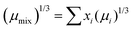Refining in silico simulation to study digestion parameters affecting the bioaccessibility of lipophilic nutrients and micronutrients
Sébastien
Marze
UR1268 Biopolymères Interactions Assemblages, INRA, F-44300 Nantes, France. E-mail: sebastien.marze@nantes.inra.fr
First published on 29th September 2014
Abstract
Despite the considerable number of in vivo and in vitro studies on the digestive fate of lipophilic nutrients, micronutrients, and bioactives, the effects of the structure and composition of foods on the physicochemical mechanisms of luminal digestion are still poorly understood. Studying them is indeed complex because the number of parameters is high and many of them are interdependent. To solve this problem, an in silico simulation based on a multi-agent system was recently proposed to study the intestinal bioaccessibility of lipophilic nutrients and micronutrients from a single oil droplet. The roles of lipolysis and solubilization in bile salt were included. The effects of several food and digestion parameters were in line with those reported in the experimental literature. The goal of the research reported in this new article was to include more digestion parameters in the simulation in order to make it more realistic against complex cases. This was done in one specific digestion condition reflecting in vitro experiments, using droplets of tricaprylin or triolein containing vitamin A. The structure and principles of the original model were kept, with independent local modifications in order to study each factor separately. First, a gastric step was added where lipolysis took place, and only a marginal effect on the following intestinal step was found. Then, the chemical form of vitamin A, either non-hydrolyzed retinyl ester or retinyl ester instantly hydrolyzed into retinol, was investigated by considering different localizations in the droplet, resulting in a higher bioaccessibility for the retinol. The case of a mixture of tricaprylin and triolein indicated an influence of the oil phase viscosity. The consideration of mixed micelles compared to simple bile salt micelles was also investigated, and resulted in a higher vitamin A bioaccessibility, especially with triolein. Finally, a full model including the most influential parameters was tested to simulate the digestion of triglyceride–limonene mixtures, giving bioaccessibility trends in very good agreement with the literature.
1. Introduction
Because of their particular physicochemical properties, lipophilic micronutrients and bioactives are generally absorbed with less efficiency than hydrophilic ones, and are thus less bioavailable. The molecular properties responsible for low absorption were identified for lipophilic drugs, but only apply partially to lipophilic micronutrients, as their lipophilicity is generally much higher.1 Moreover, their absorption also depends on the composition and multiscale structures of the food containing them.2 To study these aspects, the concept of bioaccessibility was defined as the proportion of a bioactive that is released from the food in its absorbable form during luminal digestion. Persuasive demonstrations that it influences absorption were given by measurements of the bioavailability of carotenoids from the same vegetable prepared in different forms. For example, it was shown that the bioavailability of carotenoids was increased in liquefied forms compared to the raw vegetable, because the native structures containing them were broken, enabling their release.3For lipophilic bioactives such as carotenoids, this is only the first step as it was repeatedly shown that their bioaccessibility (and subsequently their absorption) increases in the presence of lipids.2,4 In this situation, they indeed transfer from their native structures to lipid droplets, where they are efficiently co-digested with lipids to incorporate mixed micelles.5,6 There are also many foods and processed foods in which they are originally present in lipid droplets.
Thus, in most cases, studying the bioaccessibility of lipophilic bioactives consists in understanding the chemical and physical transformations during the luminal digestion of the oil phase, usually in an emulsified state (dispersed oil droplets).7 Nevertheless, this is not a trivial task as it involves many interdependent parameters related to molecular and supramolecular physicochemical properties. Typically, lipid bioaccessibility is influenced by the type and concentration of triglyceride, emulsifier (lipid, protein) and lipophilic bioactive, by the concentration (relative to their substrates) of enzymes and bile salts, and by the size of the droplets. Many sub-parameters derive from these and this makes in vitro experimental studies difficult.
To gain knowledge of the important parameters and to guide experimental work, an in silico simulation of the digestion of a single triglyceride droplet containing a lipophilic vitamin was previously designed based on a 2D multi-agent system.4 Although it was compared to other types of simulations of lipid drug delivery and digestion, it was the first time such a type of simulation was explored to obtain bioaccessibility kinetics (whereas it is more often used in biology8). The results were in very good qualitative agreement with the in vitro and in vivo experimental literature.
In this study, the objective was to increase the simulation capability against complex cases by taking more digestion parameters into account, with the longer term goal to validate the simulations using isolated droplets experiment.9 A specific digestion condition reflecting in vitro experiments was selected to create independent local modifications of the original model in order to investigate several cases separately. Parameters related to the gastric step, the chemical form of vitamin A, the formation of mixed micelles, and the use of a mixture of triglycerides or triglyceride–limonene mixtures were tested. The results are compared to those of the experimental literature.
2. Simulations
2.a. Original model
The simulation is based on a 2D multi-agent system developed previously.4 Briefly, it models a triglyceride droplet (containing a lipophilic micronutrient) in the digestive tube aqueous environment, using coarse-grained particles. Each particle represents a mass of unity for a given type of molecule. The original simulation of the small intestine digestion took four processes into account: (1) the relative diffusion of lipid particles using a random walk scheme depending on their molar mass and the oil phase viscosity, and the relative diffusion of digestive particles using position randomization in the aqueous phase, (2) the competitive adsorption/desorption of all particles using a single contact rule at the oil–water interface, (3) the lipolysis of triglyceride (yielding a diglyceride and a fatty acid as digestion products) and of diglyceride (yielding a monoglyceride and a fatty acid as digestion products) using a multiple contacts rule at the oil–water interface, inversely proportional to the relative lipolysis rates (assumed equal for triglyceride and diglyceride), (4) the solubilization of the digestion products and lipophilic micronutrient in the bile salt micelles (bioaccessibility) using a multiple contacts rule at the oil–water interface, inversely proportional to the absolute mass solubilization ratio (mass of solubilizate/mass of bile salt). For a schematic view of the simulation structure and principles, the reader is referred to the diagram in the previous article.4 Note that the time scale is not quantitative, defined as the number of computation time steps (CTS). This is why the time-related properties (diffusion coefficient and lipolysis rate) are not absolute but normalized on relative scales to enable the simulation of long-time digestion kinetics. Comparisons to an experimental setup using isolated droplets9 are currently carried out to quantify the time scale.The original simulation4 was used without any modification as the control experiment. The following conditions were used for all simulations: (a) a droplet size of 100 patches, (b) a lipophilic micronutrient content of 0.2% relative to the oil phase mass, (c) saturated bile conditions representing static in vitro protocols, where bile salt micelles get saturated with solubilizates, (d) a digestive particles/(fatty acid + monoglyceride particles) mass ratio of 2. One digestive particle represented a complex of a bile salt micelle and a pancreatic lipase. The lipophilic micronutrient was vitamin A, and the triglyceride was tricaprylin (TC) or triolein (TO). The simulation ended when all digestion products were solubilized in bile salt micelles (excess ratio) or when all bile salt micelles were saturated (limiting ratio).
The cases below were treated separately by making independent local modifications to the original model.
2.b. Gastric step
To consider the gastric step before the intestinal step, the relative lipolysis rates (relative to that of triolein under intestinal conditions) were calculated from the literature for gastric and intestinal lipolysis of TC and TO. The values are reported in Table 1. To model integer data, these values were rounded to 1/2 and 1/6 for TC and TO respectively. The gastric step was added to the simulation by considering that gastric lipolysis acts only on triglyceride, yielding a diglyceride and a fatty acid, and that no solubilization occurs in the aqueous phase. The amount of digestive particles was the same as that in the intestinal step, but here one particle represented a gastric lipase alone (no bile salt in the stomach). Based on preliminary results on time quantification (not shown), the gastric digestion was run for 900 computation time steps. This time was fixed to conform to static in vitro protocols. Then, an intestinal digestion identical to the control followed.| Molecules | Intestinal lipolysis rate4 | Gastric lipolysis rate10,11 | Solubilization ratio4 | Mixed micelles solubilization enhancement ratio15,16 |
|---|---|---|---|---|
| Caprylic lipids | 2* | 0.559 ± 0.066 (+ref. 13) | Monocaprylin 3.5* | 1 |
| Oleic lipids | 1* | 0.155 ± 0.001 (+refs. 12,14) | Monoolein 0.5* | 1 |
| Vitamin A or lipophilic drugs | Vitamin A 0.005* | Drugs 4.2 ± 1.7 (CA–MC) | ||
| 16.6 ± 3.0 (OA–MO) |
2.c. Form and localization of vitamin A
In foods, some lipophilic bioactives exist in different chemical forms. Vitamin A can be found as retinol or retinyl esters. It is known for cholesterol that the alcohol form is localized at the oil–water interface while the ester form is localized in the oil core in emulsion.17,18 So, for vitamin A, one might expect that the alcohol form is more bioaccessible than the ester form as it should be localized at the oil–water interface, where solubilization occurs.In the original simulation,4 vitamin A particles were free to diffuse in the whole oil phase throughout the digestion, thus representing a retinyl ester which is not hydrolyzed. In the alternative simulation, vitamin A particles were randomly positioned in the whole oil phase initially, but after their first contact with the oil–water interface, they were constrained to diffuse only at the interface, thus representing a retinyl ester which is instantly hydrolyzed into retinol.
2.d. Triglycerides mixture
Towards the simulation of real oils, a mixture of two triglycerides is a model system allowing a good control of the parameters. One is the lipolysis rate, the second is the solubilization ratio, and the third is the viscosity of the mixture, affecting in turn the diffusion coefficient of the triglycerides. A 50/50 mass by mass mixture of TC and TO was chosen. The lipolysis rates and solubilization ratios were the ones reported in Table 1. The viscosity of the mixture μmix was calculated using the Kendall–Monroe equation:19,20where μi is the viscosity and xi is the mole fraction of a pure component.
The viscosities of TC and TO at 37 °C were taken from the literature, and the relative diffusion coefficients of all molecules in the oil phase were scaled using the Stokes–Einstein equation with the calculated viscosity of the mixture.21
2.e. Mixed micelles
Intestinal digestion leads to the formation of mixed micelles composed of bile salts, fatty acids, monoglycerides, phospholipids and lipophilic bioactives. This structure carries the lipids from the intestinal lumen to their absorption site.14 Although lipophilic bioactives and drugs have a fair solubilization ratio in micelles only composed of bile salts,22 it was shown for some lipophilic drugs that mixed micelles including fatty acids and monoglycerides have a much higher solubilization ratio, especially with long chain fatty acids and monoglycerides.15,16 A solubilization enhancement ratio was defined as the ratio of the amount of drug solubilized in mixed micelles including fatty acids, monoglycerides, phospholipids and bile salts to the amount solubilized in simple bile salt–phospholipid micelles.16However, the solubilization enhancement ratios are not known for the lipophilic micronutrients, so they were extrapolated from those in ref. 15 and 16 for the most lipophilic drugs at fatty acids + monoglycerides concentrations between 0.4 and 2.2%. These ratios in the presence of caprylic or oleic fatty acids and monoglycerides are indicated in Table 1.
In the simulation, bile salt micelles had normal solubilization ratios initially, which were multiplied by the solubilization enhancement ratios once they were saturated with fatty acids and monoglycerides.
2.f. Triglyceride–limonene mixtures
It was recently reported that the bioaccessibility of β-carotene in nanoemulsion was much lower with essential oils (non-digestible oils) or triglyceride–essential oil mixtures than with triglyceride alone.23,24 To simulate such systems, limonene was selected as the representative molecule of citrus oils, and some triglyceride particles were replaced by limonene particles to obtain 75/25 and 50/50 mixtures (mass by mass) and also pure limonene. The viscosity under these conditions was calculated using the Kendall–Monroe equation. However, because the viscosity of limonene is low (about 0.75 mPa s at 37 °C, see ref. 25), the relative diffusion coefficients of the lipid and limonene particles were too high, and they diffused out of the oil phase boundaries using the original simulation. Based on the highest diffusion coefficient, it was found that this was solved by dividing all relative diffusion coefficients at least 9-fold. An alternative simulation where the diffusion coefficients of all particles in the oil phase were divided by 9 was thus used. Because triglyceride digestion products are able to form mixed micelles with bile salts whereas limonene, being non-digestible, is not able to do so, this simulation was based on the mixed micelles one, with reduced diffusion coefficients (which was thus run as the control for the triglyceride–limonene mixture simulations).NetLogo 4.1.3, an agent-based modeling software using the Logo programming language,26 was used to build and run the simulations. It was installed on a HP Compaq Elite 8300 PC (processor working at 3.40 GHz, RAM of 4 GB).
All simulations were run in five replicates and the results are given as averages and standard deviations. ANOVA plus hypothesis testing at 95% confidence level were performed to compare the results.
3. Results
As in the original simulation,4 the current results were obtained as a dynamic bioaccessibility, that is, the time-dependent proportion of lipophilic nutrient or micronutrient solubilized in bile salt relative to the initial mass initially inside the droplet. Bioaccessibilities of fatty acids and of monoglycerides being highly correlated, only the final bioaccessibility of fatty acids (FA bioaccessibility) is reported. The kinetics of bioaccessibility is characterized by the half life, that is, the time (given as the number of computation time steps) at which half of the final bioaccessibility is reached. The results are presented in Tables 2 and 3 for the different simulation cases and systems.| Fatty acids | TC + vitamin A | TO + vitamin A | |
|---|---|---|---|
| Control | Bioaccess. | 100.0 ± 0.0 | 93.5 ± 0.4 |
| Half life | 68 ± 4 | 334 ± 19 | |
| After gastric step | Bioaccess. | 100.0 ± 0.0 | 93.4 ± 0.6 |
| Half life | 31 ± 3 (0.46) | 343 ± 18 | |
| Vitamin A at the interface (retinol) | Bioaccess. | 100.0 ± 0.0 | 86.7 ± 1.4 (0.93) |
| Half life | 68 ± 6 | 331 ± 28 | |
| 50/50 TC–TO mixture | Bioaccess. | 100.0 ± 0.0 | 100.0 ± 0.0 (1.07) |
| Half life | 169 ± 17 (2.49) | 214 ± 16 (0.64) | |
| Mixed micelles | Bioaccess. | 100.0 ± 0.0 | 93.7 ± 0.5 |
| Half life | 65 ± 5 | 343 ± 18 | |
| Mixed micelles diffusion coeff./9 | Bioaccess. | 100.0 ± 0.0 | 94.7 ± 0.5 |
| Half life | 118 ± 10 (1.82) | 471 ± 18 (1.37) |
| Vitamin A | TC + vitamin A | TO + vitamin A | |
|---|---|---|---|
| Control | Bioaccess. | 12.4 ± 1.7 | 23.2 ± 1.9 |
| Half life | 148 ± 9 | 635 ± 66 | |
| After gastric step | Bioaccess. | 10.9 ± 1.5 | 24.7 ± 3.2 |
| Half life | 116 ± 19 (0.78) | 551 ± 56 | |
| Vitamin A at the interface (retinol) | Bioaccess. | 40.4 ± 5.5 (3.26) | 57.5 ± 6.3 (2.48) |
| Half life | 146 ± 4 | 536 ± 65 | |
| 50/50 TC–TO mixture | Bioaccess. | 27.7 ± 2.6 (2.23) | 27.7 ± 2.6 |
| Half life | 474 ± 17 (3.20) | 474 ± 17 (0.75) | |
| Mixed micelles | Bioaccess. | 15.9 ± 1.2 | 72.5 ± 5.8 (3.13) |
| Half life | 158 ± 13 | 848 ± 64 (1.34) | |
| Mixed micelles diffusion coeff./9 | Bioaccess. | 41.8 ± 7.9 (2.63) | 90.9 ± 6.8 (1.25) |
| Half life | 271 ± 33 (1.72) | 724 ± 39 (0.85) |
Concerning fatty acids, whatever the digestion case was, the final FA bioaccessibility was not significantly different from the control or only marginally (for triolein, relative to the control, a ratio of 0.93 for vitamin A at the interface, and a ratio of 1.07 for the 50/50 TC–TO mixture). In contrast, FA half life was significantly different from the control in more cases. All values for tricaprylin were significantly different from those for triolein, except the final FA bioaccessibility for the 50/50 TC–TO mixture.
Concerning vitamin A, more cases gave values that were significantly different from the control, with higher values for the final bioaccessibility. All values for vitamin A with tricaprylin were significantly lower than those with triolein.
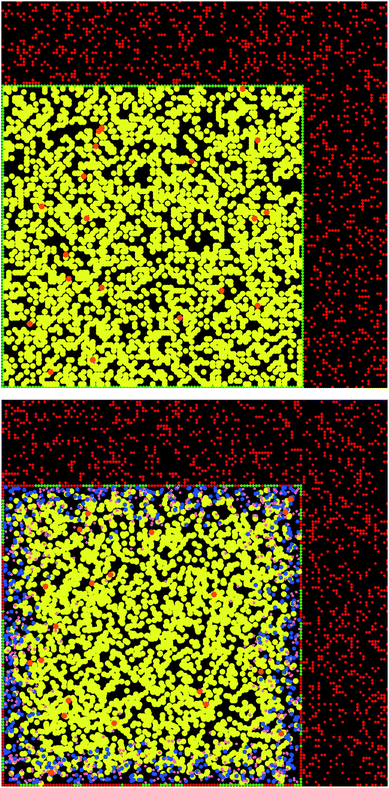
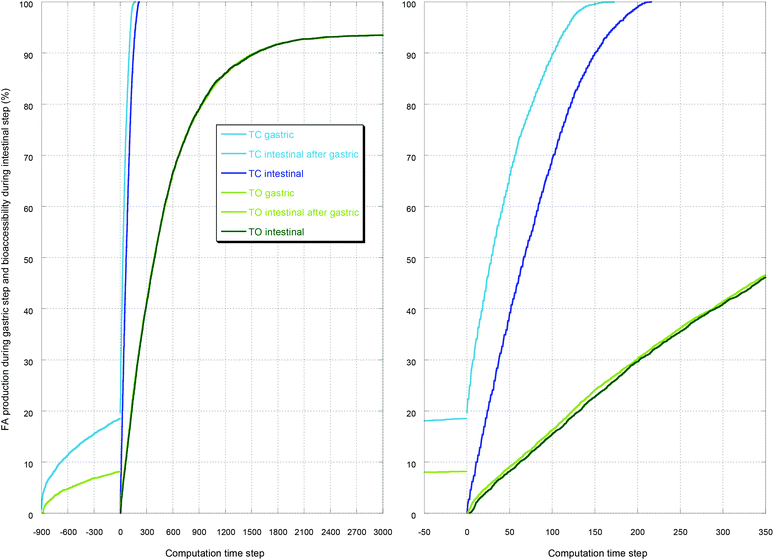
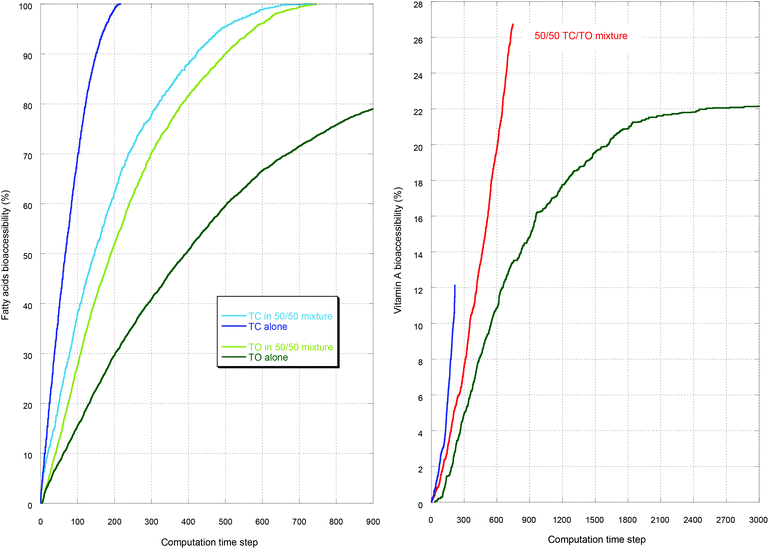
As an illustration, the case with the preceding gastric step is reported in Fig. 1–3. Fig. 1 displays the initial and the final simulation states of the gastric step for triolein and vitamin A. It shows that the diglycerides and fatty acids produced during the gastric digestion remain localized near the oil–water interface. Fig. 2 reports the full kinetics of fatty acid production during the gastric step and solubilization during the intestinal step, compared to solubilization during an intestinal step alone (control). This evidences the faster digestion kinetics of tricaprylin compared to that of triolein during both steps, with a high initial solubilization of tricaprylin digestion products in the beginning of the intestinal step. Fig. 3 reports the full kinetics of vitamin A bioaccessibility under the same conditions. It shows that this bioaccessibility is marginally affected by the gastric digestion, being significantly higher throughout the digestion only with tricaprylin (as for FA bioaccessibility).
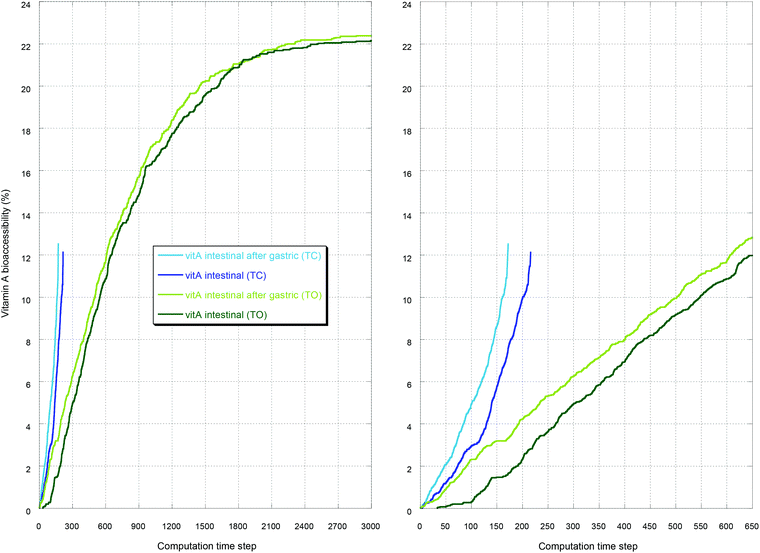 | ||
| Fig. 3 Bioaccessibility of vitamin A with tricaprylin (TC) or triolein (TO) for the same representative simulations as those presented in Fig. 2. The intestinal step is compared to the control where no gastric step preceded. On the right-hand side is a close-up of the beginning of the intestinal step. See the legend for colors. | ||
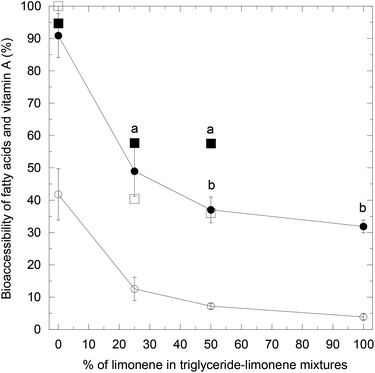
The results for the cases of triglyceride–limonene mixtures are summarized in Fig. 4 and 5. Because limonene is not digestible, a droplet structure was infinitely maintained in its presence, so FA and vitamin A always had the time to reach 100% bioaccessibility, whatever the limonene content was. The FA and vitamin A bioaccessibilities were thus taken at a given time, where the maximal FA bioaccessibility was reached for the control (100% for TC and 94.7% for TO in the mixed micelles case with reduced diffusion coefficients and no limonene). With a given triglyceride, this allowed a direct comparison of the bioaccessibility for the same digestion duration. Similarly, the relevant characteristic time was not the half life in these cases, but the time needed to reach the final bioaccessibility of the control. Overall, trends are seen for both FA and vitamin A, indicating lower bioaccessibilities and slower kinetics with increasing limonene content in the mixtures, more significantly with tricaprylin than with triolein.
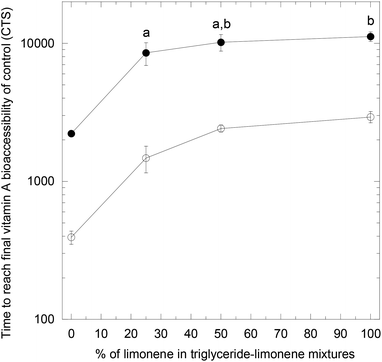 | ||
| Fig. 5 Time needed to reach the final vitamin A bioaccessibility of the control (the mixed micelles case with reduced diffusion coefficients and no limonene), with tricaprylin (empty symbol) or triolein (full symbol) in mixtures with limonene. The same comments as in Fig. 4. | ||
4. Discussion
In this part, the results for all simulation cases are examined in detail and compared with experimental data from the literature.4.a. Original model
Compared to the original article where both in vitro and in vivo conditions were explored,4 the current work focused on a specific in vitro condition where the amount of bile salts was sufficient to solubilize all digestion products of the triglyceride. Under these conditions, all digestion products of tricaprylin were solubilized whereas about 93.5% of those of triolein were solubilized (because bile salt could not solubilize all triolein digestion products plus vitamin A), and the half life was much higher for triolein. Because of that, vitamin A bioaccessibility was higher with triolein (23.2%) than with tricaprylin (12.4%), as much more time was available for solubilization.Only a few studies reported the in vitro bioaccessibility of vitamin A in emulsions.27–30 In the first study,27 vitamin A bioaccessibility ranged from 3.9 to 84.7% depending on the type of emulsion. Overall, there was a high variability between various food products with an average and a standard deviation of 35.5 ± 33.2%. In the second study by the same researchers,28 vitamin A bioaccessibility for mayonnaise was 15.0%, and the average and standard deviation for all food products were 23.3 ± 13.1%. In another study,29 vitamin A bioaccessibility for fortified milks could be estimated between 50 and 70% by calculation. In the most recent study,30 vitamin A bioaccessibility for various infant formulas and a margarine was 76.8 ± 14.5%. The simulated vitamin A bioaccessibilities for the controls are lower than that for comparable emulsions (range 50–90%),27,29,30 indicating an unrealistic modeling. In the following, the results of the different simulation cases are thus discussed in terms of estimation improvement.
4.b. Gastric step
First, the proportion of fatty acids produced during the gastric step was 18.7 ± 0.5% for tricaprylin and 8.3 ± 0.2% for triolein, which is in agreement with values of 20% and 7% reported in vitro,9,18 and in the range 5–40% reported in vivo.31 The difference arises from the fact that these triglycerides have different specific lipolysis rates.Then, only the intestinal step with the tricaprylin was affected by the gastric step, inducing a faster digestion. The literature shows that a threshold production of fatty acids during the gastric digestion is needed to induce an acceleration of the intestinal digestion through a reduction of the lag time, but the value of this threshold is not precisely known depending on the oil.11,32 This acceleration is seen with tricaprylin in Fig. 2 but not with triolein, probably because the threshold was not reached. The curves in Fig. 2 with triglycerides nevertheless display very similar kinetics to the ones obtained using real oil or fat, much faster during the intestinal step than during the gastric step.32–34
Concerning vitamin A bioaccessibility, the kinetics was also affected by the gastric step only in the case of tricaprylin. This is in qualitative agreement with the results of Lee et al.35 showing that the bioavailability of a lipophilic drug (cinnarizine) depends on the mode of administration, either orally (thus passing through stomach) or directly into the duodenum in rats. The bioavailability of the lipophilic drug was always faster and higher when administered orally, and this effect was more pronounced in the case of caprylic–capric triglyceride compared to soybean oil.
The fact that the final bioaccessibilities were not affected by the gastric step in the simulation could be due to the hypothesis that vitamin A has the same solubility in each glyceride class.4 Indeed, if the solubility was considered higher in diglyceride and fatty acid compared to triglyceride, then vitamin A would be closer to the oil–water interface at the end of the gastric step (see Fig. 1), which would likely increase its bioaccessibility.
More research is needed to understand this aspect. The role of proteolysis could also be studied, as proteins are frequently used to cover the oil–water interface. It was indeed shown in vitro that intestinal digestion lipolysis depends on the presence of hydrolyzed β-lactoglobulin for a single oil droplet,36 or on the presence of pepsin for emulsions stabilized by bovine serum albumin.37 In both cases the results indicated that the produced peptides likely inhibited lipolysis,36,37 as when no protease was present, lipolysis was similar in the absence or in the presence of β-lactoglobulin.38
4.c. Form and localization of vitamin A
Using the alcohol form of vitamin A instead of the ester form (control) greatly increased its final bioaccessibility with both tricaprylin and triolein. As for the glyceride solubility argument above, this is because retinol is localized at the oil–water interface whereas retinyl ester is in the oil core. Nevertheless, all half lives were not affected by this localization, showing that the kinetics was governed by the triglyceride digestion. The latter was only affected in the case of triolein in which the bioaccessibility was slightly lower than the control. This is because vitamin A bioaccessibility increased so much that there are less bile salts available for the solubilization of triolein digestion products.Only a few studies compared the two vitamin A forms. The results of Week and Sevigne39–42 for retinol and retinyl esters showed that retinol absorption was always more efficient than that of retinyl esters in chicks and humans, but not systematically in rats. Later, another study in rats confirmed that there was only a minor difference between the lymphatic recovery of retinol or retinyl palmitate from micellar infusates. However, a difference was clearly demonstrated in favor of retinyl palmitate when emulsions were infused.43
The final vitamin A bioaccessibility found in the case of retinol is now closer to the values reported in vitro.27,29,30 The ester form being the major one naturally present in foods, this agreement could indicate that the retinyl esters are quickly hydrolyzed into retinol, thus behaving as retinol. This was indeed the case in one in vitro study,29 where retinyl esters were entirely hydrolyzed within 1 hour. Also, in an in vivo study,43 when a lipase inhibitor was used together with retinyl palmitate, the lymphatic recovery was systematically much lower, confirming that retinol must be produced for optimal absorption.
More research is needed to evaluate whether retinyl esters hydrolysis could be a limiting step to absorption, as is suggested by the results of the current simulation. As only a few studies exist, the kinetics of hydrolysis should be investigated further and included in the simulation to make it more realistic.
4.d. Triglycerides mixture
The triglyceride digestion of the 50/50 tricaprylin–triolein mixture behaved as an ideal mixture, with increased half life of caprylic acid bioaccessibility and decreased half life of oleic acid bioaccessibility. Both FA bioaccessibilities reached 100% as there were fewer triolein digestion products to solubilize for the same quantity of bile salts. Vitamin A bioaccessibility was increased compared to the controls, although much more significantly compared to the tricaprylin control.These results apparently disagree with the literature,11,44 where mixtures of MCT and LCT displayed an intermediate FA bioaccessibility compared to MCT or LCT alone. This is because the simulation results were reported for the final FA bioaccessibility whereas it is often reported at given digestion times in the literature. When the FA bioaccessibility kinetics are compared (Fig. 6), the values for the mixture are clearly intermediate compared to each triglyceride alone, as actually reflected in the half life values. The same applies to vitamin A bioaccessibility (Fig. 6), in agreement with results for β-carotene bioaccessibility from nanoemulsions in the “low-fat” case (results in the “high-fat” case depending more on mixed micelles formation),44 and in qualitative agreement with results for the bioavailability of a lipophilic drug (halofantrine) in beagle dogs.45
4.e. Mixed micelles
As expected, the kinetics of FA bioaccessibility was not affected by the formation of mixed micelles, as it affected neither the lipolysis rate nor the FA solubilization ratio. In contrast, by increasing the vitamin A solubilization ratio, it increased its bioaccessibility, although only significantly with triolein, as its digestion products solubilization enhancement ratio is higher than that of tricaprylin digestion products.This result was even more pronounced (and now significant for both triglycerides) when using reduced diffusion coefficients for all lipids, because most half lives were then increased, reflecting slower kinetics, with more time for vitamin A to solubilize. Moreover, as lipids diffusion was slower, they spent more time near the oil–water interface once they approached it, accelerating their solubilization. The additional enhancement due to the reduced diffusion coefficients was especially pronounced with tricaprylin, because much more time was available for solubilization. As only the diffusion coefficients were changed, this simulation shows that diffusion kinetics controls digestion, as lipids must first diffuse to the oil–water interface before lipolysis can occur. It was indeed beneficial to reduce the diffusion coefficient of all lipids, in agreement with the tricaprylin–triolein mixture simulation at long times, where the final bioaccessibility of vitamin A was greatly increased compared to tricaprylin alone (the tricaprylin–triolein mixture being more viscous, the diffusion coefficients related to tricaprylin were lower).
These results are difficult to compare with the literature because no experimental work studied the same systems in the context of mixed micelles solubilization. Nevertheless, the final vitamin A bioaccessibilities are now in the range reported in vitro (50–90%),27,29,30 and also in vivo (60–90%).46 So it shows that the simulation in this case is more realistic. It still needs to be refined to incorporate the real time, and experimental work using isolated droplets generated by microfluidics is currently in progress to solve this point and produce data for the same systems and digestion conditions.
The choice of the solubilization enhancement ratios was also made by default because only one team did systematic experiments to characterize them, however for lipophilic drugs.15,16 Nevertheless, solubilization enhancement ratios between 6 and 22 could be calculated in the case of cholesterol solubilization by bile salt–sodium oleate or bile salt–monoolein mixed micelles,47–49 showing that enhancement could also be high for dietary lipophilic bioactives. More research is needed to identify the parameters affecting their solubilization enhancement ratios, as values below 2 were also reported for cholesterol and vitamin E under certain conditions.47,48,50
4.f. Triglyceride–limonene mixtures
As shown in Fig. 4 and 5, as more triglyceride was replaced by limonene in the droplet, the bioaccessibilities were decreased and the kinetics were slower. This is explained by three phenomena: (1) the increase of the diffusion coefficients of the lipids due to the decrease of viscosity as limonene content increases, as already discussed in the section above, (2) the competition for space at the oil–water interface between lipids and limonene, and (3) the reduction of the number of mixed micelles as limonene content increases.These trends are in agreement with those obtained for β-carotene bioaccessibility from mixed corn oil–citrus oil nanoemulsions.23,24 The third phenomenon was identified to explain the data, and also the assembling of the micelles, larger for glycerides–bile salt mixed micelles compared to bile salt micelles (for citrus oil alone). This effect is not directly taken into account in the simulations, but is likely included indirectly through the solubilization enhancement ratio. Thus this triglyceride–limonene mixtures simulation shows that very good agreement with the literature can be obtained when the most influential parameters are taken into account, namely the viscosity of oil mixtures and the formation of mixed micelles.
5. Conclusion
In summary, the simulation cases developed here allowed the refinement of the original simulation in two ways. First, by including the localization of either form of vitamin A and the formation of mixed micelles, it was shown that bioaccessibility estimations were closer to the literature values. In contrast, these estimations were marginally affected by the inclusion of the gastric step, perhaps because the solubility of vitamin A in digestion products and the proteolysis were not taken into account. Second, by including a law to calculate the viscosity of oil mixtures and therefore the resulting lipids diffusion coefficients, it was possible to estimate bioaccessibility in a mixture of two triglycerides and in triglyceride–limonene mixtures. The trends were in very good agreement with the literature. The results of the simulations also highlighted the need for more experimental data on the hydrolysis of vitamin esters and on the solubilization of lipophilic bioactives (vitamins, sterols, carotenoids, etc.) in mixed micelles. Experimental work on isolated droplets produced by microfluidics is currently in progress to validate the simulations under controlled conditions and in an explicit time scale.Acknowledgements
The author acknowledges the ISC-PIF (Paris Île-de-France Complex Systems Institute) for the IDEAS workshop (Ideas for Design, knowledge Engineering Applied to living complex Systems), and Alain Riaublanc for inspiring discussions.References
- C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Adv. Drug Delivery Rev., 1997, 23, 3–25 CrossRef CAS.
- S. Marze, Crit. Rev. Food Sci. Nutr., 2013, 53, 76–108 CrossRef CAS PubMed.
- J. Parada and J. M. Aguilera, J. Food Sci., 2007, 72, R21–R32 CrossRef CAS PubMed.
- S. Marze, Food Funct., 2014, 5, 129–139 CAS.
- P. Borel, P. Grolier, M. Armand, A. Partier, H. Lafont, D. Lairon and V. Azais-Braesco, J. Lipid Res., 1996, 37, 250–261 CAS.
- G. T. Rich, A. Fillery-Travis and M. L. Parker, Lipids, 1998, 33, 985–992 CrossRef CAS.
- M. Yao, H. Xiao and D. J. McClements, Annu. Rev. Food Sci. Technol., 2014, 5, 53–81 CrossRef PubMed.
- C. A. Hunt, G. E. P. Ropella, T. N. Lam, J. Tang, S. H. J. Kim, J. A. Engelberg and S. Sheikh-Bahaei, Pharm. Res., 2009, 26, 2369–2400 CrossRef CAS PubMed.
- S. Marze, H. Algaba and M. Marquis, Food Funct., 2014, 5, 1481–1488 CAS.
- S. J. DeNigris, M. Hamosh, D. K. Kasbekar, C. S. Fink, T. C. Lee and P. Hamosh, Biochim. Biophys. Acta, 1985, 836, 67–72 CrossRef CAS.
- P. Borel, M. Armand, P. Ythier, G. Dutot, C. Melin, M. Senft, H. Lafont and D. Lairon, J. Nutr. Biochem., 1994, 5, 124–133 CrossRef CAS.
- E. Rogalska, S. Ransac and R. Verger, J. Biol. Chem., 1990, 265, 20271–20276 CAS.
- E. Rogalska, C. Cudrey, F. Ferrato and R. Verger, Chirality, 1993, 5, 24–30 CrossRef CAS PubMed.
- B. Borgström and J. S. Patton, Compr. Physiol., 2011 DOI:10.1002/cphy.cp060422.
- G. A. Kossena, B. J. Boyd, C. J. H. Porter and W. N. Charman, J. Pharm. Sci., 2003, 92, 634–648 CrossRef CAS PubMed.
- G. A. Kossena, W. N. Charman, B. J. Boyd, D. E. Dunstan and C. J. H. Porter, J. Pharm. Sci., 2004, 93, 332–348 CrossRef CAS PubMed.
- S. Ekman, A. Derksen and D. M. Small, Biochim. Biophys. Acta, 1988, 959, 343–348 CrossRef CAS.
- Y. Pafumi, D. Lairon, P. Lechene de la Porte, C. Juhel, J. Storch, M. Hamosh and M. Armand, J. Biol. Chem., 2002, 277, 28070–28079 CrossRef CAS PubMed.
- J. Kendall and K. P. Monroe, J. Am. Chem. Soc., 1917, 39, 1787–1802 CrossRef CAS.
- M. A. Eiteman and J. W. Goodrum, J. Am. Oil Chem. Soc., 1994, 71, 1261–1265 CrossRef CAS.
- S. Marze, M. Choimet and L. Foucat, Soft Matter, 2012, 8, 10994–11004 RSC.
- T. S. Wiedmann and L. Kamel, J. Pharm. Sci., 2002, 91, 1743–1764 CrossRef CAS PubMed.
- C. Qian, E. A. Decker, H. Xiao and D. J. McClements, Food Chem., 2012, 135, 1440–1447 CrossRef CAS PubMed.
- J. Rao, E. A. Decker, H. Xiao and D. J. McClements, J. Sci. Food Agric., 2013, 93, 3175–3183 CrossRef CAS PubMed.
- R. A. Clara, A. C. Gomez Marigliano and H. N. Solimo, J. Chem. Eng. Data, 2009, 54, 1087–1090 CrossRef CAS.
- U. Wilensky, Center for Connected Learning and Computer-Based Modeling, Northwestern University, Evanston, IL, 1999, NetLogo. http://ccl.northwestern.edu/netlogo/ Search PubMed.
- Y. C. O'Callaghan, O. Kenny and N. M. O'Brien, Proc. Nutr. Soc., 2007, 66, 112A Search PubMed.
- Y. O'Callaghan and N. O'Brien, Int. J. Food Sci. Technol., 2010, 45, 1436–1442 CrossRef PubMed.
- M. C. Herrero-Barbudo, F. Granado-Lorencio, I. Blanco-Navarro, B. Perez-Sacristan and B. Olmedilla-Alonso, Int. Dairy J., 2009, 19, 64–67 CrossRef CAS PubMed.
- E. F. A. Brandon, M. I. Bakker, E. Kramer, H. Bouwmeester, T. Zuidema and M. Alewijn, Int. J. Food Sci. Nutr., 2014, 65, 426–435 CrossRef CAS PubMed.
- M. Armand, Curr. Opin. Clin. Nutr. Metab. Care, 2007, 10, 156–164 CrossRef CAS PubMed.
- Y. Gargouri, G. Pieroni, C. Rivière, P. A. Lowe, J. F. Saunière, L. Sarda and R. Verger, Biochim. Biophys. Acta, 1986, 879, 419–423 CrossRef CAS.
- S. Bernbäck, L. Bläckberg and O. Hernell, Biochim. Biophys. Acta, 1989, 1001, 286–293 CrossRef.
- S. Bernbäck, L. Bläckberg and O. Hernell, J. Clin. Invest., 1990, 85, 1221–1226 CrossRef PubMed.
- K. W. Y. Lee, C. J. H. Porter and B. J. Boyd, J. Pharm. Sci., 2013, 102, 3957–3965 CrossRef CAS PubMed.
- S. Marze, A. Meynier and M. Anton, Food Funct., 2013, 4, 231–239 CAS.
- H. B. Kenmogne-Domguia, A. Meynier, M. Viau, G. Llamas and C. Genot, Food Funct., 2012, 3, 1302–1309 CAS.
- S. Marze and M. Choimet, Soft Matter, 2012, 8, 10982–10993 RSC.
- E. F. Week and F. J. Sevigne, J. Nutr., 1949, 39, 233–250 CAS.
- E. F. Week and F. J. Sevigne, J. Nutr., 1949, 39, 251–257 CAS.
- E. F. Week and F. J. Sevigne, J. Nutr., 1950, 40, 563–576 CAS.
- E. F. Week and F. J. Sevigne, J. Nutr., 1950, 42, 525–537 CAS.
- E. Fernandez and B. Borgström, Lipids, 1990, 25, 549–552 CrossRef CAS.
- L. Salvia-Trujillo, C. Qian, O. Martín-Belloso and D. J. McClements, Food Chem., 2013, 139, 878–884 CrossRef CAS PubMed.
- C. J. H. Porter, A. M. Kaukonen, A. Taillardat-Bertschinger, B. J. Boyd, J. M. O'Connor, G. A. Edwards and W. N. Charman, J. Pharm. Sci., 2004, 93, 1110–1121 CrossRef CAS PubMed.
- G. F. M. Ball, Vitamins in foods: analysis, bioavailability, and stability, CRC Press, Boca Raton, FL, 2006 Search PubMed.
- W. J. Simmonds, A. F. Hofmann and E. Theodor, J. Clin. Invest., 1967, 46, 874–890 CrossRef CAS PubMed.
- T. Inoue and K. Juniper, Am. J. Dig. Dis., 1973, 18, 1067–1074 CrossRef CAS.
- J. C. Montet, M. O. Reynier, A. M. Montet and A. Gerolami, Biochim. Biophys. Acta, 1979, 575, 289–294 CrossRef CAS.
- Y. Yang and D. J. McClements, J. Colloid Interface Sci., 2013, 405, 312–321 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |