Simultaneous and selective recovery of cellulose and hemicellulose fractions from wheat bran by supercritical water hydrolysis†
Danilo A.
Cantero
ab,
Celia
Martínez
a,
M. D.
Bermejo
a and
M. J.
Cocero
*a
aHigh Pressure Processes Group, Department of Chemical Engineering and Environmental Technology, University of Valladolid, C/ Dr Mergelina s/n, 47011 Valladolid, Spain. E-mail: mjcocero@iq.uva.es; Fax: +34-983423013; Tel: +34-983423166
bDepartment of Applied and Industrial Chemistry. Faculty of Exact, Physical and Natural Sciences, National University of Cordoba, Av. Vélez Sarsfield 1611, 5000, Córdoba, Argentina. E-mail: da.cantero@iq.uva.es
First published on 22nd September 2014
Abstract
Supercritical water (SCW) has been demonstrated to be an excellent solvent and reaction medium to improve the cellulose hydrolysis selectivity by controlling the reaction time. In this study the conversion of wheat bran into soluble saccharides such as glucose, xylose and arabinose was analysed at 400 °C and 25 MPa with reaction times between 0.2 and 1 s. The process yield was evaluated for two different products: C-6 (glucose derived from cellulose) and C-5 sugars (saccharide derived from hemicellulose hydrolysis). The production of glycolaldehyde, furfural and 5-hydroxymethylfural (5-HMF) was analysed as by-product formation. Operation under supercritical conditions allows a biomass liquefaction of 84% w/w at 0.3 s of residence time. The obtained solid after the hydrolysis was composed of mainly lignin (86% w/w). The highest recovery of cellulose (C-6) and hemicellulose (C-5) as soluble sugars (73% w/w) was achieved at 0.19 s of reaction time. An increase in the reaction time decreased the yield of C-6 and C-5. A total recovery of C-5 was achieved at 0.19 s. On the other hand, the highest yield (65% w/w) of C-6 was achieved at 0.22 s of reaction time. The main hydrolysis product of C-6 and C-5 was glycolaldehyde, yielding 20% w/w at 0.22 s of reaction time. Furfural and 5-HMF production was highly inhibited under the experimental conditions, obtaining yields lower than 0.5% w/w. The hydrolysis reactions were performed in a continuous pilot plant at 400 °C, 25 MPa and residence times between 0.1 s and 0.7 s.
1. Introduction
The processes based on the conversion of biomass resources into fuels and chemicals have been intensively studied in recent years due to the necessity of changing the current production philosophy based on oil to the bioeconomy.1,2 Plant biomass is a promising raw material for the production of chemicals and fuels because it is an abundant, renewable and world-wide distributed source of carbon3 The lignocellulosic biomass is generally composed of 40–45% cellulose, 25–35% hemicellulose and 15–30% lignin.4 Even though plant biomass is one of the most abundant resources of carbon on the planet (primary production ≈1 × 1014 tons C per year5), one of the main challenges in biomass usage is the efficient depolymerisation of cellulose and hemicellulose into its composing monomers.6Water as a reaction medium presents advantages over other solvents because it is a non-expensive and environmentally friendly solvent. In addition, the medium identity can be tuned by changing the temperature and pressure in order to favour the desired reactions without using any catalyst. The use of sub and supercritical water has been proposed as a promising solvent to process biomass due to its special properties that are very promising to perform the hydrolysis reactions.1,3,7 Supercritical water refers to the state of water under pressure and temperature conditions above its critical point. The critical point of water is 374 °C and 22.1 MPa. Near its critical point, a solvent drastically changes its physical properties by simply modifying the pressure and temperature. This behaviour is a promising alternative to manage the selectivity in chemical reactions. The main variations in the properties of water can be summarized as follows: (1) in the surroundings of the critical point, the dielectric constant decreases by increasing the temperature, increasing in this way the solubility of organic compounds and (2) the ionic product of water varies from 10−10 to 10−22 when changing the temperature from 300 °C to 400 °C at 25 MPa, changing the benefited reaction mechanism from ionic to free-radical.8 In addition, the hydrothermal processing presents the following advantages: (1) the direct use of the raw material regardless of its water content, which implies an important energy saving; (2) the same reaction medium can be used for the transformation of different biomass fractions; (3) mass transfer limitations can be reduced or avoided, thus reaction rates are faster.7,9–12
The conversion of biomass components (cellulose, hemicellulose and lignin) into their constitutive monomers using supercritical water has been previously reported.13–15 The challenge then is to apply this technology to a complex biomass, in order to transform it into valuable products using a clean, safe and environmentally benign technology. The fractionation and hydrolysis of vegetal biomass in a hydrothermal medium has been studied using different kinds of reactors: batch,16–22 semi-continuous23–28 and continuous reactors.29–31 In the aforementioned studies, the yields of cellulose recovery as soluble sugars were between 3% w/w and 15% w/w; 16% w/w and 22% w/w; and 2% w/w and 6% w/w for batch, semi-continuous and continuous reactors respectively. The hemicellulose recovery yields were between 17% ww and 97% w/w; 18% w/w and 95% w/w and 25% w/w and 95% w/w for batch, semi-continuous and continuous reactors respectively. Finally, the lignin compositions of the solids in the reactor are between 45% w/w and 53% w/w and 20% w/w and 50% w/w for batch and semi-continuous reactors respectively.
Wheat bran is a by-product of the milling of wheat to produce white flour. Bran fraction constitutes around 11% of the total milling by-products, and only 10% of wheat bran available is used as fiber supplement in breakfast cereals and bakeries (human consumption) while the remaining 90% is used as animal feed.32 Compositional analysis suggests that wheat bran contains approximately 30% w/w–40% w/w of hemicellulose, 15% w/w–35% w/w of cellulose and 5% w/w–25% w/w of lignin, depending on growing conditions and varieties.33,34
Traditional hydrolysis processes using acid catalysts or enzymes have been improved in the last few years, but they still present limitations in providing high yield in moderate residence times. The hydrolysis of wheat bran was carried out by combining acid hydrolysis (0.2% w/w sulphuric acid, 160 °C for 20 min) with enzymatic hydrolysis (2% enzymes, 50 °C for 72 h) obtaining a yield of 80% w/w of sugars.35 Wheat bran hydrolysis can also be achieved exclusively by acid hydrolysis using sulphuric acid (1% w/w) as a catalyst at temperatures between 110 °C and 180 °C for 40 min, obtaining around 80% w/w of sugars.36 Other methods were proposed in order to reduce the concentration of degradation products, such as a combined method of milling, acid hydrolysis and two step enzymatic hydrolysis. In that case sulphuric acid (0.3% w/w 121 °C for 30 min) was used and also two kinds of enzymes, obtaining a yield of 63% w/w of sugars and no degradation products.37
In this work, a continuous micro-reactor was used to carry out the hydrolysis of wheat bran in supercritical water. This reactor was previously used to hydrolyse pure cellulose in supercritical water, giving as a result a total conversion of cellulose in 0.02 s of residence time yielding a sugar production of 98% w/w13. The aim of this work was to test the capability of the aforementioned reactor to hydrolyse natural biomass.
2. Results and discussion
The compositional analysis of the raw material is shown in Table 1. The percentages of moisture, extractive fraction, lignin, cellulose, hemicellulose and ash content were determined. The lignin fraction was determined as the sum of 19.6 ± 0.3% w/w−1 due to soluble lignin and 2.7 ± 0.1% w/w−1 due to insoluble lignin.| Component | Moisture | Extractives | Cellulose | Hemicellulose | Lignin | Ash | Total |
|---|---|---|---|---|---|---|---|
| g/100 g wheat bran | 8.2 ± 0.1 | 10.6 ± 0.8 | 31.4 ± 1.6 | 20.3 ± 1.0 | 22.3 ± 0.3 | 0.5 ± 0.1 | 93.4 ± 1.6 |
The wheat bran was continuously hydrolysed in supercritical water at 400 °C and 25 MPa at different reaction times. These conditions were previously chosen because they were optimized in a previous work for cellulose hydrolysis.13 The reactions of cellulose hydrolysis under supercritical water conditions are fast. In fact, the total conversion of cellulose can be achieved at a reaction time as low as 0.02 s. Therefore, the reaction time was evaluated between 0 s and 1 s. Because of the geometry of the micro reactor, flow rates and water properties, the Reynolds number developed in the reactor was around 1 × 105.38 Thus, the flow through the reactor can be considered turbulent. In fact, the mixing disposition in our reactor was set following the best arrangements developed in the literature.39,40 In those investigations on the mixing of supercritical water and room temperature water, the mixing time was calculated and represented as a function of the Richardson number (Ri = Gr/Re2). The mixing time between supercritical water and room temperature water takes values between 1 and 3 ms at Ri = 1 × 10−2. The Richardson number developed in our reactor took a value around 1 × 10−8, suggesting that the mixing time would be lower than 1 ms, thus being lower than 1% of the total time considered between the inlet and outlet of the reactor.
Each experimental point is a result of five repetitions of the analysed conditions. In Fig. S1 in the ESI† a typical temperature and pressure profile for an experiment is shown. The pressure variations over the residence time are produced due to a partial solid deposition near the depressurization valve. The reactor was maintained at 400 ± 5 °C and the pressure at 25 ± 1 MPa. The temperature of the reactor outlet (after depressurization) was around 160 °C. This stream was fed to the HE-1 and leaves it at a temperature of 150 °C. So, a post cooling was needed to take the sample at 25 °C. On the other hand, the water stream pumped into HE-1 was heated from 20 °C to 155 °C. That was the temperature at which the water stream entered the heater, and it had to be further heated until 450 °C. The use of this heat exchanger allowed the reduction of heat requirements to 20%.
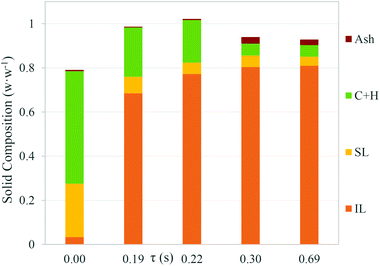
The lignin composition of the solids (soluble lignin (SL) and insoluble lignin (IL)) obtained after hydrolysis is shown in Fig. 1. The initial lignin composition was around 22% w/w. The lignin fraction was increased while the reaction time was raised. At 0.3 s of reaction time, the lignin content reached a value of 85% w/w. Increasing the reaction time up to 0.69 s did not enhance this value.
It should be taken into account that most of the plant biopolymers (mainly cellulose, hemicellulose and lignin) are present in nature in an associated way. Cellulose micro/nanofibrils interact between them by hydrogen-bond interactions forming highly associated structures like cylinders. The structure of hemicellulose is more opened and random than that of cellulose, and they interact between themselves by van der Waals and H-bond interactions. The lignin fraction is a highly amorphous polymer formed basically by phenolic units. The structure of lignin is complex, like a network, due to the random polymerization reaction when it is produced in nature. The interaction between hemicellulose and lignin takes place by covalent bonds.41 It can be considered that some fractions of cellulose are less accessible in the inner structure of lignin or linked to lignin.42 The ash content was increased from 0.5% w/w in the raw material up to 3% w/w after hydrolysis. Although the lignin composition of the solids was increased from 22% to 85% w/w, the soluble lignin fraction was decreased from 19% w/w to 5% w/w. This phenomenon occurred due to lignin hydrolysis, which occurred firstly in the sites where lignin interacts with hemicellulose.42 However, lignin remained in the solid as the main component after fractionation.
The cellulose and hemicellulose fractions in the solids decreased as the reaction time increased. However, these fractions remained constant at a value near 5% w/w when the reaction time was increased from 0.3 s to 0.7 s. In order to evaluate the solid characteristics after hydrolysis, SEM and FTIR analyses were carried out. In Fig. S2 in the ESI,† the spectrum comparison between the raw material (wheat bran) and the solid product after hydrolysis is shown. The bands at 1135 cm−1 are indicative of the aromatic C–H in-plane deformation for the syringyl type.43 This suggests that the syringyl type lignin was found in the raw material as well as in the solid product after hydrolysis. However, it exhibits aromatic C–H out of bending at 844 cm−1.43 This band was observed in the raw material but not in the solid product, suggesting that a fraction of lignin is decomposed after supercritical water hydrolysis. This agrees with the results presented in Fig. 2, in which the soluble lignin content is decreased after the process. Aromatic skeleton vibrations occurred at 1510 cm−1 and 1460 cm−1.43 The absorbance for these bands appeared in both the raw material and the product, suggesting that the aromatic properties remained in the solid products after hydrolysis. The band at 1720 cm−1 originates from the carbonyl group, including the unconjugated ketone and carbonyl group stretching.43 This band was observed in the raw material but not in the product, suggesting that typical cellulose bonds were broken or they were not present in the solid. In addition, the disappearance of the band at 1747 cm−1 indicates the rupture of the ester link of acetyl, feruloyl and p-coumaroyl between hemicellulose and lignin.44 It can also be observed that the O–H and aliphatic C–H (2870 cm−1) bonds which are the basic functional groups in biomass were present in the raw material as well as in the products.
 | ||
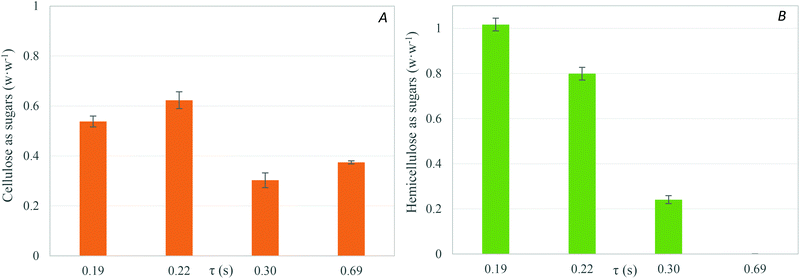
| Fig. 2 Yield of cellulose and hemicellulose recovered with residence time after supercritical water hydrolysis at 400 °C and 25 MPa. | ||
In order to analyse the structure of the hydrolysed products, SEM microscopy was applied to the samples. SEM images of the raw material are shown in Fig. S3-A and S3-C in the ESI† for a magnification of 5000× and S3-E† for a magnification of 1000×. The images corresponding to the solids obtained after hydrolysis are shown in Fig. S3-B and S3-D for a magnification of 5000× and S3-F† for a magnification of 1000×. In Fig. S3,† it is observed that the raw material presented different shapes with a smooth surface. After hydrolysis, the remaining solid showed a non-smooth surface. In addition, Fig. S3-D and S3-F† suggest that a porous solid was obtained after hydrolysis. Cellulose and hemicellulose that are located in the outer area of the particle would be rapidly hydrolysed in the process. However, the fractions of cellulose and hemicellulose situated in the inner part of the porous network of lignin are the reason for the remaining 5% w/w found (see Fig. 1) at high reaction times (0.69 s).
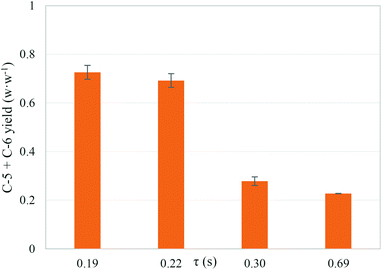
The recovery of soluble sugars from the liquid products decreased when the reaction time was increased from 0.19 s to 0.69 s. The yield of C-5 and C-6 obtained as soluble sugars after supercritical water hydrolysis is shown in Fig. 2. The maximum recovery of soluble sugar reached was 73% w/w at 0.19 s of reaction time. Although the highest yield of sugars was achieved at the lowest residence time, a decrease in the residence time would not produce a higher yield due to the uncompleted hydrolysis of cellulose and hemicellulose. In fact, the solid products after hydrolysis at 0.19 s of reaction time had a C-6 and C-5 composition of 22% w/w.
The recoveries of cellulose and hemicellulose fractions as soluble sugars over the residence time are shown in Fig. 3A and B, respectively. The maximum yield of cellulose-derived sugars (63% w/w) was achieved at 0.22 s of reaction time. The hydrolysis of pure cellulose under the same experimental conditions in this work was able to produce a yield of 98% w/w at 0.02 s of reaction time.13 The differences in the yields of cellulose hydrolysis suggest a strong effect of the cellulose interactions with the other components of the biomass over the kinetic. As explained above, it can be considered that cellulose in the natural biomass is embedded into a 3-D matrix of lignin and hemicellulose. So, the dissolution of wheat bran cellulose will be more complex and slower than the dissolution of pure cellulose due to the mass transfer limitations. In fact, pure cellulose is dissolved in supercritical water.15,38 The kinetics of cellulose hydrolysis in pressurized water takes different behaviours depending on the reaction medium conditions. At subcritical temperatures, the hydrolysis reactions occur on the surface of the cellulose grains, producing small oligosaccharides at low reaction rates. In addition, the cellulose particles obtained after partial hydrolysis at subcritical temperatures have the same crystallinity as cellulose before the treatment, which suggests that the cellulose hydrolysis takes place on the surface of cellulose grains at subcritical temperatures. However, if the reaction medium is near or supercritical water, the reaction rates are faster and the produced oligosaccharides are higher than under subcritical conditions, suggesting that the cellulose is dissolved (or partially dissolved) under those conditions. Cellulose is composed of several units of glucose linked by β-1,4 bonds, which provides the molecules of many –OH groups. These groups form intramolecular hydrogen bonds that provide the cellulose molecules of chain stiffness and molecular stability. Under supercritical conditions, water is a non-polar solvent with dielectric constant values lower than 10 and ionic products lower than 1 × 10−8 mol2 kg−2. The fact that cellulose is dissolved in near critical water suggests that cellulose presents a poor polar global structure. Although it is difficult to determine what the governing parameter is in cellulose dissolution, according to the results presented by Cantero et al.38 and Sasaki et al.,15 it can be considered that cellulose is dissolved at density values lower than 600 kg m−3 and dielectric constant values lower than 15. The cellulose dissolution might not occur with the same degree when cellulose is interacting with other components of biomass like lignin or when it is located inside a lignin network. Despite the difference between pure cellulose and wheat bran, the results obtained in this work are an improvement on those found in the literature (<25% w/w) for batch, semi-continuous or continuous fractionation.16–20,23–31 Hemicellulose was completely hydrolysed and recovered as sugars (mainly xylose) at a reaction time of 0.19 s. An increase in the reaction time caused a lower yield of C-5 recovered as sugars. In this case, a similar value of C-5 yield was found in the literature.16,23
The sugars produced after cellulose and hemicellulose hydrolysis can follow different reactions in supercritical water, such as retro-aldol condensation (RAC) or dehydration.45 The main products of cellulose hydrolysis are shown in the reaction pathway as illustrated in Fig. 4. Cellulose is hydrolysed into oligosaccharides as a first step. Then the oligosaccharides are hydrolysed into glucose. Once glucose is produced, it can be isomerised into fructose. The rate of fructose production is highly affected by the reaction medium conditions.46 Under supercritical water conditions the production of fructose from glucose is lower than under subcritical water conditions. These carbohydrates, glucose and fructose, can follow mainly two reaction pathways: dehydration and RAC. The dehydration reactions (horizontal way) produce 1,6-anhydro-glucose from glucose or 5-HMF from fructose. In these reactions, the sugar loses molecules of water. Glucose loses one molecule of water to produce 1,6-anhydro-glucose while fructose loses three molecules of water to produce 5-HMF. On the other hand, glucose and fructose can follow RAC reactions (vertical way) in which the molecules are split into two compounds. The RAC reaction takes place in the alpha carbon of the sugar. Thus, an aldose like glucose will produce a molecule of 2 carbons and a molecule of 4 carbons after the RAC reaction. On the other hand, a ketose like fructose produces two molecules of three carbons after the RAC reaction. The main product obtained from the hydrolysis of wheat bran was glycolaldehyde. The yields of production over the reaction time are shown in Fig. S4 in the ESI.† The yield of this compound at 0.19 s (highest yield of C-5 and C-6 recovery) was 20% w/w. The maximum amount of glycolaldehyde was 14% w/w at 0.22 s of reaction time. Small amounts of glycolaldehyde were also detected, which were associated with oligosaccharides. This behaviour was also observed in the hydrolysis of cellulose and cellobiose in supercritical water.
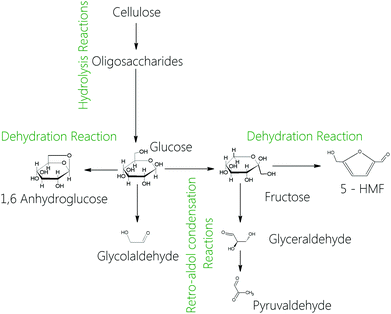 | ||
| Fig. 4 Main reactions of cellulose in supercritical water: hydrolysis, isomerization, dehydration and retro-aldol condensation. | ||
The production of 5-HMF would be undesired if a microorganism post-processing of the obtained sugars were needed.24 In Fig. S.5 in the ESI† the obtained yields of 5-HMF under the experimental conditions are shown. In the same way that it was developed in a previous work,13 the yield of 5-HMF over the whole range of residence time was lower than 0.05% w/w.
A fraction of the soluble lignin present in the starting material was hydrolysed and obtained, together with the sugars in the liquid sample. The amount of soluble lignin was determined following the method described in section 3.2.1. All the measurements of the soluble lignin gave values below 1000 ppm, which represents less than 10% w/w of carbon in the liquid sample.
The proposed method in this paper for the fractionation and hydrolysis of wheat bran shows meaningful advantages over the traditional methods of acid or enzymatic hydrolysis. In 2009, Arai et al.1 developed the concept of decentralized production of fuels and chemical compounds using renewable resources like lignocellulosic biomass as the starting material. The decentralized production proposes the use of the available biomass in the area to produce and supply this area of energy, fuels and chemical compounds. The main requirement to obtain this kind of production is the development of a compact and versatile process that can be placed in the countryside, near the biomass, in this way avoiding shipping costs. The technology developed in this work shows a promising alternative for biomass fractionation and hydrolysis due to the extremely low reaction time. The reaction time is directly linked to the reactors’ volume, which determines whether the technology is compact and versatile or not. The acid hydrolysis technology usually involves reaction times higher than 30 minutes and temperatures of around 170 °C.36,37 The enzymatic hydrolysis technology demands lower temperatures but much higher reactions times, about 70 hours.35 The reduction of the reaction time from 30 minutes or 70 hours (traditional methods) to 0.2 s (supercritical water hydrolysis) involves a substantial reduction in the reactor volumes from cubic meter to millilitres. In addition, the scale up of the process developed in this work makes the operation easier in some aspects in comparison with the lab scale: reactor volume, particle size and pumping. The reactor volume is increased from microliters to millilitres, which prevents problems of clogging in the reactor. In this work, the particle size was between 100 μm and 200 μm to prevent clogging in the reactor and the pump. A higher reactor diameter allows higher particle size, avoiding milling costs. In addition, the higher flows used in the industrial scale favour the pumping. The main issue for pumping solids on the lab scale is the size of the pump check valves. On the lab scale (1–3 L h−1) the size of the characteristic ball inside the check valve is around 1 mm, which is only ten times higher than the particle size. On the other hand, the pump check valves on the industrial scale (1–3 m3 h−1) are much higher than that on the lab scale, which makes it 100 or 1000 higher than the particle size of the biomass. Thus, on the industrial scale the clogging in the pump is highly reduced. Another important advantage of the method developed in this work is the possibility of increasing the product concentration. The reaction developed in the experimental setup described in this work is stopped through a flash decompression, lowering the temperature from 400 °C to 100 °C. The flash operation produces two phases: a liquid high with high concentration of sugars (it can be modified by changing the decompression pressure) and a vapour phase with extremely low carbon concentration (the amount of vapour can be modified by changing the decompression pressure). This cooling method has at least 3 advantages: the reaction is effectively stopped, the product concentration can be increased by taking out water as vapour and the vapour obtained as the product is almost free of carbon and contaminants, so it can be recycled to the system directly.
3. Experimental
3.1 Materials
A local supplier supplied the wheat bran used in the experiments. The particle size of the original biomass was 430 μm. In order to ensure an unstopped pumping, the particle size was reduced to 125 μm using a ball mill Retsch PM100. Distilled water was used as the reaction medium to run the experiments. The standards used in a High Performance Liquid Chromatography (HPLC) analysis were: cellobiose (≥98%), glucose (≥99%), xylose (≥99%), galactose (≥99%), mannose (≥99%), arabinose (≥99%), glyceraldehyde (≥95%), glycolaldehyde dimer (≥99%), lactic acid (≥85%), formic acid (≥98%), acetic acid (≥99%), acrylic acid (≥99%), furfural (99%) and 5-hydroxymethylfurfural (≥99%) purchased from Sigma. Milli-Q water and sulphuric acid (HPLC grade) were used as the mobile phase in the HPLC analysis. For the determination of structural carbohydrates and lignin,47 sulfuric acid (≥96%) and calcium carbonate (≥99%) supplied by Sigma were used as reagents. Milli-Q water was used in this procedure.3.2 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220 points. The SEM experiments were conducted in a JSM-820 (JOEL, Japan) operated at 20 kV of the accelerating voltage. A gold evaporator Balzers SCD003 with a gold thickness of 25 mm–30 mm was used.
220 points. The SEM experiments were conducted in a JSM-820 (JOEL, Japan) operated at 20 kV of the accelerating voltage. A gold evaporator Balzers SCD003 with a gold thickness of 25 mm–30 mm was used.
The carbon content of the products was determined by total organic carbon (TOC) analysis with Shimadzu TOC-VCSH equipment. The composition of the liquid product was determined using HPLC analysis. The column used for the separation of the compounds was Shodex SH-1011 at 50 °C, using sulphuric acid (0.01 N) as the mobile phase with a flow of 0.8 ml min−1. A Waters IR detector 2414 was used to identify the sugars and their derivatives and the Water UV-Vis detector was used to determine the 5-hydroxymethylfurfural concentrations at a wavelength of 254 nm.
The soluble oligosaccharide concentration in the samples was determined by acid hydrolysis to glucose and HPLC determination. Briefly, to 10 ml of filtered liquid aliquots was added 4 mL of 96% sulphuric acid. The sample was maintained at 30 °C over 60 min in an oven. Then it was diluted with 86 ml of deionized water and incubated at 121 °C for 60 min. Calcium carbonate was added to 20 ml of this sample to neutralize the medium, and finally the supernatant was filtered and analysed with HPLC. It should be mentioned that the oligomer concentration and the RAC and dehydration products were determined using the acid hydrolysis method. The concentration of monomers and its derived products were also determined by direct analysis of the obtained sample from the pilot plant by HPLC. In this way, the quantity of glycolaldehyde or 5-HMF that can be produced from the hydrolysis of an oligomer with a degraded end-group can also be determined by difference.
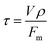 | (1) |
The yield of the main compounds (C-6 sugars, C-5 sugars, glycolaldehyde and 5-HMF) was determined in eqn (2), where ‘Ys’ is the yield of the compound ‘s’, ‘Cs’ is the concentration of ‘s’ in the liquid product in ppm and ‘Sin’ is the concentration of sugars at the inlet of the reactor in ppm, calculated as shown in eqn (3). Soluble sugars derived from cellulose (cellobiose and glucose) were called C-6, those derived from hemicellulose (xylose, mannose, galactose and arabinose) were called C-5 and the rest of the compounds were organic acids (acetic, lactic and acrylic acid), glycolaldehyde, glyceraldehyde and 5-HMF.
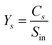 | (2) |
| Sin = Cin·ST | (3) |
In eqn (3), ‘Cin’ is the concentration of wheat bran at the reactor's inlet in ppm and ‘ST’ is the cellulose and hemicellulose fraction in the raw material in mass fraction which represents the proportion of wheat bran susceptible to being hydrolysed into sugars (see Table 1). When the yield was referred to each fraction, cellulose or hemicellulose, ‘ST’ was the portion of each fraction in the raw material.
3.3 Experimental setup
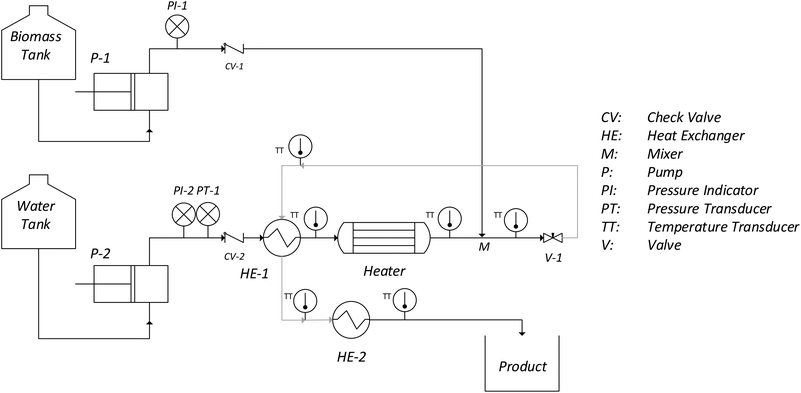
The continuous pilot plant used for this work is shown in Fig. 5. The hydrolysis pilot plan was designed to operate up to 400 °C and 30 MPa using a sudden expansion micro-reactor (SEMR) developed in a previous work.13The main advantage of this reactor is the instantaneous cooling of the products, which efficiently stops the reactions of hydrolysis at very short times. This allows the precise evaluation of the reaction time without diluting the products. In a similar way, the heating of the biomass stream is achieved instantaneously by a supercritical water injection at the reactor inlet. With this heating method, it is possible to change the temperature of a biomass stream from room temperature up to 400 °C in a mixer which is placed at the reactor inlet. In addition, the reactor is thermally isolated with Rockwool insulation, which makes it possible to consider it as isothermal. A detailed description of the pilot plant as well as the operation procedure is presented in a previous work.38 In this case, a wheat bran suspension (5% w/w) was continuously compressed and pumped up to the operation pressure (25 MPa), remaining at room temperature until the inlet of the reactor. At that point the suspension was instantaneously heated by mixing it with a supercritical water stream, and the hydrolysis reactions starts. Then the effluent was suddenly depressurized at the outlet of the reactor without previous cooling in order to instantaneously stop the hydrolysis. In this setup, a modification of the previous pilot plant13 was tested; also, the reactor outlet stream was driven to a heat exchanger (HE-1) to pre-heat the supercritical water stream. In order to ensure the cooling of the sample, a cooler (HE-2) was set after HE-1.
The aforementioned experimental setup was carefully designed following the security regulations for high pressure and temperature pilot plants. The hot and pressurised pipes of the pilot were confined inside a bunker for security reasons. This section of the setup is accessible from the back of the pilot plant as shown in Fig. S6 in the ESI.† In addition, the the setup can be completely operated by managing the control panel situated in the front of the pilot plant, opposite to the bunker access. For further information about the security aspects of the design see a previous work of Cantero et al.38
4. Conclusions
Wheat bran hydrolysis in supercritical water was analysed at 400 °C and 25 MPa at reaction times lower than 1 s. This method proved to be an effective procedure to hydrolyse cellulose and hemicellulos, at the same time with a low concentration of degradation products. This result was achieved by working at high temperature (400 °C) and low residence time (0.19 s). Controlling the reaction time was the key factor to stop the reaction before sugar degradation.The recovery yield of cellulose and hemicellulose as C-6 and C-5 was 73% w/w. The solid obtained after the hydrolysis was composed of 85% w/w of lignin. An increase in the reaction time increased the lignin content of the solid. However, a cellulose fraction (5% w/w) seems to remain occluded inside a lignin network after a reaction time increment. The obtained solid product after hydrolysis consisted of an amorphous and porous material.
Acknowledgements
The authors thank the Spanish Ministry of Economy and Competitiveness for the Projects CTQ2011-23293, CTQ2011-27347 and ENE2012-33613. The authors thank Repsol for its technical support. D.A.C. thanks the Spanish Ministry of Education for the FPU fellowship (AP2009-0402).References
- K. Arai, R. L. Smith Jr. and T. M. Aida, J. Supercrit. Fluids, 2009, 47, 628–636 CrossRef CAS PubMed
.
- BECOTEPS, European Technology Platforms for the FP7. The European Bioeconomy 2030. Delivering Sustainable Growth by addressing the Grand Societal Challenges, Accessed 10/04/2014, 2014.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS PubMed
.
- O. Bobleter, Prog. Polym. Sci., 1994, 19, 797–841 CrossRef CAS
.
- C. B. Field, M. J. Behrenfeld, J. T. Randerson and P. Falkowski, Science, 1998, 281, 237–240 CrossRef CAS
.
- J. Tollefson, Nat. News, 2008, 451, 880–883 CrossRef CAS PubMed
.
- A. A. Peterson, F. Vogel, R. P. Lachance, M. Fröling, Michael J. Antal Jr. and J. W. Tester, Energy Environ. Sci., 2008, 1, 32–65 CAS
.
- N. Akiya and P. E. Savage, Chem. Rev., 2002, 102, 2725–2750 CrossRef CAS PubMed
.
- D. Bröll, C. Kaul, A. Krämer, P. Krammer, T. Richter, M. Jung, H. Vogel and P. Zehner, Angew. Chem., Int. Ed., 1999, 38, 2998–3014 CrossRef
.
- A. Loppinet-Serani, C. Aymonier and F. Cansell, J. Chem. Technol. Biotechnol., 2010, 85, 583–589 CrossRef CAS
.
- M. Akizuki, T. Fujii, R. Hayashi and Y. Oshima, J. Biosci. Bioeng., 2014, 117, 10–18 CrossRef CAS PubMed
.
- H. Machida, M. Takesue and R. L. Smith Jr., J. Supercrit. Fluids, 2011, 60, 2–15 CrossRef CAS PubMed
.
- D. A. Cantero, M. D. Bermejo and M. J. Cocero, Bioresour. Technol., 2013, 135, 697–703 CrossRef CAS PubMed
.
- Z. Fang, T. Sato, R. L. Smith Jr., H. Inomata, K. Arai and J. A. Kozinski, Bioresour. Technol., 2008, 99, 3424–3430 CrossRef CAS PubMed
.
- M. Sasaki, T. Adschiri and K. Arai, AIChE J., 2004, 50, 192–202 CrossRef CAS
.
- A. Alfaro, F. López, A. Pérez, J. C. García and A. Rodríguez, Bioresour. Technol., 2010, 101, 7635–7640 CrossRef CAS PubMed
.
- J. Y. Lee, H. J. Ryu and K. K. Oh, Bioresour. Technol., 2013, 132, 84–90 CrossRef CAS PubMed
.
- R. Yáñez, G. Garrote and M. J. Díaz, Bioresour. Technol., 2009, 100, 6514–6523 CrossRef PubMed
.
- Y. Zhao, W.-J. Lu, H.-T. Wang and J.-L. Yang, Bioresour. Technol., 2009, 100, 5884–5889 CrossRef CAS PubMed
.
- A. Rodríguez, A. Moral, R. Sánchez, A. Requejo and L. Jiménez, Bioresour. Technol., 2009, 100, 4863–4866 CrossRef PubMed
.
- S. Marinkovic and B. Estrine, Green Chem., 2010, 12, 1929–1932 RSC
.
- T. v. Stein, P. M. Grande, H. Kayser, F. Sibilla, W. Leitner and P. D. d. María, Green Chem., 2011, 13, 1772–1777 RSC
.
- T. Ingram, T. Rogalinski, V. Bockemühl, G. Antranikian and G. Brunner, J. Supercrit. Fluids, 2009, 48, 238–246 CrossRef CAS PubMed
.
- T. Rogalinski, T. Ingram and G. Brunner, J. Supercrit. Fluids, 2008, 47, 54–63 CrossRef CAS PubMed
.
- Z. Merali, J. D. Ho, S. R. A. Collins, G. L. Gall, A. Elliston, A. Käsper and K. W. Waldron, Bioresour. Technol., 2013, 131, 226–234 CrossRef CAS PubMed
.
- C. Pronyk and G. Mazza, Bioresour. Technol., 2012, 106, 117–124 CrossRef CAS PubMed
.
- C. Pronyk and G. Mazza, Bioresour. Technol., 2011, 102, 2016–2025 CrossRef CAS PubMed
.
- C. Liu and C. E. Wyman, Bioresour. Technol., 2005, 96, 1978–1985 CrossRef CAS PubMed
.
- N. Mosier, R. Hendrickson, N. Ho, M. Sedlak and M. R. Ladisch, Bioresour. Technol., 2005, 96, 1986–1993 CrossRef CAS PubMed
.
- C. Schacht, C. Zetzl and G. Brunner, J. Supercrit. Fluids, 2008, 46, 299–321 CrossRef CAS PubMed
.
- T. Rogalinski, S. Herrmann and G. Brunner, J. Supercrit. Fluids, 2005, 36, 49–58 CrossRef CAS PubMed
.
- K. Hossain, C. Ulven, K. Glover, F. Ghavami, S. Simsek, M. S. Alamri, A. Kumar and M. Mergoum, Aust. J. Crop Sci., 2013, 7, 525–531 CAS
.
- B. P. Lamsal, R. Madl and K. Tsakpunidis, Bioenergy Res., 2011, 4, 193–200 CrossRef
.
- M. È. Seyer and P. Gélinas, Int. J. Food Sci. Technol., 2009, 44, 688–693 CrossRef CAS PubMed
.
- B. Palmarola-Adrados, P. Chotěborská, M. Galbe and G. Zacchi, Bioresour. Technol., 2005, 96, 843–850 CrossRef CAS PubMed
.
- P. Chotěborská, B. Palmarola-Adrados, M. Galbe, G. Zacchi, K. Melzoch and M. Rychtera, J. Food Eng., 2004, 61, 561–565 CrossRef
.
- L. Favaro, M. Basaglia and S. Casella, Biomass Bioenerg., 2012, 46, 605–617 CrossRef CAS PubMed
.
- D. A. Cantero, M. D. Bermejo and M. J. Cocero, J. Supercrit. Fluids, 2013, 75, 48–57 CrossRef CAS PubMed
.
- J. Sierra-Pallares, D. L. Marchisio, E. Alonso, M. Teresa Parra-Santos, F. Castro and M. José Cocero, Chem. Eng. Sci., 2011, 66, 1576–1589 CrossRef CAS PubMed
.
- T. Aizawa, Y. Masuda, K. Minami, M. Kanakubo, H. Nanjo and R. L. Smith, J. Supercrit. Fluids, 2007, 43, 222–227 CrossRef CAS PubMed
.
- C. A. S. Hill, Wood Modification: Chemical, Thermal and Other Processes, 2006.
- P. Gullón, A. Romaní, C. Vila, G. Garrote and J. C. Parajó, Biofuel. Bioproduct. Bior., 2012, 6, 219–232 CrossRef
.
- J. Zhang, H. Deng, Y. Sun, C. Pan and S. Liu, Bioresour. Technol., 2010, 101, 2311–2316 CrossRef CAS PubMed
.
- Y. Xu and B. Chen, Bioresour. Technol., 2013, 146, 485–493 CrossRef CAS PubMed
.
- M. Sasaki, K. Goto, K. Tajima, T. Adschiri and K. Arai, Green Chem., 2002, 4, 285–287 RSC
.
- T. M. Aida, Y. Sato, M. Watanabe, K. Tajima, T. Nonaka, H. Hattori and K. Arai, J. Supercrit. Fluids, 2007, 40, 381–388 CrossRef CAS PubMed
.
- J. B. Sluiter, R. O. Ruiz, C. J. Scarlata, A. D. Sluiter and D. W. Templeton, J. Agric. Food Chem., 2010, 58, 9043–9053 CrossRef CAS PubMed
.
- R. S. Fukushima and R. D. Hatfield, J. Agric. Food Chem., 2004, 52, 3713–3720 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4gc01359j |
| This journal is © The Royal Society of Chemistry 2015 |