin†
William E. S.
Hart
,
Jason B.
Harper
* and
Leigh
Aldous
*
School of Chemistry, UNSW Australia, Sydney, NSW 2052, Australia. E-mail: j.harper@unsw.edu.au; l.aldous@unsw.edu.au; Fax: +61 2 9385 6141; Tel: +61 2 9385 4692
First published on 13th October 2014
Abstract
A facile technique for the quantification of lignin solubility in ionic liquids has been established. This was used to examine the effect of the anionic and cationic components of ionic liquids on lignin solubility. Changing the cation was shown to have a significant effect on lignin solubility. Interaction of aromatic cations with the solute was significant in most, but not all, ionic liquids. The anion was required to have a minimum hydrogen bonding basicity value for lignin to dissolve, but after this point the anion effect on the overall lignin solubility was negligible relative to the cation.
Ionic liquids are salts with low melting points (arbitrarily defined as below 100 °C1). Typically they are made up of a large organic cation (usually containing either a positively charged nitrogen or phosphorus centre, often incorporated into a heterocycle in the nitrogen cases) and either an organic or an inorganic anion.2 They are generally non-volatile, non-flammable solvents that can theoretically be recycled.3 These aspects mean that ionic liquids have drawn a lot of attention as potential replacements for the environmentally hazardous molecular solvents that are currently used.1,3–5
Another property of note is that ionic liquids consist of essentially unsolvated anions and cations.2 The macroscopic behaviour that results from this feature means that they are of interest as solvents in cases where molecular solvents are deemed unfit, such as in the processing of biomass.3,6,7 Ionic liquids have been examined for their ability to assist with dissolving raw biomass7 and once biomass had been dissolved, ionic liquids can potentially be used to selectively precipitate out either cellulose6 or lignin8 (the major components of lignocellulosic biomass9).
Lignin is found in cell walls; it provides structure and resistance to degradation to the cell.10 The structure of lignin varies from source to source11 and, as such, the precise mechanism of lignin dissolution and solvation in ionic liquids is not known. A detailed study has been performed using the ionic liquid 1-allyl-3-methylimidazolium chloride and a lignin model compound, 1-(4-methoxyphenyl)-2-methoxyethanol.12 Lignin solubility in a wider range of ionic liquids was modelled by simulating the excess enthalpy of the model compound, pinoresinol,13 and then a further four model compounds.14 However, lignin's inherent heterogeneity has hindered detailed studies on lignin itself. Its heterogeneity and chemical resistance also hinders efforts to controllably fractionate lignin once it is separated from the greater biomass pulp.6 If this can be achieved, lignin can potentially be employed to make renewable polymers and feedstock chemicals for industry.15
Along with sourcing renewable feedstocks, ideally any associated solvents should be non-toxic, biodegradable, recyclable and used on a minimal scale. Ionic liquids have been recently highlighted as effective solvents for reactions involving lignin.16,17 To this end, we have investigated both the solubility and the solvation dynamics of lignin in ionic liquids, to facilitate the next generation of ionic liquids for this process. This is especially significant as ionic liquid–lignin interactions will likely influence the reactivity and reaction outcomes of lignin.18,19
The work described herein considers a range of ionic liquids with different cationic and anionic components for their ability to dissolve (raw, unmodified) lignin, in order to understand the requirements for lignin dissolution in ionic liquids. The ionic liquids were chosen to fill the current gap in understanding (notably the importance of the imidazolium ring6), as well as provide a comparison of both the anion and cation effects in the same study allowing for the comparison between different ionic liquid types, which has not been previously examined in detail. This will assist in future efforts into the use of ionic liquids to dissolve lignocellulosic biomass that, along with enhancing their potential in biomass process will enable further study of biomass reactivity in ionic liquids.
Lignin quantification in ionic liquids
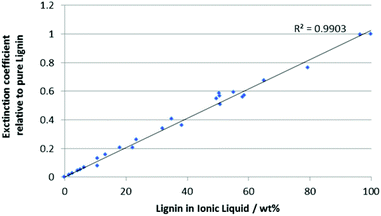
In order to determine the solubility of lignin in an ionic liquid, a sample of raw lignin was obtained from wood flour (see ESI†) as described previously.20 It should be noted that increasing either the acid concentration or the reaction time led to a decrease in the extinction coefficient of the resulting lignin, presumably due to increased degradation. Optimal extraction conditions (ca. 0.01 mol L−1 acid, 15 h) gave a high yield of lignin without significant breakdown (as judged using the extinction coefficient).UV-Visible spectroscopy has been used previously to quantify lignin in biomass by dissolving samples in ionic liquids,21 but the method was limited to very low concentrations of analyte (<0.02 wt% lignin). In this work, it has been extended to cover the range of ∼0 to ∼100 wt% lignin in a wide variety of ionic liquids, significantly enhancing its utility. This was achieved through dilution of solutions of lignin in ionic liquids in alkaline water.‡ Careful study highlighted that lignin absorption follows the Beer–Lambert Law across this range, and that ionic liquid absorption and interference on the lignin spectra could be eliminated beyond certain degrees of dilution (see ESI†). Given this it is possible to generate a calibration curve (Fig. 1, for full data and a derivation, see ESI†) by plotting the normalised extinction coefficients for a range of lignin and ionic liquid mixtures dissolved in alkaline water against the total mass of ionic liquid–lignin mixture dissolved. All samples needed to be diluted (up to 1000-fold) to ensure that the absorption was below 2. This also ensured even the largely water-immiscible ionic liquids were within their reported solubility in water.22 Each sample's extinction coefficient was also normalised against the extinction coefficient for that batch of lignin to eliminate any batch effects.
In order to determine the solubility of lignin in a given ionic liquid, samples were prepared by saturating the ionic liquid with lignin by stirring for an hour. Aliquots of the supernatant were taken and dissolved in aqueous sodium hydroxide (pH ca. 12.5). An extinction coefficient for the supernatant was determined and the calibration curve (Fig. 1) was used to determine the composition of the mixture by weight.
Effect of the anion of an ionic liquid on the solubility of lignin
The ionic liquids used in this study had both their anion and cation varied to determine the ideal combination for high lignin solubility; the effect of the anion was studied first. For the initial study into the anion effect on lignin solubility, all ionic liquids used were based on the 1-ethyl-3-methylimidazolium ([Emim]+) cation, as this has been widely employed to prepare ionic liquids capable of dissolving biomass.6,11 The anions were chosen to cover a wide range of coordinating abilities and hydrogen-bond basicity values23 since it has been previously seen that anions with high hydrogen bonding basicity are required for the dissolution of cellulose.24 The anions chosen were acetate ([CH3CO2]−), diethylphosphate ([O2P(OCH2CH3)2]−), methanesulfonate ([CH3SO3]−), thiocyanate ([NCS]−), trifluoroacetate ([CF3CO2]−), trifluoromethanesulfonate (triflate, [CF3SO3]−), tetrafluoroborate ([BF4]−), and bis(trifluoromethanesulfonyl)imide ([(SO2CF3)2N]−) and are shown in Fig. 2.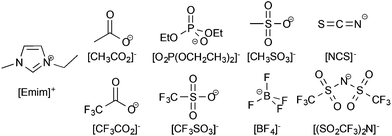 | ||
| Fig. 2 The ionic liquids used in this portion of the study were made up of the 1-ethyl-3-methylimidazolium ([Emim]+) cation and one of the anions shown here. | ||
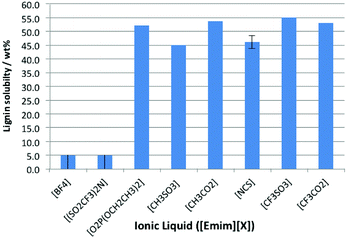
The solubility of lignin was assessed at 298 K in the above ionic liquids, and it was observed that lignin was either very soluble or it was effectively insoluble (Fig. 3). The ionic liquids containing the weakly coordinating anions ([BF4]− and [N(SO2CF3)2]−) fall into the latter category, while the remainder all dissolved ca. 50% by weight lignin. For the cases where a large amount of lignin was dissolved, the measured solubility seemed to be limited by the sample reaching a critical viscosity (which dramatically retarded mixing), rather than by the true solubility of the lignin. An early study on lignin solubility in ionic liquids utilised black liquor lignin, and highlighted that it was insoluble in [Bmim][PF6] but dissolved in [Bmim]-based ionic liquids with Cl−, Br− and [MeSO4]− anions.25
It is clear that is that the hydrogen bonding strength that has been seen to be important for cellulose dissolution,24 is not as influential here. This is seen in the case of the ionic liquid [Emim][CF3SO3]; its low hydrogen bonding basicity means that it cannot dissolve cellulose26 but is seen here as a suitable solvent for lignin dissolution. This indicates that the mechanisms for lignin and cellulose dissolution are different.
Studies at higher temperatures resulted in such high solubility values that only ionic liquid incorporated in solid lignin could be isolated. Lowering the temperature to 253 K resulted in some differentiation in solubility values (full values in ESI†) but solubility could not be clearly differentiated from viscosity issues. However, [Emim][CF3SO3] resulted in consistently high and reproducible solubility values.
The possibility to use the above method to investigate lignin extracted from whole biomass using an ionic liquid was briefly investigated. Stirring wood flour in [Emim][CF3SO3] followed by dilution in aqueous sodium hydroxide (pH ca. 12.5) allowed the clear UV-Vis observation of solubilised lignin, as described fully in the ESI.†
Effect of the cation of an ionic liquid on the solubility of lignin
Since the difference in solubility of lignin between ionic liquids containing different anions was negligible (beyond a critical point), it was considered that the cation of an ionic liquid would more likely have a significant effect upon the solubility and solvation of lignin. The trifluoromethanesulfonate ([CF3SO3]−) anion was chosen because it was seen to consistently have the largest lignin solubility and would thus be least likely to bias any cation study by limitations inherent to the anion. The cations chosen were [Emim]+, 1-butyl-3-methylimidazolium ([Bmim]+), 1-butyl-2,3-dimethylimidazolium ([Bm2im]+), tri-n-butyloctylammonium ([N4448]+) and butylpyridinium ([Bpyr]+) and are shown in Fig. 4.§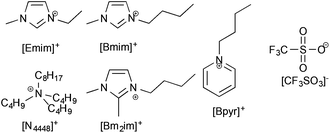 | ||
| Fig. 4 The ionic liquids used in this portion of the study were made up of the trifluoromethanesulfonate ([CF3SO3]−) anions and one of the cations shown here. | ||
These cations were chosen due to their established positions as ionic liquids for biomass dissolution ([Emim]+ and [Bmim]+),6 to indicate if any specific C2-proton interactions were present in the lignin dissolution ([Bm2im]+), to identify if access to the charged centre was important ([N4448]+) and to see if there is any variance based upon the type of aromatic system present ([Bpyr]+). The importance of steric demand and access to charged centres has been shown in interaction with solutes in reactivity studies.18,19
Particular care was taken to ensure that the ionic liquids did not contain significant alkali metal impurities (see ESI† for full details), since it has been seen that the presence of alkali metals can vary the solubility of biomass in ionic liquids.24
The saturation point for lignin in each of these ionic liquids was then measured in duplicate using the method described above except all saturation studies were undertaken at 318 K in order to keep the ionic liquids molten.¶ As can be seen in Fig. 5, there is a distinct difference in the solubility of lignin as a function of the cationic component.
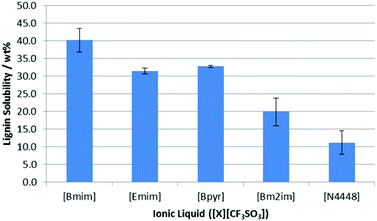 | ||
| Fig. 5 The proportion of lignin (wt%) in saturated solutions of lignin in a range of [CF3SO3]−-based ionic liquids at 318 K. Uncertainties are half the range of duplicate measurements. | ||
For [Bmim]+, [Emim]+ and [Bpyr]+-based ionic liquids, all gave high solubility values; the minor differences in solubility likely derives from their different abilities to dissolve lignin before becoming too viscous for further agitation. Lignin was seen to have the lowest solubility in [N4448][CF3SO3], which is perhaps expected given its blocked access to the cation and lack of aromatic component.16 Interestingly, methylation of the C2 carbon in [Bm2im][CF3SO3] resulted in a significant reduction in lignin solubility, which was probed in detail using NMR experiments.
NMR analysis of solvent–solute interactions
NMR spectroscopy was used to examine the solvent–solute interactions of solutions of lignin in the [Bmim]+, [Bm2im]+ and [Bpyr]+-based ionic liquids to understand the above solubility results. This was done through comparing the 1H and 13C NMR spectra of the ionic liquid with spectra of the same ionic liquid containing dissolved lignin (at ca. 1–7 wt%). An example of the changes observed in the spectra is displayed in Fig. 6 below, with the full spectra and tabulated data available in the ESI.†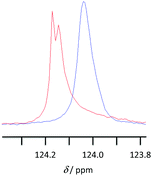 | ||
| Fig. 6 13C NMR spectra showing the signal corresponding to the carbon at 2-position of [Bmim][CF3SO3] in either [Bmim][CF3SO3] (red) or [Bmim][CF3SO3] containing 4.5 wt% lignin (blue). | ||
In the case of [Bpyr][CF3SO3], upon addition of lignin there were significant upfield shifts for the signals corresponding to all of the protons and all of the carbon atoms of the pyridinium ring. Given the largest change in the chemical shift of the signals corresponding to the hydrogen atoms in the ortho positions and the carbon atoms in the ortho and para positions, it is inferred that these changes derive from interactions between the planar cation and the aromatic components of the lignin. There is no evidence of specific or directional (hydrogen bonding) interactions at a given site, from either cation or anion. The signals corresponding to the butyl group were effectively unaltered except for a minor shift in the signals corresponding to the hydrogen and carbon atoms adjacent to the nitrogen centre. Importantly, the signal due to the carbon centre in the triflate anion undergoes no discernible shift upon addition of lignin, indicating that although the anion is likely influential in the initial dissolution process, it does not appear influential in solvation of the lignin in the ionic liquid. Therefore while a minimum hydrogen bonding basicity is clearly required for some lignin solubility (as noted above in the anion study) the role of the anion (as hydrogen-bond acceptor or some other process) is clearly not significant in the overall solubilisation of the lignin relative to the cation.
Lignin was also highly soluble in the ionic liquid [Bmim][CF3SO3]. Whilst spectral processing issues were present, likely due to the viscous nature of the sample,|| on addition of lignin to [Bmim][CF3SO3] there was an upfield shift in the signals corresponding to all the protons and all the carbon atoms in the imidazolium ring. There were smaller upfield shifts noted for the signals due to the nuclei in the butyl chain, decreasing with distance from the heterocycle. These results are consistent with, though less specific than, those previously observed for cellulose dissolved in an ionic liquid,27 where specific interactions with acidic hydrogen atoms on the cation were demonstrated. It should be noted that changes in the chemical shifts of the signals corresponding to the C4- and C5-hydrogen atoms were more pronounced than for the C2-hydrogen atom, indicating the negligible role of the so-called ‘non-innocent’ C2-hydrogen atom.28 As was the case for [Bpyr][CF3SO3], there was no change in chemical shift from the carbon nucleus in the anion, suggesting little or no interaction with the solute.
The ionic liquid [Bm2im][CF3SO3] was examined in an effort to explain its relative inability to solubilise lignin relative to the other pyridinium and imidazolium ionic liquids. The 1H NMR spectrum again showed that the most significant solute–solvent interactions were present on the imidazolium ring while the methylated C2 position showed chemical shift changes equivalent to those seen on the alkyl chains in the other ionic liquids. In [Bmim][CF3SO3], no specific C2-H interactions were noted and the addition of a methyl group would not be expected to significantly interfere with the ability of [Bm2im]+ to experience planar interactions. However, methylating the C2 position is widely known to result in the relative localisation of anions above and below the planar [Bm2im]+ cation,18,29 altering the physical properties of the overall ionic liquid,29,30 and ultimately influencing the reactivity of solutes.18,19 Methylation of the C2 position thus reduces the potential sites of interaction on the cation, hence introducing competition between anions and lignin for access to [Bm2im]+, and hence the overall solubility of lignin.
Having identified the key role of the cation in lignin solubility and solvation, investigations can now be performed to identify to what degree specific cation–lignin interactions alter lignin reactivity and reaction outcomes in ionic liquids.
Conclusions
A technique for the quantification of lignin solubility in ionic liquids was developed (spanning from ∼0 to ∼100 wt% lignin in the ionic liquid) and used to evaluate the effect of both anion and cation structure upon the solubility of lignin. It was shown that while anions above a certain threshold hydrogen bond basicity are required to get any dissolution of lignin, the cation has a much more subtle effect upon lignin solubility, with planar cations being most effective. NMR evidence suggests an interaction between the solute (likely the planar aromatic portions) and the cation. Importantly, this work demonstrates the structural components of an ionic liquid necessary for high lignin solubility. These key solute–solvent interactions, can subsequently be used to understand and alter reactivity in these systems.Acknowledgements
This work was supported by the Australian Research Council through Projects DE130100770 and DP130102331.Notes and references
- J. Wilkes, Green Chem., 2002, 4, 73–80 RSC.
- K. Seddon, J. Chem. Technol. Biotechnol., 1997, 68, 351–356 CrossRef CAS.
- M. Gericke, P. Fardim and T. Heinze, Molecules, 2012, 17, 7458–7502 CrossRef PubMed.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, 2 Volume Set, Wiley, Weinheim, 2007 Search PubMed.
- K. V. Bhaskar, J. Pharm. Biomed. Res., 2012, 1, 7–12 Search PubMed.
- A. Brandt, J. Grasvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 RSC.
- A. Brandt, M. J. Ray, T. Q. To, D. J. Leak, R. J. Murphy and T. Welton, Green Chem., 2011, 13, 2489–2499 RSC.
- S. H. Lee, T. V. Doherty, R. J. Linhardt and J. S. Dordick, Biotechnol. Bioeng., 2009, 102, 1368–1376 CrossRef CAS PubMed.
- M. G. Freire, A. R. R. Teles, M. A. A. Rocha, B. Schroder, C. M. S. S. Neves, P. J. Carvalho, D. V. Evtuguin, L. M. N. B. F. Santos and J. A. P. Coutinho, J. Chem. Eng. Data, 2011, 56, 4813–4822 CrossRef CAS.
- S. E. Lebo, J. D. Gargulak and T. J. McNally, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., London, 2000 DOI:10.1002/0471238961.12090714120914.a01.pub2.
- P. Mäki-Arvela, I. Anugwom, P. Virtanen, R. Sjöholm and J. P. Mikkola, Ind. Crop. Prod., 2010, 32, 175–201 CrossRef PubMed.
- W. Ji, Z. Ding, J. Liu, Q. Song, X. Xia, H. Gao, H. Wang and W. Gu, Energy Fuels, 2012, 26, 6393–6403 CrossRef CAS.
- A. Casas, J. Palomar, M. V. Alonso, M. Oliet, S. Omar and F. Rodriguez, Ind. Crop. Prod., 2012, 37, 155–163 CrossRef CAS PubMed.
- A. Casas, S. Omar, J. Palomar, M. Oliet, M. V. Alonso and F. Rodriguez, RSC Adv., 2013, 3, 3453–3460 RSC.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed.
- M. M. Hossain and L. Aldous, Aust. J. Chem., 2012, 65, 1465–1477 CrossRef CAS.
- G. Chatel and R. D. Rogers, ACS Sustainable Chem. Eng., 2014, 2, 322–339 CrossRef CAS.
- E. E. L. Tanner, H. M. Yau, R. R. Hawker, A. K. Croft and J. B. Harper, Org. Biomol. Chem., 2013, 11, 6170–6175 CAS.
- S. T. Keaveney, K. S. McHale Schaffarczyk, R. S. Haines and J. B. Harper, Org. Biomol. Chem., 2014, 12, 7092–7099 CAS.
- P. M. Froass, A. J. Ragauskas and J.-e. Jiang, J. Wood Chem. Technol., 1996, 16, 347–365 CrossRef CAS.
- L. M. Kline, D. G. Hayes, A. R. Womac and N. Labbé, BioResearch, 2010, 5, 1366–1383 CAS.
- M. G. Freire, P. J. Carvalho, R. L. Gardas, I. M. Marrucho, L. M. N. B. F. Santos and J. A. P. Coutinho, J. Phys. Chem. B, 2008, 112, 1604–1610 CrossRef CAS PubMed.
- R. Bini, O. Bortolini, C. Chiappe, D. Pieraccini and T. Siciliano, J. Phys. Chem. B, 2006, 111, 598–604 CrossRef PubMed.
- A. Xu, J. Wang and H. Wang, Green Chem., 2010, 12, 268–275 RSC.
- Y. Pu, N. Jiang and A. J. Ragauskas, J. Wood Chem. Technol., 2007, 27, 23–33 CrossRef CAS.
- J. Vitz, T. Erdmenger, C. Haensch and U. S. Schubert, Green Chem., 2009, 11, 417–424 RSC.
- B. Lu, A. Xu and J. Wang, Green Chem., 2014, 16, 1326–1335 RSC.
- S. Sowmiah, V. Srinivasadesikan, M.-C. Tseng and Y.-H. Chu, Molecules, 2009, 14, 3780–3813 CrossRef CAS PubMed.
- Y. Zhang and E. J. Maginn, Phys. Chem. Chem. Phys., 2012, 14, 12157–12164 RSC.
- S. Katsuta, Y. Shiozawa, K. Imai, Y. Kudo and Y. Takeda, J. Chem. Eng. Data, 2009, 55, 1588–1593 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Procedure for the extraction of lignin from wood flour, extraction of lignin from wood using [Emim][CF3SO3], lithium NMR spectroscopy for quantification of lithium impurities in ionic liquids, synthesis of ionic liquids and their precursors, derivation of the relationship between observed extinction coefficient and lignin concentration, raw data for Fig. 1, 3 and 5, and NMR spectra and analyses. See DOI: 10.1039/c4gc01888e |
| ‡ All ionic liquids used in this study were water soluble at the concentrations used here. This includes the largely water-immiscible ionic liquid [Emim][(SO2CF3)2N], which when diluted ca. 1000 fold is far above the reported mutual solubility of this ionic liquid and water.22 |
| § Other trifluoromethanesulfonate-based ionic liquids were considered, such as those based on the 1-butyl-1-methylpyrrolidinium, tetrabutylammonium and butylmethylmorpholinium cations, but not used as they are solid at the temperature used in the experiments. |
| ¶ The melting point of [Bpyr][CF3SO3] is 38 °C. |
| || Signal broadening was significant in both the presence and the absence of lignin. Duplication of signals was noted in some cases, which may indicate two cation environments. |
| This journal is © The Royal Society of Chemistry 2015 |