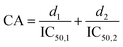Utilisation of the isobole methodology to study dietary peptide–drug and peptide–peptide interactive effects on dipeptidyl peptidase IV (DPP-IV) inhibition†
Alice B.
Nongonierma
and
Richard J.
FitzGerald
*
Department of Life Sciences and Food for Health Ireland (FHI), University of Limerick, Castletroy, Limerick, Ireland. E-mail: dick.fitzgerald@ul.ie; Fax: +353 (0) 61 331490; Tel: +353 (0) 61 202598
First published on 26th November 2014
Abstract
Inhibition of dipeptidyl peptidase-IV (DPP-IV) is used as a means to regulate post-prandial serum glucose in type 2 diabetics. The effect of drug (Sitagliptin®)/peptide and binary peptide mixtures on DPP-IV inhibition was studied using an isobole approach. Five peptides (Ile-Pro-Ile-Gln-Tyr, Trp-Lys, Trp-Pro, Trp-Arg and Trp-Leu), having DPP-IV half maximum inhibitory concentration values (IC50) < 60 μM and reported to act through different inhibition mechanisms, were investigated. The dose response relationship of Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) peptide (1
peptide (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 0
0, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852, 1
852, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426 and 1
426 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1704 on a molar basis) and binary Ile-Pro-Ile-Gln-Tyr
1704 on a molar basis) and binary Ile-Pro-Ile-Gln-Tyr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) peptide (1
peptide (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 0
0, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on a molar basis) mixtures for DPP-IV inhibition was characterised. Isobolographic analysis showed, in most instances, an additive effect on DPP-IV inhibition. However, a synergistic effect was observed with two Sitagliptin
1 on a molar basis) mixtures for DPP-IV inhibition was characterised. Isobolographic analysis showed, in most instances, an additive effect on DPP-IV inhibition. However, a synergistic effect was observed with two Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ile-Pro-Ile-Gln-Tyr (1
Ile-Pro-Ile-Gln-Tyr (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426 and 1
426 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852) mixtures and an antagonistic effect was seen with one Sitagliptin
852) mixtures and an antagonistic effect was seen with one Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Pro (1
Trp-Pro (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852) mixture, and three binary peptide mixtures (Ile-Pro-Ile-Gln-Tyr
852) mixture, and three binary peptide mixtures (Ile-Pro-Ile-Gln-Tyr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Lys (1
Trp-Lys (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and Ile-Pro-Ile-Gln-Tyr
1) and Ile-Pro-Ile-Gln-Tyr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Leu (1
Trp-Leu (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)). The results show that Sitagliptin and food protein-derived peptides can interact, thereby enhancing overall DPP-IV inhibition. Combination of Sitagliptin with food protein-derived peptides may help in reducing drug dosage and possible associated side-effects.
2)). The results show that Sitagliptin and food protein-derived peptides can interact, thereby enhancing overall DPP-IV inhibition. Combination of Sitagliptin with food protein-derived peptides may help in reducing drug dosage and possible associated side-effects.
1. Introduction
The increasing global prevalence of type 2 diabetes (T2D) has led the scientific community to investigate different strategies in order to slow down its evolution. Dipeptidyl peptidase IV (DPP-IV) inhibitors belong to a new class of drugs with an antidiabetic action, with Sitagliptin® (Januvia®, Merck & Co., Inc. USA) being the first DPP-IV inhibitor launched on the market. DPP-IV cleaves incretins such as glucose dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) in vivo. Inhibition of DPP-IV therefore increases the half-life of incretins, thereby promoting insulin secretion from pancreatic beta cells.1Food protein-derived bioactive peptides have been shown to positively affect biomarkers of T2D such as postprandial glycaemia and insulin secretion.2–4 It is thought that the antidiabetic properties of specific food protein hydrolysates may arise from their DPP-IV inhibitory activity.5,6 Food protein hydrolysates, originating mostly from milk, have been reported for their DPP-IV inhibitory potential.7 The peptides therein may inhibit DPP-IV through different modes of inhibition.8–10 In a physiological situation, it is expected that different food protein-derived peptides may concomitantly inhibit DPP-IV. However, the contribution of multiple food protein-derived peptides, as present in food protein hydrolysates, to overall DPP-IV inhibition has not been determined. The combination of milk protein-derived peptides with Sitagliptin was recently shown to have an additive effect on DPP-IV inhibition.11 However, to date the interactive effects of peptide–peptide and peptide–drug combinations on DPP-IV inhibition does not appear to have been extensively studied.
The interactive effects of drug mixtures is conventionally studied using an isobole methodology.12,13 It has been recently proposed that using combinations of antidiabetic drugs and phytochemicals may be a new approach to help reduce the side-effects observed during drug intake.13 Synergistic antidiabetic activity has been shown in vivo when combinations of phytochemicals (ferulic acid) and antidiabetic drugs (metformin and thiazolidinedione) were employed.14 To our understanding, the isobole method has not been previously applied to determine interactive effects between drug–peptide or binary peptide mixtures. The aim of this study was therefore to utilise an isobole methodology to study the interactions between Sitagliptin and food protein-derived DPP-IV inhibitory peptides, and between binary mixtures of DPP-IV inhibitory peptides.
2. Materials and methods
2.1. Reagents
Porcine DPP-IV (≥10 Units mg−1 protein), Gly-Pro-pNA, tris(hydroxymethyl)aminomethane (TRIS), Ile-Pro-Ile and Sitagliptin were from Sigma Aldrich (Dublin, Ireland). Trp-Pro, Trp-Arg and Ile-Pro-Ile-Gln-Tyr were obtained from Thermo Fisher Scientific (Ulm, Germany) while Trp-Leu and Trp-Lys were from Bachem (Bubendorf, Switzerland). Hydrochloric acid (HCl) and high-performance liquid chromatography (HPLC) grade water were from VWR (Dublin, Ireland).2.2. In silico analysis of food proteins
The occurrence of the five DPP-IV inhibitory peptides used in this study was determined in silico in 72 dietary proteins15 (ESI Table S1†). The sequences of the mature proteins (without the propeptide) were obtained from UniProt using the ExPASy resource portal. The occurrence of the peptides was determined using an in-house generated Matlab programme (version R2014b, MathWorks, Inc, Natick, MA, USA). Proteins with the five peptides were further subjected to in silico digestion with gastrointestinal enzymes (pepsin, trypsin, chymotrypsin and elastase) using the Peptide Cutter facility in Matlab.2.3. Experimental design to study Sitagliptin–peptide and peptide–peptide interactions
Stock solutions of peptides (900 μM) and Sitagliptin (1056 nM) were prepared to yield ∼80% DPP-IV inhibition. The ratios studied for the binary peptide mixtures were as described by Tallarida.16 The same volumetric mixtures of peptide stock solutions (i.e. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were also employed for the Sitagliptin/peptide mixtures. For the binary peptide mixtures, only the combinations with the most potent substrate-type competitive DPP-IV inhibitor, Ile-Pro-Ile-Gln-Tyr (IC50 value of 23 μM), and non-competitive (Trp-Lys, Trp-Pro and Trp-Arg) and competitive (Trp-Leu) DPP-IV inhibitors were studied.
1) were also employed for the Sitagliptin/peptide mixtures. For the binary peptide mixtures, only the combinations with the most potent substrate-type competitive DPP-IV inhibitor, Ile-Pro-Ile-Gln-Tyr (IC50 value of 23 μM), and non-competitive (Trp-Lys, Trp-Pro and Trp-Arg) and competitive (Trp-Leu) DPP-IV inhibitors were studied.
The mixtures consisted of aqueous Sitagliptin/peptide solutions with the following ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1
0, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426, 1
426, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852, 1
852, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1704 and 0
1704 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on a molar basis. Similarly, binary mixtures of peptides consisting of Ile-Pro-Ile-Gln-Tyr and another peptide (Trp-Lys, Trp-Pro, Trp-Arg or Trp-Leu) in the ratios of 1
1 on a molar basis. Similarly, binary mixtures of peptides consisting of Ile-Pro-Ile-Gln-Tyr and another peptide (Trp-Lys, Trp-Pro, Trp-Arg or Trp-Leu) in the ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1
0, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 0
2 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
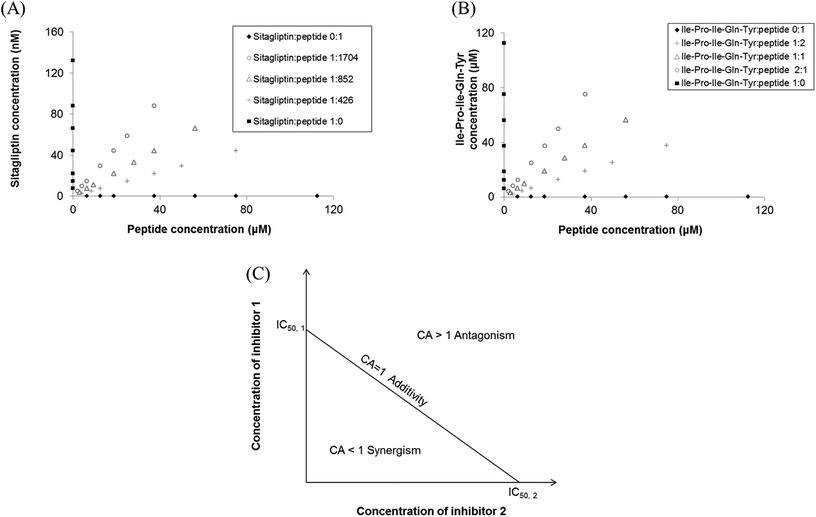
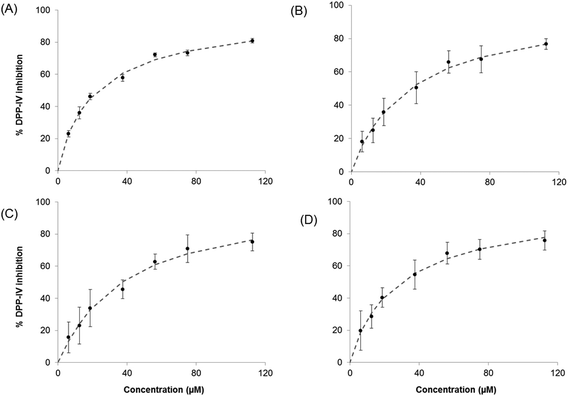
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on a molar basis, were studied. The dose response for DPP-IV inhibition (n = 3) was determined with each of the previous mixtures diluted in HPLC water at 7 different concentrations (Fig. 1A and B).
1 on a molar basis, were studied. The dose response for DPP-IV inhibition (n = 3) was determined with each of the previous mixtures diluted in HPLC water at 7 different concentrations (Fig. 1A and B).
2.4. DPP-IV inhibition assay
The DPP-IV inhibition assay was carried out essentially as described by Nongonierma & FitzGerald.9 Briefly, the Sitagliptin/peptide or binary peptide mixtures (25 μL) were pipetted onto a 96 well microplate (Sarstedt, Dublin, Ireland) containing Gly-Pro-pNA (final concentration 0.200 mM). The negative control contained 100 mM Tris-HCl buffer pH 8.0 (25 μL) and Gly-Pro-pNA. The reaction was initiated by the addition of DPP-IV (final concentration 0.0025 U mL−1). The microplate was incubated at 37 °C for 60 min in a microplate reader (Biotek Synergy HT, Winoosky, VT, USA) and absorbance of the released pNA was monitored at 405 nm. Each sample was analysed in triplicate (n = 3). The half maximum inhibitory concentration (IC50) for DPP-IV was determined by plotting the percentage inhibition as a function of the concentration of test compounds.2.5. Determination of the isobole diagram at 50% DPP-IV inhibition
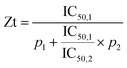
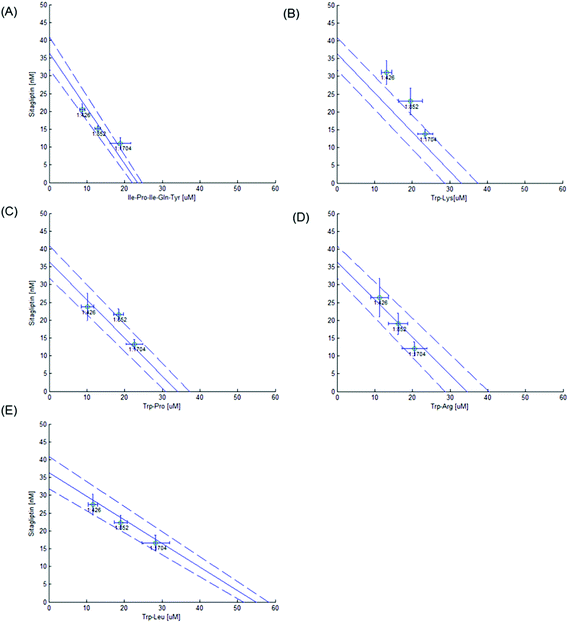
The isobole diagrams for 50% DPP-IV inhibition were plotted for the different Sitagliptin/peptide or binary peptide mixtures. Each isobole showed the IC50 value for the inhibitors on the x and y axes. The line between the two IC50 values corresponds to the line of additivity (Fig. 1C). The concentration addition (CA) effect is described by the following equation:12where d1 and d2 are the concentrations of inhibitors 1 and 2, respectively, in a mixture yielding 50% DPP-IV inhibition; IC50,1 and IC50,2 are the half maximum inhibitory concentrations of inhibitors 1 and 2, respectively.
The mixture of inhibitors 1 and 2 can have an additive (CA = 1), synergistic (CA < 1) or antagonistic effect (CA > 1) on DPP-IV inhibition (Fig. 1C). The theoretical total additivity concentration (Zt) of the mixture was determined as described elsewhere17 using an in-house Matlab program. Zt corresponds to the theoretical concentration of the mixture which should yield 50% DPP-IV inhibition if the two inhibitors have an additive effect. Zt was calculated as follows:
2.6. Statistical analysis
Means comparison was carried out with a one way ANOVA followed by a Student Newman–Keuls test using SPSS (version 22, SPSS Inc., Chicago, IL, USA) at a significance level P < 0.05. For each mixture, Zt was compared to the apparent IC50 value using a Student test (P < 0.05) as described elsewhere.123. Results
3.1. Occurrence of the DPP-IV inhibitory peptides in 72 dietary food proteins
The five DPP-IV inhibitory peptides studied were found within 50% of the dietary proteins considered (ESI Table S1†). The in silico digestion of the dietary proteins predicted that 4 out of the 5 peptides may be released from 14 of the dietary proteins studied. It is interesting to note that 86% of these proteins are plant-derived. Although Trp-Pro was present within 16 of the proteins studied, it was not predicted to be released by gastrointestinal enzymes (Table 1). The outcome of the in silico analysis suggested that 4 of the target peptides may be released during the digestion of foods. Therefore, they may play a role in DPP-IV inhibition following oral ingestion.| Peptidea | Protein fragment | Enzyme | Protein source | Protein |
|---|---|---|---|---|
| a Peptide sequence using the three letter code. b RuBisCO: Ribulose bisphosphate carboxylase. | ||||
| Trp-Lys | 40-41 | Pepsin | Oat (Avena sativa) | Avenin |
| Trp-Arg | 212-213 | Trypsin | Wheat (Triticum aestivum) | Large subunit RuBisCOb |
| Trp-Arg | 212-213 | Trypsin | Barley (Hordeum vulgare) | Large subunit RuBisCO |
| Trp-Arg | 212-213 | Trypsin | Oat (Avena sativa) | Large subunit RuBisCO |
| Trp-Arg | 212-213 | Trypsin | Corn (Zea mays) | Large subunit RuBisCO |
| Trp-Arg | 212-213 | Trypsin | Rice (Oryza sativa subsp. Japonica) | Large subunit RuBisCO |
| Trp-Arg | 212-213 | Trypsin | Sorghum (Sorghum vulgare) | Large subunit RuBisCO |
| Trp-Arg | 171-172 | Trypsin | Quinoa (Chenopodium quinoa) | RuBisCO large chain |
| Trp-Arg | 212-213 | Trypsin | Amaranth (Amaranthus hypochondriacus) | Large subunit RuBisCO |
| Trp-Arg | 207-208 | Trypsin | Palmaria palmata (Rhodymenia palmata) | Allophycocyanin α chain |
| Trp-Arg | 171-172 | Trypsin | Palmaria palmata (Rhodymenia palmata) | Allophycocyanin β chain |
| Trp-Arg | 212-213 | Trypsin | Palmaria palmata (Rhodymenia palmata) | Phycocyanin α |
| Trp-Leu | 104-105 | Elastase | Bovine milk (Bos taurus) | α-Lactalbumin |
| Ile-Pro-Ile-Gln-Tyr | 26-30 | Chymotrypsin | Bovine milk (Bos taurus) | κ-Casein |
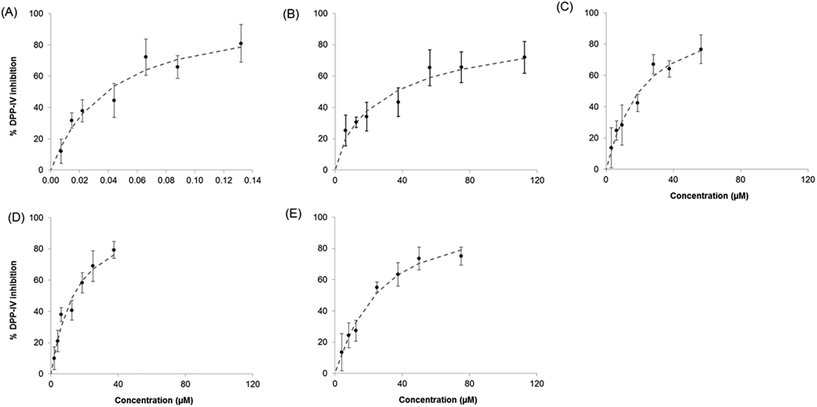
3.2. Dose–response relationship for the Sitagliptin/peptide and the binary peptide mixtures
The five DPP-IV inhibitory peptides studied were selected based on differences in their mode of inhibition and the fact that they were relatively potent food protein-derived DPP-IV inhibitors (IC50 value <60 μM).8,18 The IC50 values obtained during this study were of the same order as previously described8,18 (ESI Table S2†). Mixtures of Sitagliptin/peptides and binary peptides were evaluated for their ability to inhibit DPP-IV as outlined in section 2.4. The dose–response curves obtained for the Sitagliptin/Trp-Lys mixtures are illustrated on Fig. 2 and that for the binary peptide mixtures Ile-Pro-Ile-Gln-Tyr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Lys are shown on Fig. 3. A dose response relationship was seen with Sitagliptin and Ile-Pro-Ile-Gln-Tyr alone, and with all Sitagliptin/peptide and binary peptide mixtures (Fig. 2, 3 and data not shown).
Trp-Lys are shown on Fig. 3. A dose response relationship was seen with Sitagliptin and Ile-Pro-Ile-Gln-Tyr alone, and with all Sitagliptin/peptide and binary peptide mixtures (Fig. 2, 3 and data not shown).
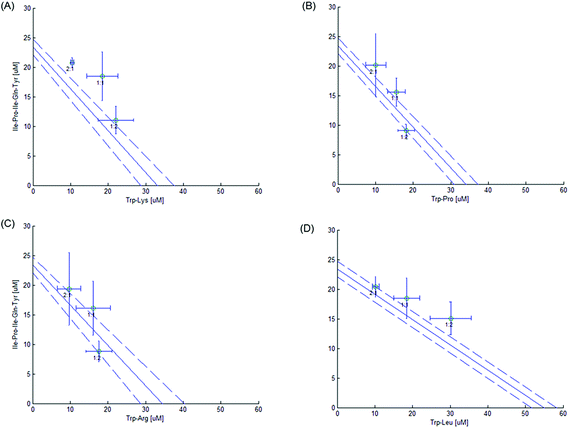
3.3. Sitagliptin–peptide and peptide–peptide interactions
The 50% isobole diagram shows the IC50 value for Sitagliptin or Ile-Pro-Ile-Gln-Tyr on the y axis and that of the peptide on the x axis (Fig. 4 and 5). In a few instances, the apparent IC50 value for the mixture was close to the line of additivity for Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ile-Pro-Ile-Gln-Tyr and Sitagliptin
Ile-Pro-Ile-Gln-Tyr and Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Pro (1
Trp-Pro (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426 and 1
426 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852), Sitagliptin
852), Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Arg and Sitagliptin
Trp-Arg and Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Leu (1
Trp-Leu (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426, 1
426, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852 and 1
852 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1704), Ile-Pro-Ile-Gln-Tyr
1704), Ile-Pro-Ile-Gln-Tyr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Arg and Ile-Pro-Ile-Gln-Tyr
Trp-Arg and Ile-Pro-Ile-Gln-Tyr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Pro (1
Trp-Pro (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and Ile-Pro-Ile-Gln-Tyr
1) and Ile-Pro-Ile-Gln-Tyr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Leu (2
Trp-Leu (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). For the other mixtures, the values were either in the area of the isobole corresponding to an antagonistic effect or in the area corresponding to a synergistic effect.
1). For the other mixtures, the values were either in the area of the isobole corresponding to an antagonistic effect or in the area corresponding to a synergistic effect.
Most Zt values were not significantly different (P > 0.05) from the apparent IC50 value (Table 2), suggesting an additive effect of the mixture on DPP-IV inhibition. However, three Sitagliptin/peptide mixtures (Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ile-Pro-Ile-Gln-Tyr (1
Ile-Pro-Ile-Gln-Tyr (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426 and 1
426 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852) and Sitagliptin
852) and Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Pro (1
Trp-Pro (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852)) had apparent IC50 values which were significantly different (P < 0.05) from that of Zt (12.9 vs. 13.8, 8.8 vs. 9.9 and 18.4 vs. 16.9 μM, respectively), indicating a synergistic effect for the Sitagliptin
852)) had apparent IC50 values which were significantly different (P < 0.05) from that of Zt (12.9 vs. 13.8, 8.8 vs. 9.9 and 18.4 vs. 16.9 μM, respectively), indicating a synergistic effect for the Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ile-Pro-Ile-Gln-Tyr mixtures and an antagonistic effect for the Sitagliptin
Ile-Pro-Ile-Gln-Tyr mixtures and an antagonistic effect for the Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Pro mixture on DPP-IV inhibition. Similarly, three binary peptide mixtures (Ile-Pro-Ile-Gln-Tyr
Trp-Pro mixture on DPP-IV inhibition. Similarly, three binary peptide mixtures (Ile-Pro-Ile-Gln-Tyr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Lys (1
Trp-Lys (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and Ile-Pro-Ile-Gln-Tyr
1) and Ile-Pro-Ile-Gln-Tyr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Leu (1
Trp-Leu (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)) had apparent IC50 values significantly higher than that of Zt (36.9 vs. 27.3; 31.2 vs. 25.8 and 45.2 vs. 37.8 μM, respectively), also suggesting an antagonistic effect of the binary peptide mixture on DPP-IV inhibition.
2)) had apparent IC50 values significantly higher than that of Zt (36.9 vs. 27.3; 31.2 vs. 25.8 and 45.2 vs. 37.8 μM, respectively), also suggesting an antagonistic effect of the binary peptide mixture on DPP-IV inhibition.
| Peptide | |||||||
|---|---|---|---|---|---|---|---|
| Ile-Pro-Ile-Gln-Tyr | Trp-Lys | Trp-Pro | Trp-Arg | Trp-Leu | |||
a Values represent the mean of triplicate determination (n = 3) of the theoretical additivity concentration (Zt) ± confidence interval (P = 0.05) and the apparent half maximum inhibitory concentration (IC50) ± confidence interval (P = 0.05) for different Ile-Pro-Ile-Gln-Tyr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) peptide (1 peptide (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2 2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and Sitagliptin 1) and Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) peptide (1 peptide (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852, 1 852, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426 and 1 426 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1704) mixtures. ns: the apparent IC50 value of the mixture is not significantly different from Zt (P > 0.05). *: the apparent IC50 value of the mixture is significantly different from Zt (P < 0.05). na: not applicable. 1704) mixtures. ns: the apparent IC50 value of the mixture is not significantly different from Zt (P > 0.05). *: the apparent IC50 value of the mixture is significantly different from Zt (P < 0.05). na: not applicable.
|
|||||||
Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) peptide ratio (on a molar basis)a peptide ratio (on a molar basis)a |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1704 1704 |
Zt (μM) | 17.3 ± 0.7 | 22.5 ± 3.3 | 22.6 ± 1.3 | 22.6 ± 2.0 | 30.4 ± 2.0 |
| IC50 (μM) | 18.9 ± 2.3ns | 23.5 ± 1.5ns | 22.6 ± 1.7ns | 20.5 ± 2.6ns | 28.3 ± 2.8ns | ||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852 852 |
Zt (μM) | 13.8 ± 0.7 | 17.1 ± 2.7 | 16.9 ± 1.1 | 16.9 ± 1.2 | 21.12 ± 1.9 | |
| IC50 (μM) | 12.9 ± 0.6* | 19.6 ± 2.4ns | 18.4 ± 1.0* | 16.2 ± 2.0ns | 19.1 ± 1.3ns | ||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426 426 |
Zt (μM) | 9.9 ± 0.8 | 11.5 ± 2.0 | 11.4 ± 1.0 | 11.4 ± 1.0 | 13.2 ± 1.5 | |
| IC50 (μM) | 8.8 ± 0.5* | 13.3 ± 1.1ns | 10.1 ± 1.3ns | 11.3 ± 1.8ns | 11.7 ± 1.0ns | ||
Ile-Pro-Ile-Gln-Tyr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) peptide ratio (on a molar basis)a peptide ratio (on a molar basis)a |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Zt (μM) | na | 28.8 ± 2.5 | 29.5 ± 2.1 | 29.5 ± 2.5 | 37.9 ± 2.0 |
| IC50 (μM) | na | 33.1 ± 5.4ns | 27.2 ± 2.5ns | 26.4 ± 4.0ns | 45.2 ± 6.3* | ||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Zt (μM) | na | 27.1 ± 2.0 | 27.6 ± 0.4 | 27.6 ± 1.8 | 32.8 ± 1.7 | |
| IC50 (μM) | na | 36.9 ± 6.3* | 31.2 ± 3.6ns | 32.2 ± 7.0ns | 36.9 ± 5.3ns | ||
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Zt (μM) | na | 25.6 ± 1.7 | 26.1 ± 1.5 | 26.0 ± 1.3 | 28.9 ± 1.6 | |
| IC50 (μM) | na | 31.2 ± 1.1* | 30.2 ± 6.2ns | 29.0 ± 7.1ns | 30.6 ± 2.0ns | ||
4. Discussion
Confirmatory studies conducted with synthetic peptides, following mass spectrometric identification frequently show that several peptide sequences identified within active fractions of food protein hydrolysates display DPP-IV inhibitory properties.5,6,10,19 This indicates that the overall DPP-IV inhibitory effect seen in food protein hydrolysates originates from a mixture of peptides rather than a single peptide. The isobole methodology has been mainly utilised to study interactive effects between drugs, fertilisers, pesticides and phytochemicals13 with a limited number of examples applied to antimicrobial peptide mixtures.20,21 An additive effect of Sitagliptin (when studied at one level) and peptide mixtures on DPP-IV inhibitory properties has previously been shown.11 However, to our knowledge, study of the effect of drug–peptide and binary peptide mixtures on DPP-IV inhibition has not previously been described using an isobolographic approach.The synthetic substrate, Gy-Pro-pNA, used herein for the DPP-IV inhibitory assay has a different N-terminal amino acid sequence than that of the incretins (His-Ala for GLP-1 and Tyr-Ala for GIP). However, in the case of the synthetic substrate and the incretins, the presence of a Pro or Ala at position P1 is consistent with the sequence of DPP-IV preferred substrates.22,23 Therefore, the results described herein may be extrapolated to a physiological situation where food protein-derived peptides may inhibit DPP-IV, preventing incretin degradation.
Most Sitagliptin/peptide and binary peptide mixtures showed an additive effect (Table 2 and Fig. 4 and 5). However, the Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Pro (1
Trp-Pro (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852) mixture showed an antagonistic effect on DPP-IV inhibition. The extent of apparent IC50 increase compared to Zt was 9% for the Sitagliptin
852) mixture showed an antagonistic effect on DPP-IV inhibition. The extent of apparent IC50 increase compared to Zt was 9% for the Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Pro (1
Trp-Pro (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852) mixture. In the case of the Sitagliptin
852) mixture. In the case of the Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ile-Pro-Ile-Gln-Tyr (1
Ile-Pro-Ile-Gln-Tyr (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426 and 1
426 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852) mixtures, a synergistic effect was seen with a reduction of the IC50 value compared to Zt of 7 and 11%, respectively. Although the peptides studied have different modes of inhibition (competitive, non-competitive, true or substrate-type inhibitor), there did not seem to be a clear trend showing specific types of interactions in the mixtures in one instance or the other. However, it is interesting to note that, the synergistic effect was seen with a mixture of competitive DPP-IV inhibitors (Sitagliptin and Ile-Pro-Ile-Gln-Tyr). While most antagonistic effects involved a non-competitive DPP-IV inhibitor (Trp-Lys and Trp-Pro). In addition, it was not clear why the antagonistic effect was only seen for certain ratios of the DPP-IV inhibitors studied (Table 2). A number of in silico approaches have suggested that non-competitive DPP-IV inhibitors may interact at a secondary binding site located in the neighbourhood of the active site.24,25 Binding of non-competitive inhibitors to a secondary binding site may, in some instances, restrict access to the active site for competitive DPP-IV inhibitors.
852) mixtures, a synergistic effect was seen with a reduction of the IC50 value compared to Zt of 7 and 11%, respectively. Although the peptides studied have different modes of inhibition (competitive, non-competitive, true or substrate-type inhibitor), there did not seem to be a clear trend showing specific types of interactions in the mixtures in one instance or the other. However, it is interesting to note that, the synergistic effect was seen with a mixture of competitive DPP-IV inhibitors (Sitagliptin and Ile-Pro-Ile-Gln-Tyr). While most antagonistic effects involved a non-competitive DPP-IV inhibitor (Trp-Lys and Trp-Pro). In addition, it was not clear why the antagonistic effect was only seen for certain ratios of the DPP-IV inhibitors studied (Table 2). A number of in silico approaches have suggested that non-competitive DPP-IV inhibitors may interact at a secondary binding site located in the neighbourhood of the active site.24,25 Binding of non-competitive inhibitors to a secondary binding site may, in some instances, restrict access to the active site for competitive DPP-IV inhibitors.
Ile-Pro-Ile-Gln-Tyr behaves like a substrate type DPP-IV inhibitor.8 This may explain the overall increase in DPP-IV inhibition seen in the Sitagliptin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ile-Pro-Ile-Gln-Tyr (1
Ile-Pro-Ile-Gln-Tyr (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852 and 2
852 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426) mixtures. Trp-Lys is an hydrophilic and positively charged peptide, while Ile-Pro-Ile-Gln-Tyr (pI 5.5) is negatively charged at the assay pH (8.0). It may be possible that some electrostatic interactions between Trp-Lys and Ile-Pro-Ile-Gln-Tyr may have reduced the amount of inhibitors available for DPP-IV inhibition. Surprisingly, no antagonistic effect was seen with Trp-Arg, which has very similar characteristics to Trp-Lys. An antagonistic effect was also seen in the Ile-Pro-Ile-Gln-Tyr
426) mixtures. Trp-Lys is an hydrophilic and positively charged peptide, while Ile-Pro-Ile-Gln-Tyr (pI 5.5) is negatively charged at the assay pH (8.0). It may be possible that some electrostatic interactions between Trp-Lys and Ile-Pro-Ile-Gln-Tyr may have reduced the amount of inhibitors available for DPP-IV inhibition. Surprisingly, no antagonistic effect was seen with Trp-Arg, which has very similar characteristics to Trp-Lys. An antagonistic effect was also seen in the Ile-Pro-Ile-Gln-Tyr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Trp-Leu (1
Trp-Leu (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture. Both peptides are competitive DPP-IV inhibitors and compete for binding at the same site on DPP-IV. This may explain why an antagonistic effect was seen when Trp-Leu was present at the highest concentration.
2) mixture. Both peptides are competitive DPP-IV inhibitors and compete for binding at the same site on DPP-IV. This may explain why an antagonistic effect was seen when Trp-Leu was present at the highest concentration.
The antagonistic activity of peptide mixtures on DPP-IV inhibition could result in the activity of specific peptides being “masked” by the presence of other peptides. This may have consequences in particular in bioassay driven fractionation approaches where specific fractions may be erroneously disregarded even though they contain relatively potent DPP-IV inhibitory peptides. Similar results have been described where the immunomodulatory properties of an hydrolysate was less than that of its associated isoelectric focusing fractions when tested at the same concentration.26 This was explained by the fact that some peptides may interact through physicochemical interactions,27 making them unavailable as bioactive components.
A well-known example of a food drug interaction is the combination of grapefruit juice and drugs. Furanocoumarin from grapefruit juice has been shown to inhibit the drug metabolising enzyme, cytochrome P450 (CYP) 34A.28 In terms of antidiabetic activity, small animal studies have demonstrated that the ingestion of Leu-Pro-Gln-Asn-Ile-Pro-Pro-Leu (β-casein f70-77, DPP-IV IC50 = 160 μM) or a tryptic β-lactoglobulin hydrolysate containing Val-Ala-Gly-Thr-Trp-Tyr (β-lg f15-20, DPP-IV IC50 = 174 μM) could lower plasma glucose following an oral glucose tolerance test.5,6 Recently, it was shown that a porcine skin gelatin hydrolysate could inhibit plasma DPP-IV in rats as well as reducing serum glucose in the post-prandial phase.29 However, little or no data appears to exist on the effect of foods on the pharmacokinetics of Sitagliptin in vivo following food intake.30 There is therefore a need to evaluate the peptide sequences studied herein in humans to assess their in vivo biological activity. The interactions reported with the Sitaglitpin–peptide mixtures suggest that it may be possible to lower drug intake level when combined with food protein-derived DPP-IV inhibitory peptides. This may help to reduce the possible side-effects associated with drug intake.31
5. Conclusion
A systematic approach has been utilised to study the effect of Sitagliptin/peptide and binary peptide mixtures on DPP-IV inhibition using an isobole methodology. It was shown in most cases that there was an additive effect of the mixtures on overall DPP-IV inhibition. However, in some instances antagonistic or synergistic effects were observed. Since the ability of food protein-derived peptides to inhibit DPP-IV has been demonstrated in vitro, the interactive effects described herein may therefore be relevant to the post-prandial regulation of serum glucose and to the pharmacokinetics of antidiabetic drugs. In addition, the isobolographic approach used herein may aid in the formulation of foods with a desired DPP-IV inhibitory profile which in turn may complement the effects of T2D preventative and therapeutic agents. In vivo studies are required to test these hypotheses.Acknowledgements
The work described herein was supported by Enterprise Ireland under grant number TC2013-0001.Notes and references
- D. J. Drucker, Endocrinology, 2006, 147, 3171–3172 CrossRef CAS PubMed.
- R. J. F. Manders, S. F. E. Praet, R. C. R. Meex, R. Koopman, A. L. de Roos, A. J. M. Wagenmakers, W. H. M. Saris and L. J. C. van Loon, Diabetes Care, 2006, 29, 2721–2722 CrossRef CAS PubMed.
- C. Gaudel, A. B. Nongonierma, S. Maher, S. Flynn, M. Krause, B. A. Murray, P. M. Kelly, A. W. Baird, R. J. FitzGerald and P. Newsholme, J. Nutr., 2013, 143, 1109–1114 CrossRef CAS PubMed.
- M. Morifuji, M. Ishizaka, S. Baba, K. Fukuda, H. Matsumoto, J. Koga, M. Kanegae and M. Higuchi, J. Agric. Food Chem., 2010, 58, 8788–8797 CrossRef CAS PubMed.
- M. Uchida, Y. Ohshiba and O. Mogami, J. Pharm. Sci., 2011, 117, 63–66 CrossRef CAS.
- H. Uenishi, T. Kabuki, Y. Seto, A. Serizawa and H. Nakajima, Int. Dairy J., 2012, 22, 24–30 CrossRef CAS PubMed.
- I. M. E. Lacroix and E. C. Y. Li-Chan, Mol. Nutr. Food Res., 2014, 58, 61–78 CAS.
- A. B. Nongonierma and R. J. FitzGerald, Food Chem., 2014, 145, 845–852 CrossRef CAS PubMed.
- A. B. Nongonierma and R. J. FitzGerald, Peptides, 2013, 39, 157–163 CrossRef CAS PubMed.
- I. M. E. Lacroix and E. C. Y. Li-Chan, Peptides, 2014, 54, 39–48 CrossRef CAS PubMed.
- A. B. Nongonierma and R. J. FitzGerald, Int. Dairy J., 2013, 32, 33–39 CrossRef CAS PubMed.
- R. J. Tallarida, J. Pharmacol. Exp. Ther., 2001, 298, 865–872 CAS.
- P. Prabhakar, A. Kumar and M. Doble, Phytomedicine, 2014, 21, 123–130 CrossRef CAS PubMed.
- P. K. Prabhakar, R. Prasad, S. Ali and M. Doble, Phytomedicine, 2013, 20, 488–494 CrossRef CAS PubMed.
- A. B. Nongonierma and R. J. FitzGerald, Food Chem., 2014, 165, 489–498 CrossRef CAS PubMed.
- R. J. Tallarida, J. Pharmacol. Exp. Ther., 2012, 342, 2–8 CrossRef CAS PubMed.
- R. J. Tallarida, Pain, 2002, 98, 163–168 CrossRef CAS.
- A. B. Nongonierma and R. J. FitzGerald, Food Funct., 2013, 4, 1843–1849 CAS.
- S. T. Silveira, D. Martínez-Maqueda, I. Recio and B. Hernández-Ledesma, Food Chem., 2013, 141, 1072–1077 CrossRef CAS PubMed.
- N. B. Last and A. D. Miranker, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 6382–6387 CrossRef CAS PubMed.
- K. Luna-Ramírez, M.-A. Sani, J. Silva-Sanchez, J. M. Jiménez-Vargas, F. Reyna-Flores, K. D. Winkel, C. E. Wright, L. D. Possani and F. Separovic, Biochim. Biophys. Acta, Biomembr., 2013, 1838, 2140–2148 CrossRef PubMed.
- M. Engel, T. Hoffmann, L. Wagner, M. Wermann, U. Heiser, R. Kiefersauer, R. Huber, W. Bode, H.-U. Demuth and H. Brandstetter, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 5063–5068 CrossRef CAS PubMed.
- K. Kühn-Wache, J. W. Bär, T. Hoffmann, R. Wolf, J.-U. Rahfeld and H.-U. Demuth, Biol. Chem., 2011, 392, 223–231 CrossRef PubMed.
- A. B. Nongonierma, C. Mooney, D. C. Shields and R. J. FitzGerald, Food Chem., 2013, 141, 644–653 CrossRef CAS PubMed.
- S. Lorey, A. Stöckel-Maschek, J. Faust, W. Brandt, B. Stiebitz, M. D. Gorrell, T. Kähne, C. Mrestani-Klaus, S. Wrenger, D. Reinhold, S. Ansorge and K. Neubert, Eur. J. Biochem., 2003, 270, 2147–2156 CrossRef CAS.
- A. Mercier, S. F. Gauthier and I. Fliss, Int. Dairy J., 2004, 14, 175–183 CrossRef CAS PubMed.
- P. E. Groleau, P. Morin, S. F. Gauthier and Y. Pouliot, J. Agric. Food Chem., 2003, 51, 4370–4375 CrossRef CAS PubMed.
- M. F. Paine, W. W. Widmer, H. L. Hart, S. N. Pusek, K. L. Beavers, A. B. Criss, S. S. Brown, B. F. Thomas and P. B. Watkins, Am. J. Clin. Nutr., 2006, 83, 1097–1105 CAS.
- S.-L. Huang, C.-C. Hung, C.-L. Jao, Y.-S. Tung and K.-C. Hsu, J. Funct. Foods, 2014, 11, 235–242 CrossRef CAS PubMed.
- A. Bergman, D. Ebel, F. Liu, J. Stone, A. Wang, W. Zeng, L. Chen, S. Dilzer, K. Lasseter, G. Herman, J. Wagner and R. Krishna, Biopharm. Drug Dispos., 2007, 28, 315–322 CrossRef CAS PubMed.
- C. F. Deacon, Drug Dev. Eval., 2007, 16, 533–545 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4fo00883a |
| This journal is © The Royal Society of Chemistry 2015 |