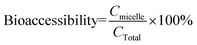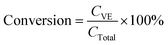Enhancing vitamin E bioaccessibility: factors impacting solubilization and hydrolysis of α-tocopherol acetate encapsulated in emulsion-based delivery systems
Ying
Yang
a,
Eric Andrew
Decker
ab,
Hang
Xiao
a and
David Julian
McClements
*ab
aDepartment of Food Science, University of Massachusetts, Amherst, Massachusetts 01003, USA. E-mail: mcclements@foodsci.umass.edu; Fax: +1 (413) 545-1262; Tel: +1 (413) 545-1019
bDepartment of Biochemistry, Faculty of Science, King Abdulaziz University, P. O. Box 80203, Jeddah 21589, Saudi Arabia
First published on 14th October 2014
Abstract
Oil-soluble vitamins are often encapsulated within emulsion-based delivery systems to facilitate their incorporation into aqueous-based products. We have examined the influence of carrier oil type and simulated small intestinal fluid (SSIF) composition on the bioaccessibility of emulsified vitamin E using a gastrointestinal model. Oil-in-water emulsions containing vitamin E acetate were prepared using bile salts as emulsifier, and either long chain triacylglycerols (glyceryl trioleate, LCT) or medium chain triacylglycerols (glyceryl trioctanoate, MCT) as carrier oils. The addition of calcium (CaCl2) to the SSIF increased the extent of lipid digestion in LCT-emulsions, but had little impact in MCT-emulsions. The bioaccessibility of vitamin E increased in the presence of calcium and phospholipids (DOPC) in LCT-emulsions, but decreased in MCT-emulsions. The highest bioaccessibility (≈ 66%) was achieved for LCT-emulsions when the SSIF contained both calcium and phospholipids. The conversion of α-tocopherol acetate to α-tocopherol after in vitro digestion was considerably higher for LCT-emulsions when calcium ions were present in the SSIF, but was not strongly affected by SSIF composition for MCT-emulsions. In general, this research provides important information about the factors influencing the bioaccessibility of emulsified vitamin E, which could be used to design more effective emulsion-based delivery systems for increasing the oral bioavailability of this important bioactive component.
1. Introduction
The term “vitamin E” refers to a group of naturally occurring compounds that have common molecular, physicochemical, and biological features, with α-tocopherol being the most biological active form.1 As well as its important role as an essential nutrient, vitamin E may also provide additional health benefits, such as reducing cardiovascular disease, diabetes, and cancer due to its antioxidant and non-antioxidant biological activities.2–4 Vitamin E can also protect lipids in foods against oxidation due to the ability of α-tocopherol to trap peroxyl radicals, which are responsible for the initiation of lipid oxidation.5,6 The food industry is therefore interested in fortifying functional foods and beverages with vitamin E due to its antioxidant activity and potential health benefits.7 However, the incorporation of α-tocopherol into many commercial products is a challenge because of its relatively low chemical stability, water-solubility, and bioavailability.2–4Emulsion-based delivery systems are especially suitable for encapsulating, protecting and delivering lipophilic bioactive components, such as ω-3 fatty acids, carotenoids, curcuminoids phytosterols, and oil-soluble vitamins.7–9 A considerable amount of research has already been carried out to identify the major factors affecting the bioavailability of lipophilic bioactive molecules encapsulated within emulsion-based delivery systems, such as particle size, physical state, and interfacial properties.10–13 It is important that any encapsulated bioactive component has a high oral bioavailability so that it can effectively deliver its health benefits after ingestion. However, a number of physicochemical and physiological processes occur within the human gastrointestinal tract that impact the oral bioavailability of lipophilic vitamins.14,15 After ingestion, vitamin E is usually released from the food matrix, solubilized within mixed micelles in the small intestine, and then transported to the epithelium cells where it is absorbed.16,17 Mixed micelles are complex colloidal structures formed from bile salts and phospholipids present in the intestinal fluids, as well as free fatty acids and monoacylglycerols generated by lipid hydrolysis. The bioaccessibility of oil-soluble nutraceuticals, vitamins and drugs has previously been shown to increase when the amount of mixed micelles present within the intestinal fluids increases, which typically occurs as the amount of co-ingested digestible lipids (triacylglycerol hydrolysis products) increases.18–20 The bioaccessibility also depends on the nature of the mixed micelles formed after lipid digestion, i.e., the composition and nature of the colloidal structures formed.19,21–23 Indeed, highly lipophilic components encapsulated using delivery systems containing long chain triglycerides (LCT) have been reported to have a higher bioaccessibility than those containing medium chain triglycerides (MCT), which can be attributed to the ability of the mixed micelles formed by LCT to incorporate larger lipophilic molecules.24,25
The chemical form of the vitamin E present within a food or beverage product also influences its bioavailability. Vitamin E is often incorporated into foods in an esterified form (α-tocopherol acetate) because it has a higher chemical stability than the non-esterified form (α-tocopherol).26 However, the esterified form of vitamin E has a lower bioaccessibility than the free form, presumably because it is more difficult to incorporate into mixed micelles.27–30 Consequently, the bioavailability of vitamin E would be increased if there were greater conversion of α-tocopherol acetate to α-tocopherol in the gastrointestinal tract due to the presence of digestive enzymes.
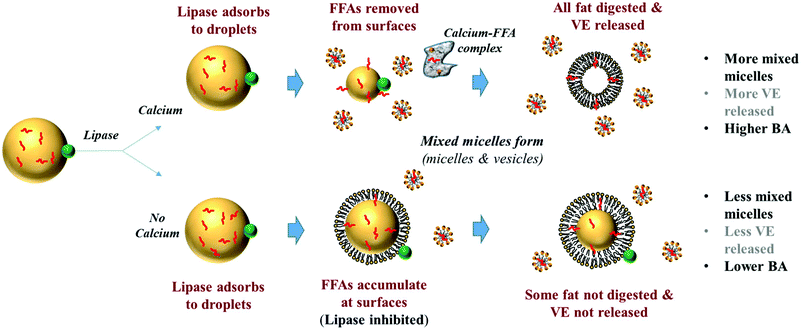
Numerous factors influence the digestion of emulsified triglycerides by pancreatic lipase and the subsequent formation of mixed micelles, including droplet surface area, interfacial composition, carrier oil type, calcium ions, bile salts, and phospholipids.31–33 Calcium ions have been identified as playing a particularly important role in the digestion of emulsified LCTs. In the absence of calcium, long-chain free fatty acids (FFAs) accumulate at the oil–water interface and inhibit further lipid digestion, presumably by preventing lipase from reaching non-digested lipids at the core of the emulsion droplets.34 The presence of calcium ions facilitates lipid digestion due to the formation of insoluble calcium soaps that remove long-chain FFAs from the emulsion droplet surfaces, thereby allowing the lipase to remain in close contact with the non-digested lipids.35–40 One might expect the bioaccessibility of oil-soluble vitamins to increase in the presence of calcium ions since then more LCTs would be digested, leading to the release of more vitamin molecules from the lipid droplets and to the formation of more mixed micelles capable of solubilizing them. On the other hand, calcium ions may interact with mixed micelles and form insoluble complexes that actually reduce the bioaccessibility of oil-soluble vitamins by preventing them from being absorbed.41 The rate and extent of lipid digestion in emulsion-based delivery systems comprised of MCT (rather than LCT) have been shown to be much less sensitive to calcium ions.32 Research is therefore needed to determine the potential effect of carrier oil type and calcium ions on the bioaccessibility of emulsified oil-soluble vitamins.
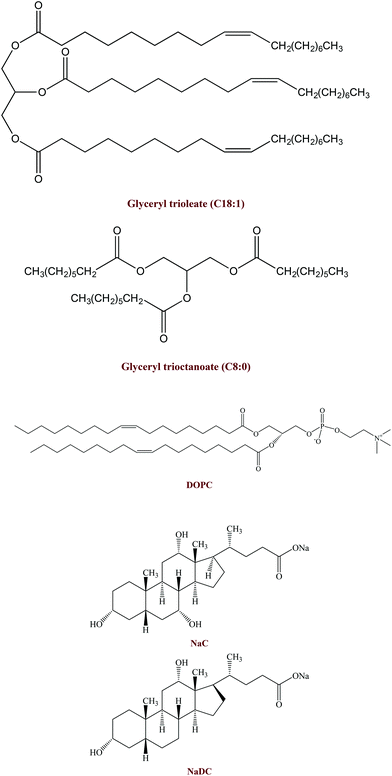
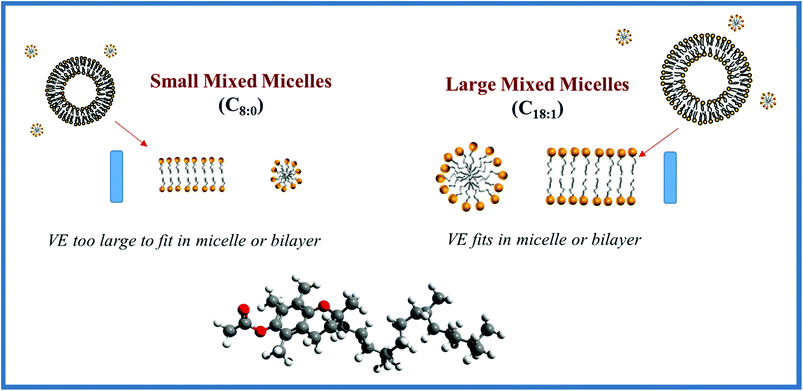
In a recent study using a simulated gastrointestinal tract (GIT) model, we found that the bioaccessibility of vitamin E was higher in emulsions prepared using LCTs than in those prepared using MCTs.24 The LCT (corn oil) and MCT used in that study were food-grade oils containing a mixture of different triacylglycerols. In a follow up study, we used model mixed micelles assembled from well-defined fatty acids (C8:0 or C18:1) (Fig. 1) to provide further insights into the impact of MCT and LCT digestion products on vitamin E solubilization.42 However, we did not find an appreciable difference between the vitamin E solubilization capacity of mixed micelles prepared from C8:0 or C18:1. The apparent discrepancy between these two studies may have been due to differences in the nature of the lipids used or due to differences in the simulated gastrointestinal conditions used. For example, mixed micelles were formed by digesting food-grade MCTs and LCTs in simulated intestinal fluids in the initial study, resulting in a complex mixture of free fatty acids and monoacylglycerols, which would interact with the bile extract. However, the mixed micelles were formed by simply mixing pure free fatty acids (C8:0 or C18:1) with pure bile salts (sodium cholate and sodium deoxycholate) (Fig. 1) in the follow up study.
The purpose of the current study was therefore to use a simulated GIT model to establish the influences of carrier oil type (C8:0 versus C18:1) and small intestinal composition (bile type, calcium, and phospholipids) on the bioaccessibility of emulsified vitamin E (Fig. 1). The information gained from this study will be useful for designing more effective emulsion-based delivery systems for these important lipophilic bioactive components.
2. Materials and methods
2.1 Materials
Sodium cholate (NaC), sodium deoxycholate (NaDC), glyceryl trioleate, and glyceryl trioctanoate were purchased from the Sigma Chemical Company (St Louis, MO). 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC) was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Vitamin E acetate was kindly supplied by BASF (Florham Park, NJ). Lipase from porcine pancreas pancreatin (activity >2.0 USP units mg−1, Type II, L3126, Batch # SLBC9250 V) was obtained from the Sigma Chemical Company (St Louis, MO). Here, 1 USP unit will hydrolyze 1.0 microequivalent of fatty acid from triacetin in 1 h at pH 7.4 at 37 °C. Bile extract (B8631, Batch # MKBQ8333 V) was also obtained from the Sigma Chemical Company (St Louis, MO). This material was reported to contain glycine and taurine conjugates of hyodeoxycholic acid and other bile salts. All other chemicals used were of analytical grade. Double distilled water was used for the preparation of all solutions and emulsions.2.2 Vitamin E emulsion preparation
Vitamin E emulsions were prepared by homogenizing 2.5 wt% lipid phase (Vitamin E acetate–triacylglycerol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 97.5 wt% aqueous phase. The aqueous phase was comprised of surfactant (0.5 wt% bile salt) and buffer solution phosphate-buffered saline, pH 7.0). A coarse emulsion premix was prepared by blending the lipid and aqueous phases together using a high-speed mixer (Bamix, Biospec Products, Bartlesville, OK) for 2 min at room temperature. Fine vitamin E emulsions were formed by passing the coarse emulsions through an air-driven microfluidizer (Microfluidics, Newton, MA, USA). The coarse emulsions were passed through the homogenizer for 4 passes at 9000 psi. The resulting systems were designed to represent the emulsified lipids that are present within the small intestine after ingestion of fatty foods, which typically consist of lipid droplets coated by bile salts (since these biological surfactants typically displace the original surfactants from the lipid droplet surfaces in the gastrointestinal tract).
1) with 97.5 wt% aqueous phase. The aqueous phase was comprised of surfactant (0.5 wt% bile salt) and buffer solution phosphate-buffered saline, pH 7.0). A coarse emulsion premix was prepared by blending the lipid and aqueous phases together using a high-speed mixer (Bamix, Biospec Products, Bartlesville, OK) for 2 min at room temperature. Fine vitamin E emulsions were formed by passing the coarse emulsions through an air-driven microfluidizer (Microfluidics, Newton, MA, USA). The coarse emulsions were passed through the homogenizer for 4 passes at 9000 psi. The resulting systems were designed to represent the emulsified lipids that are present within the small intestine after ingestion of fatty foods, which typically consist of lipid droplets coated by bile salts (since these biological surfactants typically displace the original surfactants from the lipid droplet surfaces in the gastrointestinal tract).
2.3 Particle characterization
Mean particle sizes and particle size distributions of initial emulsions and samples exposed to GIT conditions were measured using static light scattering (Mastersizer 2000, Malvern Instruments, Malvern, UK), while their electrical charge (ζ-potential) was measuring by electrophoretic mobility (Nano-ZS, Malvern Instruments, Worcestershire, UK). The mean particle diameter, particle size distribution, and electrical charge of mixed micelles were determined by dynamic light scattering and electrophoretic mobility (Nano-ZS, Malvern Instruments, Malvern, UK). Samples were equilibrated for 1 min inside the instrument before data were collected over at least 10 sequential readings and analyzed using the Smoluchowski model.2.4 In vitro small intestine digestion
Samples (10 mL) were added to a clean beaker, mixed with 20 mL phosphate-buffered saline (PBS, 10 mM, pH 7.0), incubated in a water bath (37 °C) for 10 min, and then adjusted to pH 7.0 using NaOH solution (range from 0.05 to 1 M). The mixture was then incubated for 2 h at 37 °C with simulated small intestinal fluids (SSIF) of different compositions (Table 1). SSIFs with five different compositions were prepared: bile extract without CaCl2; bile extract with CaCl2; bile salts (NaC and NaDC) without CaCl2; bile salts (NaC and NaDC) with CaCl2; bile salts (NaC and NaDC) with CaCl2 and DOPC. A pH-stat (Metrohm, USA Inc.) was used to monitor and control the pH (at pH 7.0) of the digestion solution after the sample and SSIF were mixed.32 The amount of alkali solution (0.25 M NaOH) that had to be added to the reaction chamber to maintain the pH at 7.0 was recorded, and used to determine the percentage of free fatty acids (FFA) released from the system (McClements & Li, 2010a). A control (containing bile salts but no oil) was run under the same conditions as the samples, and the amount of alkali titrated into the reaction chamber for the control was subtracted from that for the samples before calculating the FFA released. Samples were also taken for physicochemical and structural characterization after 2 h incubation in the small intestinal stage.| SSIF | Bile extract | NaC | NaDC | DOPC | CaCl2 | Lipase |
|---|---|---|---|---|---|---|
| Bile extract | 4 mL (187.5 mg) | 0 | 0 | 0 | 0 | 2.5 mL (60 mg) |
| Bile extract & Ca2+ | 4 mL (187.5 mg) | 0 | 0 | 0 | 1 mL (110 mg) | 2.5 mL (60 mg) |
| Bile salts | 0 | 4 mL (73.22 mg NaC and 114.06 mg NaDC) | 0 | 0 | 2.5 mL (60 mg) | |
| Bile salts & Ca2+ | 0 | 4 mL (73.22 mg NaC and 114.06 mg NaDC) | 0 | 1 mL (110 mg) | 2.5 mL (60 mg) | |
| Bile salts & Ca2+ & DOPC | 0 | 4 mL (73.22 mg NaC, 114.06 mg NaDC and 36 mg DOPC) | 1 mL (110 mg) | 2.5 mL (60 mg) | ||
In this study, each sample was only tested using a simulated small intestine model so that we could focus on the physicochemical events occurring in this region of the gastrointestinal tract (GIT), without having to consider structural or compositional changes occurring in the mouth or stomach phases, which would have complicated interpretation of the results. Nevertheless, it would be useful in future studies to pass samples through a full GIT model (mouth, stomach, and small intestine) to more accurately represent the changes occurring in the human GIT.
2.5 Bioaccessibility determination
Vitamin E is solubilized within mixed micelles present in the intestinal lumen before uptake into intestinal epithelial cells.43 The fraction of lipophilic bioactive compounds solubilized within the mixed micelle phase is usually regarded as the bioaccessibility.44,45 The bioaccessibility of vitamin E was determined using a method described previously.24 Briefly, the digesta resulting from small intestine digestion of the samples was collected and then centrifuged (4000 rpm; CL10 centrifuge, Thermo Scientific, Waltham, MA, USA) at 25 °C for 40 min. Samples after centrifugation separated into an optically opaque sediment phase at the bottom, a relatively clear aqueous phase in the middle, and sometimes a thin oily or creamed phase at the top. The middle phase was assumed to be the “micelle” phase that solubilized the vitamin E. Vitamin E was extracted from the middle phase using an organic solvent mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 isopropanol and isooctane) at 1
3 isopropanol and isooctane) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and then centrifuged at 1750 rpm for another 10 min. One mL of the top layer was removed and dried using nitrogen evaporation and stored in the −80 °C refrigerator prior to further analysis. Before detection by HPLC, samples were dissolved in 200 μL methanol. The vitamin E concentrations in the samples were determined using HPLC (Shimadzu, Kyoto, Japan). A C18 reverse phase column (150–4.6 mm, 5 μm, Beckman Coulter) was used for the chromatographic separation of α-tocopherol acetate and α-tocopherol. The flow rate of the mobile phase was 1.0 mL min−1. An isocratic elution was carried out using HPLC-grade solvent (95% methanol and 5% double distilled water containing 0.5% phosphoric acid). The α-tocopherol acetate and α-tocopherol contents were determined using a PDA detector at 295 nm. Tocopherol quantification was determined using external standards. The overall bioaccessibility of vitamin E was estimated using the following expression:
5 and then centrifuged at 1750 rpm for another 10 min. One mL of the top layer was removed and dried using nitrogen evaporation and stored in the −80 °C refrigerator prior to further analysis. Before detection by HPLC, samples were dissolved in 200 μL methanol. The vitamin E concentrations in the samples were determined using HPLC (Shimadzu, Kyoto, Japan). A C18 reverse phase column (150–4.6 mm, 5 μm, Beckman Coulter) was used for the chromatographic separation of α-tocopherol acetate and α-tocopherol. The flow rate of the mobile phase was 1.0 mL min−1. An isocratic elution was carried out using HPLC-grade solvent (95% methanol and 5% double distilled water containing 0.5% phosphoric acid). The α-tocopherol acetate and α-tocopherol contents were determined using a PDA detector at 295 nm. Tocopherol quantification was determined using external standards. The overall bioaccessibility of vitamin E was estimated using the following expression:Here Cmicelle and CTotal represent the total concentration of vitamin E (α-tocopherol acetate + α-tocopherol) in the micelle phase and in the overall system after digestion, respectively. The percentage of specific forms of vitamin E solubilized within the micelle phase was also calculated using the same expression, but for α-tocopherol acetate and for α-tocopherol separately. The conversion of α-tocopherol acetate to α-tocopherol in the overall system after digestion was calculated from the following expression:
Here CVE and CTotal represent the concentration of α-tocopherol and the total concentration of vitamin E (α-tocopherol acetate + α-tocopherol) in the overall system after digestion, respectively. A preliminary experiment was carried out to estimate the recovery of the total tocopherols using the solvent extraction and HPLC analysis method described above. The recovery of the total tocopherols (VE + VE acetate) was always >90%, which indicates that the methods used were appropriate.
2.6 Statistical analysis
All measurements were performed on at least two freshly prepared samples (i.e., new samples were prepared for each series of experiments), and two separate measurements were made per sample, leading to four measurements in total. The means and standard deviations were calculated from this data. Statistical differences were performed by ANOVA analysis.3. Results and discussion
3.1 Impact of SSIF composition and carrier oil on gastrointestinal fate of emulsions
Initially, we studied the influence of SSIF composition and carrier oil type on the potential gastrointestinal fate of emulsion-based delivery systems using a simulated small intestine model (pH-stat). Vitamin-fortified emulsions containing 2.5 wt% lipid phase were produced using either long chain triglycerides (C18:1) or medium chain triglycerides (C8:0) as carrier oil. The emulsions were then mixed with SSIFs with different compositions: bile extract (with and without CaCl2); pure bile salts (with and without CaCl2); and, pure bile salts with CaCl2 and phospholipids (DOPC). The influence of SSIF composition and carrier oil type on particle characteristics after digestion were then measured.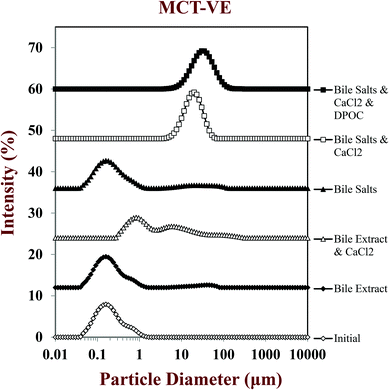 | ||
| Fig. 3 Influence of carrier oil type and simulated small intestine fluid composition on the particle size distribution of MCT-VE emulsions passing through the simulated small intestine tract. | ||
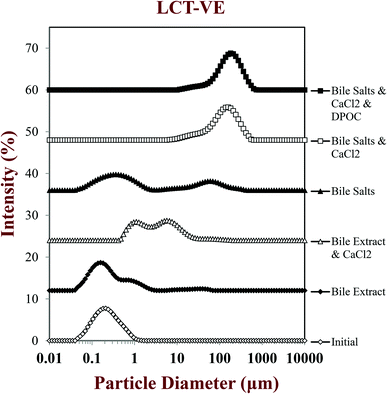 | ||
| Fig. 4 Influence of carrier oil type and simulated small intestine fluid composition on the particle size distribution of LCT-VE emulsions passing through the simulated small intestine tract. | ||
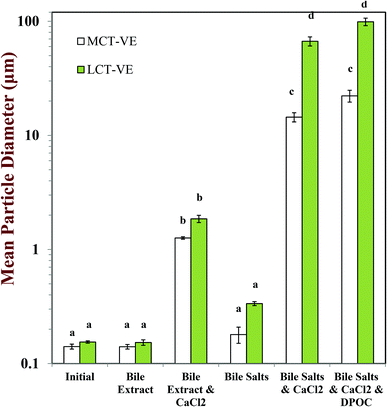
After passage through the simulated small intestinal stages, the mean particle diameters of all samples increased and there was evidence of large particles in the particle size distributions (Fig. 2–4). The composition of the simulated small intestinal fluids (SSIFs) had a pronounced influence on the particle size of the emulsions after digestion. The presence of bile extract in the SSIF caused little change in particle size, but the presence of pure bile salts (NaC and NaDC) caused an appreciable increase in particle size. The presence of calcium ions in the SSIFs caused a large increase in mean particle diameter (Fig. 2) and there was evidence of large particles in the particle size distributions for both MCT- and LCT-emulsions (Fig. 3 and 4). The presence of these large particles indicates a marked change in the structure of the systems after exposure to small intestinal conditions, which may be due to several physicochemical phenomena. The pancreatic lipase in the SSIFs will adsorb to the lipid droplet surfaces and convert the triacylglycerols (TAGs) into free fatty acids (FFAs) and monoacylglycerols (MAGs). The products of lipid hydrolysis may move into the surrounding aqueous phase or remain at the droplet surfaces depending on their water-dispersibility, which is related to their chain length.19,22 Long chain FFAs tend to remain at the droplet surface (in the absence of bile salts or calcium), whereas short and medium chain FFAs tend to move into the aqueous phase. Lipid digestion may therefore reduce the size of the initial lipid droplets due to removal of FFA and MAG digestion products from their surfaces.14,15 On the other hand, partially digested lipid droplets may be more prone to droplet coalescence due to the change in their interfacial properties, which would lead to an increase in particle size.14,15 It should also be noted that the light scattering instrument is sensitive to all kinds of particles that scatter light within the sample, which includes any mixed micelles or insoluble calcium complexes formed after digestion.40
The measured particle size did not change much after vitamin-fortified MCT- or LCT-emulsions were exposed to SSIFs in the absence of calcium, regardless of whether bile extract or pure bile salts were used (Fig. 2–4). This may have occurred because some of the lipid droplets were not digested and retained their original size, but this is unlikely since the pH-stat measurements (described below) indicated that lipid digestion had occurred. It is therefore possible that the mixed micelles formed by lipid digestion were of a similar size to the original lipid droplets in the system.
In general, the droplet sizes were appreciably larger when the SSIFs contained bile salts than when they contain bile extract (Fig. 2). Bile extract from porcine is a complex mixture that contains various bile salts, phospholipids and other components, whereas the bile salts only contained pure NaC and NaDC. Hence, the concentration of actual bile salts in the system would be higher for the pure bile salts than for the bile extract, which may have led to the formation of more insoluble complexes with calcium. In addition, the mixed micelles formed by pure bile salts may have been larger than those formed by bile extract, e.g., pure bile salts may have formed large vesicle structures, whereas some of the components in bile extract may have promoted disruption of these structures. Nevertheless, further work is required using microscopy methods to identify the precise nature of the mixed micelles formed by different sources of bile salts.
Another complication associated with interpreting the results of light scattering measurements in complex colloidal dispersions is associated with data analysis. The software used to calculate the particle size distribution of a colloidal dispersion from its light scattering pattern usually assumes that the particles are spherical, dilute, and have well-defined refractive indices. However, the colloidal dispersions resulting from lipid digestion contain a complex mixture of particles with different compositions and structures, such as undigested lipid droplets, partially digested lipid droplets, micelles, vesicles, and various other colloidal structures. Light scattering results should therefore be treated with some caution for this type of complex colloidal dispersion.
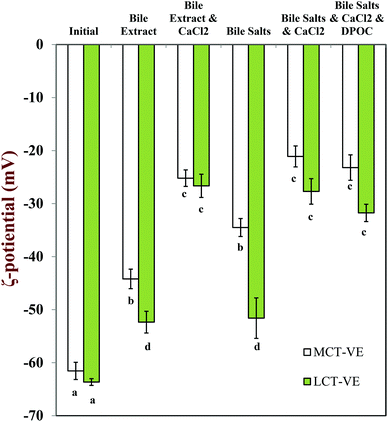
The charge on the particles present in the digesta was less negative in the presence of calcium ions (−21 to −32 mV) than in their absence (−44 to −52 mV) for both carrier oil types, which can be attributed to binding of cationic Ca2+ ions to the surfaces of the anionic droplets and mixed micelles, as well as some electrostatic screening effects.47 The negative charge was higher on the particles present in LCT-emulsions than those present in MCT-emulsions after digestion (Fig. 5) which may be due to the fact that glyceryl trioleate contains long chain fatty acids (C18:1) that accumulate at oil–water interfaces, whereas glyceryl trioctanoate contains medium chain fatty acids (C8:0) that tend to move into the surrounding aqueous phase.48
It should be noted that it is not clear exactly what types of particles are detected by an electrophoresis instrument in a complex colloidal dispersion that contains different types of charged particles that scatter light. Moreover, since measurements were made on diluted and stirred samples, the nature of the particles in the measurement cells might be different from the particles in the original undiluted samples. One should therefore be cautious when interpreting the results from electrophoresis measurements on this type of complex colloidal dispersion.
3.2 Impact of SSIF composition and carrier oil type on lipid digestion
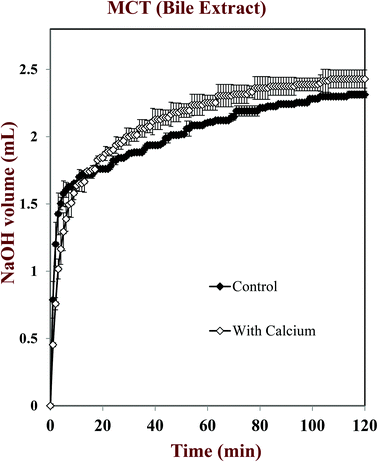
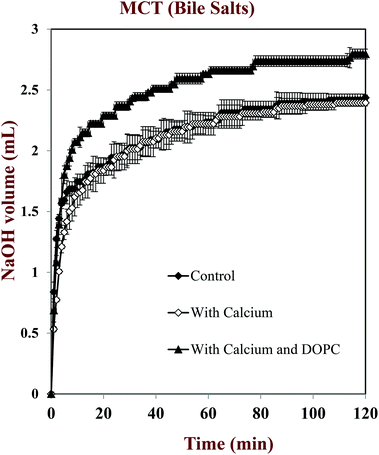
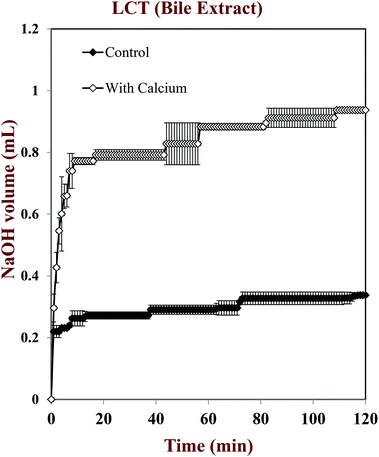
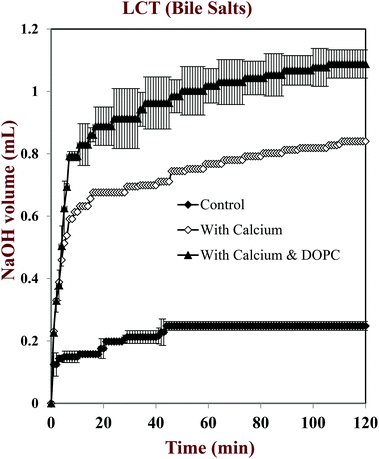
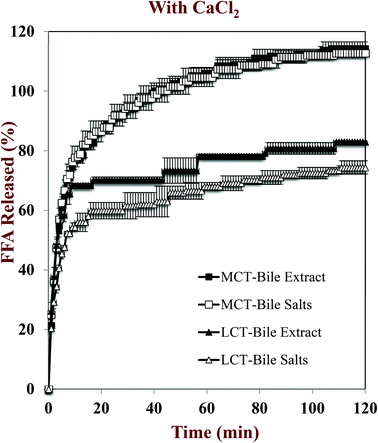
In this part of the study, the influence of carrier oil type and SSIF composition on the rate and extent of lipid digestion was measured using the pH-stat method, which is widely used in pharmaceutical and food research for this purpose.32,35,49–53 The principle of the pH-stat method is to measure the volume of alkaline solution (0.25 M NaOH) required to neutralize the free fatty acids (FFAs) released from a sample when incubated in SSIFs containing lipase. This information is then used to calculate the percentage of FFAs released from the sample, assuming that a maximum of two FFAs are released per triglyceride molecule.Generally, there was a rapid initial increase in the volume of NaOH added to the emulsions during the first few minutes of incubation in SSIFs, followed by a more gradual increase at longer times (Fig. 6–10). This result suggests that lipases in the SSIFs were able to quickly adsorb to the lipid droplet surfaces and convert encapsulated TAGs into FFAs and MAGs. Nevertheless, vitamin-fortified emulsions prepared using different types of carrier oil exhibited quite different behavior. In the absence of calcium, the rate and extent of lipid digestion was appreciably higher for MCT-emulsions than for LCT-emulsions (Fig. 6–10), which is in good agreement with previous studies.19,22,31 As mentioned earlier, long-chain FFAs accumulate at lipid droplet surfaces in the absence of calcium ions,48 which inhibits digestion by preventing lipase molecules from reaching non-digested TAGs at the core of the lipid droplets.34
The influence of calcium and phospholipid addition on the titration of FFAs during in vitro digestion was also studied (Fig. 6–10). For MCT-emulsions, the presence of calcium ions in the SSIF did not cause an appreciable alteration in lipid digestion (Fig. 6 and 7). However, for LCT-emulsions, the addition of calcium ions led to an appreciable increase in the final amount of lipid digestion products generated (Fig. 8 and 9). These measurements clearly show that calcium ions have a major impact on lipid digestion in LCT-emulsions but not in MCT-emulsions, which is in agreement with previous research.40 This result may be explained by a number of physicochemical mechanisms. First, a certain amount of calcium is required as a co-factor to activate pancreatic lipase.54,55 Thus, the lower extent of FFA production in the absence of calcium may be partly due to the fact that the enzyme was not in its most active form. However, this is unlikely to be important because lipid digestion still occurred in the MCT-emulsions in the absence of calcium. Second, calcium ions bind to long-chain FFAs generated during the digestion of emulsified LCT and form insoluble calcium soaps that remove them from the droplet surfaces.35,36,39,40 The precipitation of these long-chain fatty acids enables lipase molecules to come into close contact with the remaining non-digested lipids and facilitate their digestion.56 The digestion of emulsified MCT is less dependent on calcium ions because the lipid digestion products (medium-chain FFAs) are more water-dispersible and rapidly move into the surrounding aqueous phase, thereby enabling lipase to continue operating at the droplet surfaces. Calcium ions may also impact lipid digestion by affecting other characteristics of emulsions, such as droplet aggregation.57,58 Anionic lipid droplets may become flocculated in the presence of cationic calcium ions due to ion binding and electrostatic scattering screening effects, which may reduce the ability of lipase to interact with the lipid droplet surfaces.59,60
For both MCT-VE and LCT-VE emulsions, the addition of phospholipids (DOPC) into the SSIFs increased the final extent of lipid digestion. This may have occurred because phospholipids facilitated the ability of the lipase to interact with the emulsified TAGs, or because the phospholipids were themselves hydrolyzed and released FFAs. Based on the amount of DOPC (36 mg) present in the SSIF, and the assumption that one FFA is released per phospholipid molecule, we calculated that about 0.18 mL of 0.25 M NaOH would be required to neutralize any fatty acids produced due to phospholipid hydrolysis. This value is quite close to the difference between the volumes of NaOH required to neutralize calcium-containing samples in the presence and absence of DOPC (Fig. 7 and 9). We therefore conclude that the increased amount of alkali required for the samples containing DOPC is mainly due to the hydrolysis of the phospholipid by digestive enzymes in the SSIFs.
3.3 Impact of SSIF composition and carrier oil type on vitamin E bioaccessibility
The impact of SSIF composition and carrier oil type on the bioaccessibility of vitamin E after passage through the simulated small intestine was also examined. The bioaccessibility was determined by incubating the emulsions in SSIFs for 2 hours, centrifuging the resulting digesta, and then determining the concentration of vitamin E in the micelle phase and overall digesta using HPLC.The overall appearance of the digesta after exposure to SSIFs depended on calcium content (Fig. 11). In the absence of calcium, samples separated into a thick white layer at the top (“cream”), a clear or slightly turbid layer in the middle (“micelle phase”), and a thin white layer at the bottom (“sediment”). The white layer at the top probably consisted of non-digested fat droplets and possibly some large mixed micelle structures (which are less dense than water), while the white layer at the bottom probably contained insoluble matter such as bile salt or protein complexes (which are denser than water). In the presence of calcium, we only observed a single white layer (“sediment”) at the bottom of the samples with a clear or slightly turbid layer above (“micelle phase”). The fact that a cream layer was not observed in the samples containing calcium can be attributed to two factors. First, calcium promoted digestion of the lipid phase (Fig. 6–9), and so there would be less non-digested lipid droplets present. Second, cationic calcium ions (Ca2+) formed dense insoluble aggregates with anionic species, such as bile salts and free fatty acids, which sedimented to the bottom of the tubes.
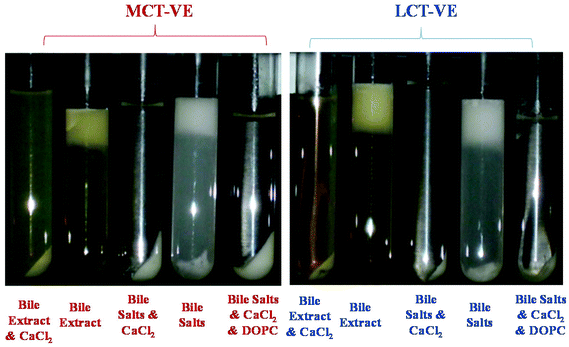 | ||
| Fig. 11 Influence of carrier oil type and SSIF composition on the appearance of the micelle phase after in vitro digestion of vitamin E fortified emulsions. | ||
The mixed micelles formed in the human body are compositionally and structurally complex colloidal dispersions whose properties depend on the nature of any co-ingested lipids.18,19 Mixed micelles contain bile salts, phospholipids, and cholesterol from the small intestinal fluids, as well as monoacylglycerols (MAG) and free fatty acids (FFAs) from any lipid digestion products. These surface-active lipids self-assemble into the mixed micelle phase, which may contain micelles, vesicles, and liquid crystalline phases that vary in composition, dimensions, and structure.61,62 The micelle phase was therefore passed through a 450 nm pore size filter before measuring the bioaccessibility to more closely simulate gastrointestinal conditions.63,64 Lipophilic bioactive components solubilized within mixed micelles must pass through the mucus layer before they can be absorbed by the human body. The mucus layer acts as a biological filter that only allows particles smaller than about 400 nm to pass through.63 Filtering the micelle layer prior to analysis may therefore provide a more accurate representation of the potential bioavailability of a lipophilic compound that needs to be transported by mixed micelles through the mucus layer.
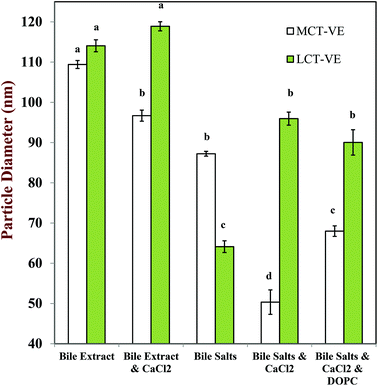
In the presence of calcium ions, the size of the particles in the micelle phases collected from the LCT-emulsions were slightly larger than those collected from the MCT-emulsions (Fig. 12), which suggests that the colloidal structures in the micelle phase formed by LCT digestion products were larger than these formed by MCT digestion products. One possible explanation for this observation is that long chain fatty acids form more vesicles or liquid crystals (which are larger than simple micelles) than medium chain fatty acids, but microscopy analysis of the mixed micelle phases would be required to demonstrate this.
The nature of the bile salts present in the SSIFs also had an influence on the size of the structures formed in the mixed micelle phase. The particle size was larger when the SSIF contained bile extract, than when it contained pure bile salts (Fig. 12). Bile extract contains a mixture of bile salts, phospholipids, and other components,40,65 which may have led to the formation of larger mixed micelles. Indeed, when phospholipids (DOPC) were added to the SSIF containing bile salts and calcium ions there was an appreciable increase in the size of the colloidal structures present in the micelle phase (Fig. 12). In addition, bile extract may have contained some insoluble matter that also contributed to the light scattering signal.
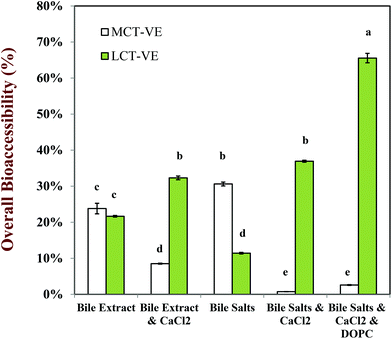
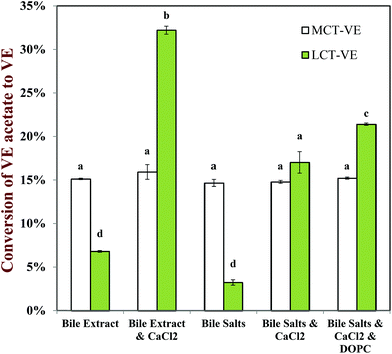
The bioaccessibility of the vitamin E was determined by measuring the concentrations of α-tocopherol acetate and α-tocopherol in the micelle phase and within the total digesta after in vitro digestion (Fig. 13). Calcium ions had a major impact on the bioaccessibility of vitamin E, which depended strongly on the nature of the carrier oil used. For the MCT-emulsions, the addition of calcium ions to the SSIF led to an appreciable decrease in the bioaccessibility of vitamin E, regardless of the nature of bile salts used. For example, the vitamin bioaccessibility decreased from around 24% to 9% when calcium was added to SSIFs containing bile extract, whereas it decreased from around 31% to 1% when calcium was added to SSIFs containing pure bile salts. It is possible that cationic calcium ions formed insoluble precipitates with mixed micelles containing solubilized vitamin E, thereby reducing the amount of vitamin E present within the micelle phase. Conversely, for the LCT-emulsions, the addition of calcium ions to the SSIF led to an appreciable increase in the bioaccessibility of vitamin E. For example, the vitamin bioaccessibility increased from around 22% to 32% when calcium was added to SSIFs containing bile extract, whereas it increased from around 11% to 37% when calcium was added to SSIFs containing pure bile salts. In addition, there was a further increase (to around 66%) when phospholipids (DOPC) were incorporated into the SSIFs for the LCT-emulsions. Previous researchers have also reported that phospholipids can increase the bioavailability of lipophilic bioactive components.66–69 The solubilization of lipophilic components in the micelle phase usually depends on the total amount of mixed micelles available for transporting them across the mucus layer. Our pH-stat experiments show that a higher amount of lipid digestion products (FFAs and MAGs) are formed when calcium is present during the digestion of LCT-emulsions, and so there should be a greater amount of mixed micelles present to solubilize the vitamin E. In addition, a greater amount of vitamin E should have been released from the lipid droplets when more TAGs were digested. The addition of calcium ions to the LCT-emulsions may therefore increase the amount of vitamin E in the mixed micelle phase. Nevertheless, one might still expect the calcium ions to cause some precipitation of the mixed micelles (as with the MCT-emulsions), which would reduce the amount of vitamin E in the micelle phase. Our results suggest that the greater release of vitamin E from the lipid droplets and the higher amount of mixed micelles formed in the presence of calcium, outweigh the precipitation effect for the LCT-emulsions. These results may have important implications for the design of effective emulsion-based delivery systems for vitamin E. Calcium is normally present within the fluids secreted by the human gastrointestinal tract. This calcium may reduce the bioavailability of vitamin E delivered in MCT-emulsions, which might be overcome by incorporating calcium chelating agents in the delivery system (such as EDTA or alginate). On the other hand, calcium may increase the bioavailability of vitamin E in LCT-emulsions, and therefore it may be advantageous to avoid the presence of calcium chelating agents in these delivery systems or to supplement them with additional calcium.
Under realistic digestion conditions (i.e., samples containing bile salts, calcium and phospholipids), the bioaccessibility of vitamin E was appreciably higher when it was encapsulated in LCT-emulsions than in MCT-emulsions (Fig. 13). The long-chain free fatty acids (C18:1) arising from the lipolysis of glycerol trioleate (LCT) can presumably form colloid structures with a larger solubilization capacity than the medium chain fatty acids (C8:0) generated from the hydrolysis of glycerol trioctanoate (MCT). The α-tocopherol molecule has a non-polar chain with 14 carbon atoms (C14), which is presumably too long to be accommodated into the micelles or vesicles formed by medium-chain fatty acids, but short enough to be incorporated into those formed by long chain fatty acids. Researchers in the pharmaceutical area have also reported that the bioaccessibility of some highly oil-soluble drugs is greater when LCT was used as a carrier oil rather than MCT.70,71 It should be noted that it is the dimensions of the lipophilic structures within the mixed micelle phase that can incorporate α-tocopherol molecules, such as the hydrophobic core of simple micelles or the bilayers of vesicles, rather than their overall dimensions that is important. For example, a vesicle can have relatively large overall dimensions (>100 nm), but it can only accommodate a lipophilic molecule if it can fit within the bilayers formed by the fatty acid tails (a few nm).
3.4 Impact of SSIF composition and carrier oil type on vitamin E conversion
The esterified form of α-tocopherol is often used in foods and other commercial products rather than the free form because it is more stable to oxidation during processing, transportation and storage.72,73 Previous research has shown that the molecular form of vitamin E has a major impact on the bioaccessibility of vitamin E, i.e., α-tocopherol versus α-tocopherol acetate.27–30 Thus, we determined the amount of α-tocopherol acetate converted to α-tocopherol after in vitro digestion (Fig. 14). Our previous research using food-grade oils showed that carrier oil type had an appreciable impact on the hydrolysis of α-tocopherol acetate to α-tocopherol, with the extent of conversion being about 29% for LCT-emulsions and 17% for MCT-emulsions.24 This result suggests that the conversion of α-tocopherol acetate to α-tocopherol occurred more readily when LCT was used as the carrier oil than when MCT was used.24 In the current study, we used purified LCT and MCT carrier oils to provide further insights into this important effect.For MCT-emulsions, the SSIF composition did not have an appreciable impact on the conversion of α-tocopherol acetate to α-tocopherol (Fig. 14). Conversely, for LCT-emulsions, the SSIF composition had a major impact on α-tocopherol acetate hydrolysis. For the LCT-emulsions, the conversion of α-tocopherol acetate to α-tocopherol increased when calcium and phospholipid were incorporated into the SSIFs (Fig. 14). There therefore appeared to be a correlation between the bioaccessibility of vitamin E and the hydrolysis of α-tocopherol acetate. It is likely that α-tocopherol acetate can only be hydrolyzed by digestive enzymes after it is released from the interior of the fat droplets.74 Hydrolysis may occur at the lipid droplet surfaces or after the α-tocopherol acetate is incorporated into mixed micelles, which would account for the increase in hydrolysis with increasing bioaccessibility. This effect may also account for that fact that the extent of hydrolysis was greater for the LCT-emulsions than the MCT-emulsions in the presence of calcium ions (Fig. 14).
4. Conclusions
The purpose of this study was to identify the key factors impacting the bioaccessibility of emulsified α-tocopherol acetate using a simulated small intestine model. We have shown that the rate and extent of lipid digestion was higher for MCT-emulsions than for LCT-emulsions, which was attributed to differences in the water-dispersibility of the medium and long chain fatty acids formed during lipolysis. The addition of calcium ions to the SSIFs greatly increased the extent of lipid digestion for LCT-emulsions, but had little effect on MCT-emulsions, which was attributed to the ability of calcium ions to remove long-chain fatty acids from droplet surfaces. The addition of calcium ions and phospholipids into the SSIFS also had a major impact on the bioaccessibility of vitamin E depending on carrier oil type. The addition of calcium ions greatly improved the bioaccessibility of vitamin E in LCT-emulsions, but reduced it in MCT-emulsions. Finally, calcium addition increased the conversion of α-tocopherol acetate to α-tocopherol after in vitro digestion of LCT-emulsions, but had little effect on α-tocopherol acetate hydrolysis in MCT-emulsions. A schematic representation of the important physicochemical events occurring with the gastrointestinal tract based on our results is shown in Fig. 15 and 16.In summary, our results suggest that the bioaccessibility of vitamin E encapsulated in emulsion-based delivery systems is strongly influence by carrier oil type, bile salt type, calcium ions, and phospholipids. This information is important for developing effective emulsion-based delivery systems for oil-soluble vitamins and testing their potential efficacy.
Acknowledgements
The authors thank BASF for providing the vitamin E acetate used in these experiments. This material is based upon work supported by the Cooperative State Research, Extension, Education Service, United State Department of Agriculture, Massachusetts Agricultural Experiment Station and United States Department of Agriculture, CREES, NRI, AFRI (2011-03539, 2013-03795, 2011-67021, and 2014-67021) grants.References
- M. Meydani, Lancet, 1995, 345, 170–175 CrossRef CAS.
- R. Brigelius-Flohé and F. Galli, Mol. Nutr. Food Res., 2010, 54, 583–587 Search PubMed.
- G. W. Burton and M. G. Traber, Annu. Rev. Nutr., 1990, 10, 357–382 CrossRef CAS PubMed.
- J.-M. Zingg and A. Azzi, Curr. Med. Chem., 2004, 11, 1113–1133 CrossRef CAS.
- E. Niki, T. Saito, A. Kawakami and Y. Kamiya, J. Biol. Chem., 1984, 259, 4177–4182 CAS.
- E. Niki, Y. Yamamoto, E. Komuro and K. Sato, Am. J. Clin. Nutr., 1991, 53, 201S–205S CAS.
- L. Sagalowicz and M. E. Leser, Curr. Opin. Colloid Interface Sci., 2010, 15, 61–72 CrossRef CAS PubMed.
- D. J. McClements, Annu. Rev. Food Sci. Technol., 2010, 1, 241–269 CrossRef CAS PubMed.
- K. P. Velikov and E. Pelan, Soft Matter, 2008, 4, 1964–1980 RSC.
- P. Grolier, S. Agoudavi and V. Azaisbraesco, Nutr. Res., 1995, 15, 1507–1516 CrossRef CAS.
- A. M. Nik, S. Langmaid and A. J. Wright, Food Funct., 2012, 3, 234–245 CAS.
- A. M. Nik, A. J. Wright and M. Corredig, J. Am. Oil Chem. Soc, 2011, 88, 1397–1407 CrossRef.
- H. S. Ribeiro, J. M. M. Guerrero, K. Briviba, G. Rechkemmer, H. P. Schuchmann and H. Schubert, J. Agric. Food Chem., 2006, 54, 9366–9369 CrossRef CAS PubMed.
- D. J. McClements, E. A. Decker and Y. Park, Crit. Rev. Food Sci. Nutr., 2009, 49, 48–67 CrossRef PubMed.
- H. Singh, A. Q. Ye and D. Horne, Prog. Lipid Res., 2009, 48, 92–100 CrossRef CAS PubMed.
- A. Rigotti, Mol. Aspects Med., 2007, 28, 423–436 CrossRef CAS PubMed.
- D. Ullrey, J. Anim. Sci., 1972, 35, 648–657 CAS.
- C. J. H. Porter, C. W. Pouton, J. F. Cuine and W. N. Charman, Enhancing intestinal drug solubilisation using lipid-based delivery systems, San Antonio, TX, 2006 Search PubMed.
- C. J. H. Porter, N. L. Trevaskis and W. N. Charman, Nat. Rev. Drug Discovery, 2007, 6, 231–248 CrossRef CAS PubMed.
- C. Chitchumroonchokchai, K. Kamonpatana, M. Feruzzi, E. Harrison and M. Failla, Ann. Nutr. Metab., 2009, 55, 198–198 Search PubMed.
- T. Huo, M. G. Ferruzzi, S. J. Schwartz and M. L. Failla, J. Agric. Food Chem., 2007, 55, 8950–8957 CrossRef CAS PubMed.
- C. W. Pouton and C. J. H. Porter, Adv. Drug Delivery Rev., 2008, 60, 625–637 CrossRef CAS PubMed.
- M. L. Failla, C. Chitchumronchokchai, M. G. Ferruzzi, S. R. Goltz and W. W. Campbell, Food Funct., 2014, 5, 1101–1112 CAS.
- Y. Yang and D. J. McClements, Food Chem., 2013, 141, 473–481 CrossRef CAS PubMed.
- C. Qian, E. A. Decker, H. Xiao and D. J. McClements, Food Chem., 2012, 135, 1440–1447 CrossRef CAS PubMed.
- C. Lauridsen, M. S. Hedemann and S. K. Jensen, J. Nutr. Biochem., 2001, 12, 219–224 CrossRef CAS.
- Y. Chung, D. Mahan and A. Lepine, J. Anim. Sci., 1992, 70, 2485–2492 CAS.
- S. Eicher, J. Morrill and J. Velazco, J. Dairy Sci., 1997, 80, 393–399 CrossRef CAS.
- N. Hidiroglou, L. Laflamme and L. McDowell, J. Anim. Sci., 1988, 66, 3227–3234 CAS.
- N. Hidiroglou, L. McDowell and R. Pastrana, Int. J. Vitam. Nutr. Res., 1988, 58, 189 CAS.
- Y. Li, M. Hu and D. J. McClements, Food Chem., 2011, 126, 498–505 CrossRef CAS PubMed.
- Y. Li and D. J. McClements, J. Agric. Food Chem., 2010, 58, 8085–8092 CrossRef CAS PubMed.
- A. M. Nik, A. J. Wright and M. Corredig, Colloids Surf., B, 2011, 83, 321–330 CrossRef PubMed.
- G. Fave, T. Coste and M. Armand, Cell. Mol. Biol., 2004, 50, 815–831 CAS.
- M. Armand, P. Borel, P. Ythier, G. Dutot, C. Melin, M. Senft, H. Lafont and D. Lairon, J. Nutr. Biochem., 1992, 3, 333–341 CrossRef CAS.
- S. Hwang, S. Lee, I.-S. Ahn and J.-K. Jung, Biocatal. Biotransform., 2009, 27, 290–295 CrossRef CAS.
- J. Patton, R. Vetter, M. Hamosh, B. Borgstrom, M. Lindstrom and M. Carey, Food Microstruct., 1985, 29–41 CAS.
- J. S. Patton and M. C. Carey, Science, 1979, 204, 145–148 CAS.
- N. H. Zangenberg, A. Müllertz, H. Gjelstrup Kristensen and L. Hovgaard, Eur. J. Pharm. Sci., 2001, 14, 237–244 CrossRef CAS.
- N. H. Zangenberg, A. Müllertz, H. G. Kristensen and L. Hovgaard, Eur. J. Pharm. Sci., 2001, 14, 115–122 CrossRef CAS.
- J. K. Lorenzen, S. Nielsen, J. J. Holst, I. Tetens, J. F. Rehfeld and A. Astrup, Am. J. Clin. Nutr., 2007, 85, 678–687 CAS.
- Y. Yang and D. J. McClements, J. Colloid Interface Sci., 2013, 405, 312–321 CrossRef CAS PubMed.
- M. G. Traber, I. Goldberg, E. Davidson, N. Lagmay and H. J. Kayden, Gastroenterology, 1990, 98, 96–103 CAS.
- S. Marze, Crit. Rev. Food Sci. Nutr., 2012, 53, 76–108 CrossRef PubMed.
- E. Reboul, M. Richelle, E. Perrot, C. Desmoulins-Malezet, V. Pirisi and P. Borel, J. Agric. Food Chem., 2006, 54, 8749–8755 CrossRef CAS PubMed.
- J. Maldonado-Valderrama, P. Wilde, A. Macierzanka and A. Mackie, Adv. Colloid Interface Sci., 2011, 165, 36–46 CrossRef CAS PubMed.
- D. McClements, Food Emulsions: Principles, Practices, and Techniques, CRC Press, Boca Raton, Florida, 2005 Search PubMed.
- L. Sek, B. J. Boyd, W. N. Charman and C. J. Porter, J. Pharm. Pharmacol., 2006, 58, 809–820 CrossRef CAS PubMed.
- A. Dahan and A. Hoffman, Pharm. Res., 2006, 23, 2165–2174 CrossRef CAS PubMed.
- A. Dahan and A. Hoffman, J. Controlled Release, 2008, 129, 1–10 CrossRef CAS PubMed.
- N. O. Nilsson and P. Belfrage, J. Lipid Res., 1979, 20, 557–560 CAS.
- C. J. H. Porter, N. L. Trevaskis and W. N. Charman, Nat. Rev. Drug Discovery, 2007, 6, 231–248 CrossRef CAS PubMed.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carriere, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, S. Marze, D. J. McClements, O. Menard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and A. Brodkorb, Food Funct., 2014, 5, 1113–1124 CAS.
- T. F. Whayne and J. M. Felts, Circ. Res., 1971, 28, 649–654 CrossRef CAS.
- H. Kimura, Y. Futami, T. Sei-ichiro and T. Shinomiya, J. Biochem., 1982, 92, 243–251 CAS.
- K. J. MacGregor, J. K. Embleton, J. E. Lacy, E. A. Perry, L. J. Solomon, H. Seager and C. W. Pouton, Adv. Drug Delivery Rev., 1997, 25, 33–46 CrossRef CAS.
- A. Ye, J. Lo and H. Singh, J. Colloid Interface Sci., 2012, 378, 184–190 CrossRef CAS PubMed.
- E. Dickinson, Food Hydrocolloids, 2011, 25, 1966–1983 CrossRef CAS PubMed.
- H. Singh, A. Ye and D. Horne, Prog. Lipid Res., 2009, 48, 92–100 CrossRef CAS PubMed.
- M. Golding and T. J. Wooster, Curr. Opin. Colloid Interface Sci., 2010, 15, 90–101 CrossRef CAS PubMed.
- J. Patton, Physiol. Gastrointest. Tract, 1981, 2, 1123–1139 Search PubMed.
- Y. F. Shiau, Physiol. Gastrointest. Tract, 1987, 2, 2 Search PubMed.
- R. A. Cone, Adv. Drug Delivery Rev., 2009, 61, 75–85 CrossRef CAS PubMed.
- L. M. Ensign, R. Cone and J. Hanes, Adv. Drug Delivery Rev., 2012, 64, 557–570 CrossRef CAS PubMed.
- P. Augustijns and M. Brewster, Solvent systems and their selection in pharmaceutics and biopharmaceutics, Springer, 2007 Search PubMed.
- Q. Tan, S. Liu, X. Chen, M. Wu, H. Wang, H. Yin, D. He, H. Xiong and J. Zhang, AAPS PharmSciTech, 2012, 13, 534–547 CrossRef CAS PubMed.
- S. Bhattacharyya, S. M. Ahammed, B. P. Saha and P. K. Mukherjee, AAPS PharmSciTech, 2013, 14, 1025–1033 CrossRef CAS PubMed.
- Q. Peng, Z.-R. Zhang, X. Sun, J. Zuo, D. Zhao and T. Gong, Mol. Pharm., 2010, 7, 565–575 CrossRef CAS PubMed.
- N. Domoto, M. E. Koenen, R. Havenaar, A. Mikajiri and B. S. Chu, Food Sci. Nutr., 2013, 1, 409–415 CrossRef CAS PubMed.
- G. A. Kossena, B. J. Boyd, C. J. H. Porter and W. N. Charman, J. Pharm. Sci., 2002, 92, 634–648 CrossRef PubMed.
- P. Nielsen, A. Müllertz, T. Norling and H. Kristensen, Int. J. Pharm., 2001, 222, 217–224 CrossRef CAS.
- Y. Yoon and E. Choe, Food Sci. Biotechnol., 2009, 18, 356–361 CAS.
- M. Gawrysiak-Witulska, A. Siger and M. Nogala-Kalucka, J. Food Lipids, 2009, 16, 524–539 CrossRef CAS PubMed.
- C. Chitchumroonchokcai and M. L. Failla, J. Nutr., 2006, 136, 588–594 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |