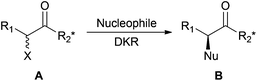
Faster and cleaner dynamic kinetic resolution via mechanochemistry†
Thomas-Xavier
Métro
*,
Xavier J.
Salom-Roig
,
Maëva
Reverte
,
Jean
Martinez
and
Frédéric
Lamaty
*
Institut des Biomolécules Max Mousseron (IBMM), UMR 5247 CNRS-Universités Montpellier 1 et 2-ENSCM, Bâtiment Chimie (17), Université Montpellier 2, Place Eugène Bataillon, 34095 Montpellier cedex 5, France. E-mail: txmetro@univ-montp2.fr; frederic.lamaty@univ-montp2.fr; Fax: (+33) 4-6714-4866
First published on 15th October 2014
Abstract
Application of the ball-milling techniques to dynamic kinetic resolution accelerates reactions while avoiding the use of toxic organic solvents and reactants commonly required in these processes. In this way, dynamic kinetic resolutions can be both faster and “cleaner” in the sense that mechanochemistry enables the reduction of their environmental impact.
Controlling the optical purity of molecules is essential in many areas, particularly in pharmaceutical research where biological activity highly depends on the chirality of active compounds. Numerous highly stereoselective methodologies have been developed to access enantiopure molecules and among them kinetic resolution provides an attractive approach. In this process, two enantiomers of a racemate are transformed into chiral products at different rates. When the resolution is efficient, one of the enantiomers of the racemic mixture is transformed into the desired product while the other is recovered unchanged. However, this procedure is limited to the maximum theoretical yield of 50%. To overcome this limitation, an in situ epimerization of the chirally labile substrate can be combined with the kinetic resolution to become a dynamic kinetic resolution (DKR).1 However, the inherent necessity for the product formation to be slower than the substrate epimerization usually makes dynamic kinetic resolution quite a slow process.
α-Halo carbonyls (A, Scheme 1) can be taken as a good illustration of this process: the configurationally labile halogen atom in the α position of the carbonyl can be irreversibly substituted by a nucleophile. Stereo-differentiation in the SN2 halogen displacement can be controlled by the chiral environment on R2.
Whereas DKR of such substrates allows access to products of type B with good yield and diastereoselectivity, long reaction times are needed. As many other examples of SN2 reactions, this DKR requires polar and aprotic solvents such as toxic DCM or THF,2 and is performed with organic, toxic and corrosive bases such as Et3N to epimerise the substrate.3 Thus, reaction conditions allowing shorter reaction times and avoiding the use of problematic solvents and reagents would be highly preferable. Following our general objective to find greener alternatives to the otherwise environmentally troublesome chemical reactions,4 we envisioned performing DKR avoiding the use of undesirable solvents and bases. Due to its relevance for a laboratory scale study, the Ecoscale score5 was chosen as the green metric of choice to evaluate the environmental impact of the new reaction conditions. The Ecoscale is a score ranging from 0 (totally failed reaction) to 100 (ideal reaction) that is based on yield, cost, safety, technical set up, the temperature and time of reaction, work up and purification aspects. To each of these parameters are attributed penalty points that are subtracted from the ideal score of 100 to give the Ecoscale score of the studied reaction. The reaction conditions are ranked excellent if the Ecoscale score is >75, acceptable if >50 and inadequate if <50.
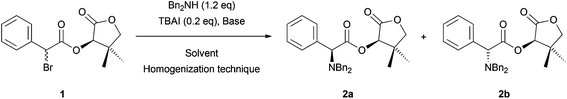
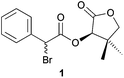
As the first example, we studied the DKR reaction of α-bromo-(R)-pantolactone ester 1 with Bn2NH that was previously described by Durst and coworkers.6 Thus, we treated compound 1 with Et3N, a catalytic amount of TBAI and Bn2NH to obtain the corresponding α-dibenzylamino ester 2a in 59% yield with an excellent diastereoisomeric ratio (>98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (Table 1, entry 1). As described in the literature, this reaction was set up using an excess of Et3N (2.0 eq.) in a toxic solvent (THF is suspected to be carcinogenic), accounting for a low Ecoscale score of 42.5, which corresponds to an inadequate synthesis.5 To reduce the environmental impact of this reaction, we envisioned replacing the problematic Et3N and THF with innocuous NaHCO3 and water.7 Under these conditions, the Ecoscale score was barely improved to 54, mainly due to a low yield of 34% (50% brsm; Table 1, entry 2). In addition, the reaction was much slower as 6 h were necessary to reach 50% conversion and 2a was obtained with a lower diastereomeric ratio (87
2) (Table 1, entry 1). As described in the literature, this reaction was set up using an excess of Et3N (2.0 eq.) in a toxic solvent (THF is suspected to be carcinogenic), accounting for a low Ecoscale score of 42.5, which corresponds to an inadequate synthesis.5 To reduce the environmental impact of this reaction, we envisioned replacing the problematic Et3N and THF with innocuous NaHCO3 and water.7 Under these conditions, the Ecoscale score was barely improved to 54, mainly due to a low yield of 34% (50% brsm; Table 1, entry 2). In addition, the reaction was much slower as 6 h were necessary to reach 50% conversion and 2a was obtained with a lower diastereomeric ratio (87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13). When a preferable solvent such as EtOAc8 was used in place of water, the reaction proceeded with a satisfying Ecoscale score of 62.5, and furnished 2a in a good yield of 73% with an excellent diastereomeric ratio (>98
13). When a preferable solvent such as EtOAc8 was used in place of water, the reaction proceeded with a satisfying Ecoscale score of 62.5, and furnished 2a in a good yield of 73% with an excellent diastereomeric ratio (>98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (Table 1, entry 3). Even when careful attention was paid to use the minimum amount of solvent enabling proper agitation of this heterogeneous reaction media, 6 days were necessary to reach full conversion of the substrate. At this point of the study, we considered that using innocuous NaHCO3 as the base would force us to use more polar solvents such as EtOH, THF or DMF. The use of THF or EtOH as the solvent was disappointing since 24 h and more than 6 days of reaction were respectively required to reach 95% of conversion (Table 1, entries 4 and 5). The use of DMF as the solvent resulted in a much shorter reaction time; only 2 h were required for a complete consumption of substrate 1 (Table 1, entry 6). Nevertheless, an unsatisfying Ecoscale score of 50.5 was obtained mainly due to the low yield (55%) and the fact that DMF presents high health-related risks, which hamper its environmental impact.9 Except for DMF, these relatively long reaction times could be attributed to the low solubility of either NaHCO3 in organic solvents or α-bromo ester 1 in water, resulting in heterogeneous reaction mixtures that may lead to mass transfer limitations. As heterogeneity of the reaction mixture could be responsible for a low speed of reaction, the transformation was performed using the ball-milling technology.10 In this kind of apparatus, reagents (liquid or solid) are introduced into a jar with one or more balls. Rapid movements of the jar create repeated and violent contacts between reagents, balls and walls allowing for a very efficient mixing of solid-containing reaction mixtures.
2) (Table 1, entry 3). Even when careful attention was paid to use the minimum amount of solvent enabling proper agitation of this heterogeneous reaction media, 6 days were necessary to reach full conversion of the substrate. At this point of the study, we considered that using innocuous NaHCO3 as the base would force us to use more polar solvents such as EtOH, THF or DMF. The use of THF or EtOH as the solvent was disappointing since 24 h and more than 6 days of reaction were respectively required to reach 95% of conversion (Table 1, entries 4 and 5). The use of DMF as the solvent resulted in a much shorter reaction time; only 2 h were required for a complete consumption of substrate 1 (Table 1, entry 6). Nevertheless, an unsatisfying Ecoscale score of 50.5 was obtained mainly due to the low yield (55%) and the fact that DMF presents high health-related risks, which hamper its environmental impact.9 Except for DMF, these relatively long reaction times could be attributed to the low solubility of either NaHCO3 in organic solvents or α-bromo ester 1 in water, resulting in heterogeneous reaction mixtures that may lead to mass transfer limitations. As heterogeneity of the reaction mixture could be responsible for a low speed of reaction, the transformation was performed using the ball-milling technology.10 In this kind of apparatus, reagents (liquid or solid) are introduced into a jar with one or more balls. Rapid movements of the jar create repeated and violent contacts between reagents, balls and walls allowing for a very efficient mixing of solid-containing reaction mixtures.
| Entry | Homogenization technique | Base (eq.) | Solvent | Time to reach >95% conversiona | Yieldb (%) | drc (2a)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (2b) (2b) |
Ecoscale score |
|---|---|---|---|---|---|---|---|
a Determined by HPLC.
b Isolated yield.
c Determined using 300 MHz 1H NMR.
d Time to reach 50% conversion.
e Obtained as a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 molar mixture of 2a 50 molar mixture of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b and 1.
f Based on recovered starting material (brsm).
g Not determined.
h The η ratio is defined as the amount of added liquid to the sum of the mass of reactants. It is expressed in μL mg−1. 2b and 1.
f Based on recovered starting material (brsm).
g Not determined.
h The η ratio is defined as the amount of added liquid to the sum of the mass of reactants. It is expressed in μL mg−1.
|
|||||||
| 1 | Magnetic stirring | Et3N (2.0) | THF | 5 h | 59 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
42.5 |
| 2 | Magnetic stirring | NaHCO3 (1.2) | Water | 6 hd | 34e (50)f | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
54 |
| 3 | Magnetic stirring | NaHCO3 (1.2) | EtOAc | 6 days | 73 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
62.5 |
| 4 | Magnetic stirring | NaHCO3 (1.2) | THF | 24 h | —g | —g | —g |
| 5 | Magnetic stirring | NaHCO3 (1.2) | EtOH | >6 days | —g | —g | —g |
| 6 | Magnetic stirring | NaHCO3 (1.2) | DMF | 2 h | 55 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
50.5 |
| 7 | Ball-milling | NaHCO3 (1.2) | Solvent-free | 30 min | 62 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
63 |
| 8 | Ball-milling | NaHCO3 (1.2) | Water (η = 2)h | 15 min | 96 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 |
| 9 | Ball-milling | NaHCO3 (1.2) | Water (η = 1)h | 15 min | 94 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
79 |
Indeed, 30 min of vigorous agitation were enough for a complete conversion of α-bromo ester 1 into amino-ester 2a when 1 was placed in a 10 mL jar with one 10 mm diameter ball, NaHCO3, dibenzylamine, and TBAI in the absence of any solvent (Table 1, entry 7). Under these conditions, dibenzylamino ester 2a was isolated in 62% yield with an excellent diastereomeric ratio (>98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and a satisfying Ecoscale score of 63. Obtainable benefits from adding a liquid in a grinded reaction mixture are now well established.10b,11,12 Indeed, when adding small amounts of water in the jar (η ratio of 2 μL mg−1)13 only 15 min were necessary to obtain complete conversion of the substrate while dibenzylamino ester 2a was isolated in excellent yield and diastereomeric ratio (96% yield, dr > 98
2) and a satisfying Ecoscale score of 63. Obtainable benefits from adding a liquid in a grinded reaction mixture are now well established.10b,11,12 Indeed, when adding small amounts of water in the jar (η ratio of 2 μL mg−1)13 only 15 min were necessary to obtain complete conversion of the substrate while dibenzylamino ester 2a was isolated in excellent yield and diastereomeric ratio (96% yield, dr > 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; Table 1, entry 8). In these conditions, the highest Ecoscale score was obtained (80), corresponding to an excellent synthesis.5 Calculations leading to this high Ecoscale score include the solvent used during work-up and chromatographic purification (details on the Ecoscale calculations are available in the ESI†). Reducing the amount of water in the reaction media to 1 μL mg−1 had little effect on the course of the reaction as 2a was obtained in 94% yield and >98
2; Table 1, entry 8). In these conditions, the highest Ecoscale score was obtained (80), corresponding to an excellent synthesis.5 Calculations leading to this high Ecoscale score include the solvent used during work-up and chromatographic purification (details on the Ecoscale calculations are available in the ESI†). Reducing the amount of water in the reaction media to 1 μL mg−1 had little effect on the course of the reaction as 2a was obtained in 94% yield and >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr (Table 1, entry 9). We postulate that solving the suspected mass transfer limitations using the tremendous ability of the ball-mill technology to mix the solid-containing mixtures allowed:
2 dr (Table 1, entry 9). We postulate that solving the suspected mass transfer limitations using the tremendous ability of the ball-mill technology to mix the solid-containing mixtures allowed:
– improvement of the yield without hampering the diastereoselectivity;
– reduction of reaction time;
– utilisation of the least problematic base (NaHCO3) and solvent (H2O) regardless of their solubility or solubilizing capacities.
To our knowledge, this is the first example of DKR to be performed through ball-milling technology.
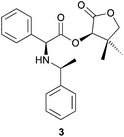
At this stage of the study, we became interested in comparing the efficiency and the environmental impact of solution-based DKR reactions with the mechanochemistry-mediated approach on other known DKR reactions implying a SN2 halogen substitution. When treated with (S)-(–)-α-methylbenzylamine in THF in the presence of Et3N as the base, α-bromo ester 1 was transformed into 3 in 84% yield and >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr (Table 2, entry 1).6 The use of THF and Et3N resulted in a low Ecoscale score of 52. Replacing these problematic chemicals with water and NaHCO3 while mixing the reaction mixture with a vibrating ball-mill allowed for the production of 3 with much better Ecoscale score (72), high yield (80%) and diastereoselectivity (>98
2 dr (Table 2, entry 1).6 The use of THF and Et3N resulted in a low Ecoscale score of 52. Replacing these problematic chemicals with water and NaHCO3 while mixing the reaction mixture with a vibrating ball-mill allowed for the production of 3 with much better Ecoscale score (72), high yield (80%) and diastereoselectivity (>98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
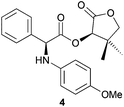
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (Table 2, entry 2). Treating α-bromo ester 1 with p-anisidine in a classical solution-based approach6 furnished amine 4 in 75% yield and >98
2) (Table 2, entry 2). Treating α-bromo ester 1 with p-anisidine in a classical solution-based approach6 furnished amine 4 in 75% yield and >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr, though with a low Ecoscale score of 40.5. When applying our ball-mill mediated approach, the Ecoscale score could be improved up to 54, yet with a slightly lower yield (64%) and a drop in diastereoselectivity (88
2 dr, though with a low Ecoscale score of 40.5. When applying our ball-mill mediated approach, the Ecoscale score could be improved up to 54, yet with a slightly lower yield (64%) and a drop in diastereoselectivity (88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
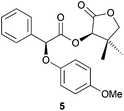
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) (Table 2, entry 4). Oxygen-based nucleophiles such as p-methoxyphenol could also be used to perform DKR on α-bromo ester 1. Indeed, Durst and coworkers utilised NaH and THF to produce 5 with 70% yield and 95
12) (Table 2, entry 4). Oxygen-based nucleophiles such as p-methoxyphenol could also be used to perform DKR on α-bromo ester 1. Indeed, Durst and coworkers utilised NaH and THF to produce 5 with 70% yield and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, yet with a low Ecoscale score of 41 (Table 2, entry 5).14 Treatment of α-bromo ester 1 with p-methoxyphenol in a ball-mill with water and NaHCO3 instead of THF and NaH resulted in the production of ester 5 with a better yield and an Ecoscale score of 86% and 62, respectively, albeit with a moderate 72
5 dr, yet with a low Ecoscale score of 41 (Table 2, entry 5).14 Treatment of α-bromo ester 1 with p-methoxyphenol in a ball-mill with water and NaHCO3 instead of THF and NaH resulted in the production of ester 5 with a better yield and an Ecoscale score of 86% and 62, respectively, albeit with a moderate 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
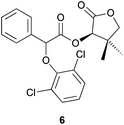
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 dr (Table 2, entry 6). When 2,6-dichlorophenol was used as a nucleophile, the reaction time, diastereoselectivity and the Ecoscale score were improved while the yield remained similar (Table 2, entries 7 and 8).15
28 dr (Table 2, entry 6). When 2,6-dichlorophenol was used as a nucleophile, the reaction time, diastereoselectivity and the Ecoscale score were improved while the yield remained similar (Table 2, entries 7 and 8).15
| Entry | Substrate | Product | Homogenization technique | Base | Solvent | Time | Yielda (%) | dr | Ecoscale score |
|---|---|---|---|---|---|---|---|---|---|
| a Isolated yield. b Not indicated in the original publication. c Diastereomeric ratios were determined using 300 MHz 1H NMR. d 2.16 eq. of Bn2NH were used. e 2.5 eq. of Bn2NH were used. | |||||||||
| 1 |
|
|
Magnetic stirring | Et3N | THF | —b | 84 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
52 |
| 2 | Ball-milling | NaHCO3 | Water | 30 min | 80 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2c 2c |
72 | ||
| 3 | — |
|
Magnetic stirring6 | Et3N | THF | —b | 75 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
40.5 |
| 4 | Ball-milling | NaHCO3 | Water | 6 h | 64 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12c 12c |
54 | ||
| 5 | — |
|
Magnetic stirring14 | NaH | THF | 6 h | 70 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
41 |
| 6 | Ball-milling | NaHCO3 | Water | 2 h | 86 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28c 28c |
62 | ||
| 7 | — |
|
Magnetic stirring15 | NaH | THF | 7 h | 78 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
23.5 |
| 8 | Ball-milling | NaHCO3 | Water | 1 h 30 | 77 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44c 44c |
47.5 | ||
| 9 |
|
|
Magnetic stirring16 | Et3N | CH2Cl2 | 24 h | 81 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
51 |
| 10 | Ball-milling | NaHCO3 | Water | 13 h | 75d | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2c 2c |
68.5 | ||
| 11 |
|
|
Magnetic stirring17 | Bn2NHe | THF | 10 days | 74 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
58 |
| 12 | Ball-milling | Bn2NHe | Water | 7 h | 71 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2c 2c |
66.5 | ||
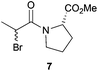
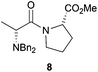
After having changed the nature of nucleophiles, we focused our attention on other types of substrates such as α-bromo amide 7 and α-bromo ketone 9. When 1-(2-bromo-1-oxopropyl)-L-proline methyl ester 7 was reacted with Bn2NH in CH2Cl2 in the presence of Et3N for 24 h, N,N-dibenzyl-D-alanyl-L-proline methyl ester 8 was obtained in 81% yield with 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 dr, accounting for an Ecoscale score of 51 (Table 2, entry 9).16 Once again, utilising the ball-milling technology avoided the use of problematic CH2Cl2 and Et3N. Thus, the reaction of α-bromo amide 7 with Bn2NH in the presence of NaHCO3 and water in a vibrating ball-mill furnished product 8 with a similar yield (75%) and with an improvement in the Ecoscale score (68.5 vs. 51 with the solution-based approach) (Table 2, entry 10). It is worth noting that utilisation of the ball-mill also resulted in improving the diastereomeric ratio from 87
13 dr, accounting for an Ecoscale score of 51 (Table 2, entry 9).16 Once again, utilising the ball-milling technology avoided the use of problematic CH2Cl2 and Et3N. Thus, the reaction of α-bromo amide 7 with Bn2NH in the presence of NaHCO3 and water in a vibrating ball-mill furnished product 8 with a similar yield (75%) and with an improvement in the Ecoscale score (68.5 vs. 51 with the solution-based approach) (Table 2, entry 10). It is worth noting that utilisation of the ball-mill also resulted in improving the diastereomeric ratio from 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 to >98
13 to >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
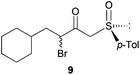
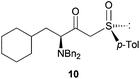
Finally, this approach was applied to the DKR reaction of γ-bromo-β-ketosulfoxide 9 with Bn2NH. Salom-Roig and coworkers17 reported that treatment of 9 with 2.5 equivalents of Bn2NH required 10 days in THF to reach reaction completion furnishing γ-dibenzylamino-β-ketosulfoxide 10 with 74% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr (Table 2, entry 11). Using Bn2NH as the nucleophile and the base to deprotonate the HBr salts generated during the course of the reaction, the authors already avoided the use of the flammable, corrosive and toxic Et3N, thus reducing the environmental impact of the reaction. This particularly resulted in a good Ecoscale score of 58. Yet, when the same reaction was performed in a ball-mill with water replacing THF, the time for reaction completion was dramatically reduced to only 7 h, while furnishing γ-dibenzylamino-β-ketosulfoxide 10 with a similar yield of 71% and diastereoselectivity (Table 2, entry 12). Replacing THF with water and reducing the required time to reach reaction completion resulted in an Ecoscale score improvement up to 66.5.
5 dr (Table 2, entry 11). Using Bn2NH as the nucleophile and the base to deprotonate the HBr salts generated during the course of the reaction, the authors already avoided the use of the flammable, corrosive and toxic Et3N, thus reducing the environmental impact of the reaction. This particularly resulted in a good Ecoscale score of 58. Yet, when the same reaction was performed in a ball-mill with water replacing THF, the time for reaction completion was dramatically reduced to only 7 h, while furnishing γ-dibenzylamino-β-ketosulfoxide 10 with a similar yield of 71% and diastereoselectivity (Table 2, entry 12). Replacing THF with water and reducing the required time to reach reaction completion resulted in an Ecoscale score improvement up to 66.5.
Conclusions
In conclusion, the great capacity of the ball-milling technology to mix solid-containing reaction mixtures allowed the design of reaction conditions that could mitigate the environmental impact of dynamic kinetic resolutions. This reduction was evaluated by calculating the Ecoscale score of every studied reaction conditions. Thus, we have shown that problematic chemicals such as THF or Et3N could be replaced by innocuous water and NaHCO3 without dramatically hampering the performance of the DKR reactions. In all cases, the time required to completion was severely reduced and in some cases yields and/or diastereomeric ratios were also improved. Exemplification of utilisation of the ball-milling technology to reduce the environmental impact of other problematic reactions is currently under progress.We thank CNRS, Université Montpellier 1 and Université Montpellier 2 for financial support. François Métro is gratefully acknowledged for producing graphical abstract artwork.
References
- (a) H. Pellissier, Tetrahedron, 2003, 59, 8291–8327 CrossRef CAS; (b) H. Pellissier, Tetrahedron, 2008, 64, 1563–1601 CrossRef CAS PubMed; (c) Y. S. Park, Tetrahedron: Asymmetry, 2009, 20, 2421–2427 CrossRef CAS PubMed; (d) H. Pellissier, Tetrahedron, 2011, 67, 3769–3802 CrossRef CAS PubMed.
- DCM stands for dichloromethane and THF for tetrahydrofuran. DCM and THF are classified as toxic and carcinogenic (Category 2) according to Regulation (EC) No. 1272/2008.
- Triethylamine is classified as highly flammable (R11), corrosive (R35) and harmful (R20/21/22) according to EU Directives 67/548/EEC or 1999/45/EC.
- (a) V. Declerck, P. Nun, J. Martinez and F. Lamaty, Angew. Chem., Int. Ed., 2009, 48, 9318–9321 CrossRef CAS PubMed; (b) T.-X. Métro, J. Bonnamour, T. Reidon, J. Sarpoulet, J. Martinez and F. Lamaty, Chem. Commun., 2012, 48, 11781–11783 RSC; (c) J. Bonnamour, T.-X. Métro, J. Martinez and F. Lamaty, Green Chem., 2013, 15, 1116–1120 RSC.
- K. Van Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem., 2006, 2, 3 CrossRef PubMed.
- R. N. Ben and T. Durst, J. Org. Chem., 1999, 64, 7700–7706 CrossRef CAS . Reaction times were not indicated in the publication.
- NaHCO3 is considered to be a not hazardous substance as defined by the European regulation No. 1272/2008 and not classified as dangerous according to Directive 67/548/EEC.
- (a) K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31–36 RSC; (b) R. K. Henderson, C. Jiménez-González, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 13, 854–862 RSC; (c) D. Prat, O. Pardigon, H.-W. Flemming, S. Letestu, V. Ducandas, P. Isnard, E. Guntrum, T. Senac, S. Ruisseau, P. Cruciani and P. Hosek, Org. Process Res. Dev., 2013, 17, 1517–1525 CrossRef CAS.
- DMF is classified as presenting reproductive toxicity according to Regulation (EC) No. 1272/2008.
- (a) B. Rodríguez, A. Bruckmann, T. Rantanen and C. Bolm, Adv. Synth. Catal., 2007, 349, 2213–2233 CrossRef; (b) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC; (c) G. W. Wang, Chem. Soc. Rev., 2013, 42, 7668–7700 RSC.
- (a) E. Boldyreva, Chem. Soc. Rev., 2013, 42, 7719–7738 RSC; (b) G. A. Bowmaker, Chem. Commun., 2013, 49, 334–348 RSC.
- T. Friščić, S. L. Childs, S. A. A. Rizvi and W. Jones, CrystEngComm, 2009, 11, 418–426 RSC.
- The η ratio is defined as the amount of added liquid to the sum of the mass of reactants. It is expressed in μL mg−1.
- K. Koh and T. Durst, J. Org. Chem., 1994, 59, 4683–4686 CrossRef CAS.
- G. Massolini, G. Fracchiolla, E. Calleri, G. Carbonara, C. Temporini, A. Lavecchia, S. Cosconati, E. Novellino and F. Loiodice, Chirality, 2006, 18, 633–643 CrossRef CAS PubMed.
- J. Nam, J.-Y. Chang, K.-S. Hahm and Y. S. Park, Tetrahedron Lett., 2003, 44, 7727–7730 CrossRef CAS PubMed.
- P.-Y. Géant, J. Martinez and X. J. Salom-Roig, Eur. J. Org. Chem., 2011, 1300–1309 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedures, characterisation data of all synthesized compounds and details on the calculations of Ecoscale scores. See DOI: 10.1039/c4gc01416b |
| This journal is © The Royal Society of Chemistry 2015 |