Research highlights: antibiotic resistance genes: from wastewater into the environment
David T.
Tan
*a and
Danmeng
Shuai
b
aDepartment of Civil, Environmental, and Geo-Engineering, University of Minnesota, 500 Pillsbury Drive SE, Minneapolis, Minnesota 55455, USA. E-mail: tanxx253@umn.edu
bDepartment of Civil and Environmental Engineering, The George Washington University, 639 Phillips Hall, The Academic Center, 801 22nd Street NW, Washington DC 20052, USA
First published on 1st May 2015
The increasing prevalence of antibiotic resistant pathogens and the dwindling development of new antibiotics is a critical public health issue, prompting initiatives such as the Joint Programming Initiative on Antimicrobial Resistance launched in Europe in 2010 and an Executive Order issued in the United States in 2014. While the recent development of a new method for growing uncultured bacteria opens the possibility of a new wave of antibiotic discoveries,1,2 nonetheless it remains necessary to address the development and spread of antibiotic resistance. Antibiotic resistance spreads not only through the use or presence of antibiotics, but also through the ability of bacteria to share antibiotic resistance genes (ARGs) via conjugation and to uptake extracellular ARGs (natural transformation). Municipal wastewater is an important source of ARGs to the environment.3,4 Understanding the pathways for transport to the environment and the subsequent fate of these genes will enable us to target key processes and implement appropriate mitigation strategies. In this article we highlight the effects of treated and untreated wastewater on ARGs in the environment, attenuation of ARGs following land application of wastewater solids, and a quantitative model for natural transformation.Irrigation with untreated wastewater
Irrigation with untreated wastewater is common practice in many developing countries. While this practice can alleviate water shortages and provides nutrients for crops, it also introduces a variety of health risks to farmers and consumers. One potential risk is the spread of antibiotic resistant pathogens due to repeated application of pathogens, antibiotics, antibiotic resistant bacteria, and heavy metals (known to provide selective pressure for efflux pumps, which contribute to antibiotic resistance) to the soil. Jechalke et al. examined the effects of this practice in the Mezquital Valley, Mexico, where wastewater irrigation has been practised for up to 100 years.5 In addition to enumeration of a variety of resistance genes, the group also targeted class 1 integrons (intI1) and IncP-1 plasmids, which are both associated with multiple antibiotic resistance.Absolute abundance of all target genes except for tet(Q) increased with duration of irrigation (Fig. 1a), with increases of up to three log values over unirrigated soil. These increases were due to accumulation rather than enrichment: no positive correlation was found between normalized abundance (to the 16S rRNA gene) of target genes and duration of irrigation, even though antibiotics had accumulated in the soil (Fig. 1b). Likewise, abundance of heavy metals had no positive correlation with the relative abundance of the target genes. Nonetheless, the results clearly show that attenuation rates of these genes are not sufficient to prevent accumulation of ARGs even over short irrigation periods.
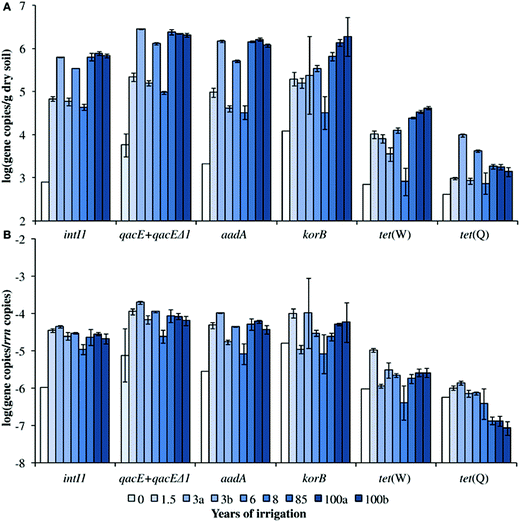 | ||
| Fig. 1 Concentrations of ARGs and intI1 in soils irrigated with untreated wastewater. Absolute concentrations are shown in (A), and concentrations normalized to the 16S rRNA gene are shown in (B). Figure reproduced from ref. 5: S. Jechalke, M. Broszat, F. Lang, C. Siebe, K. Smalla and E. Grohmann, Front. Microbiol., 2015, 6, 1 in accordance with Creative Commons guidelines. | ||
Antibiotics and antibiotic resistance genes: from hospital to river
While wastewater contributes to the spread of antibiotic resistance, eliminating this risk through treatment remains a challenge. Understanding the scope and magnitude of existing risks is an ongoing process, illustrated by the recent proliferation of studies examining antibiotics and ARGs in wastewaters and receiving environments. One such study by Rodriguez-Mozaz et al. monitored sixty-two antibiotics and five ARGs representing different classes of antibiotics in wastewater of a hospital, the receiving wastewater treatment plant, and the receiving river in Girona, Spain.6Hospital wastewater had higher concentrations of fluoroquinolones than influent wastewater at the treatment site, but had approximately equivalent or lower concentrations of macrolides and cephalosporins. Concentrations of antibiotics generally decreased during treatment but were still sufficient to increase downstream concentrations in the receiving river. ARG concentration trends (Fig. 2) generally mirrored that of antibiotic concentrations. Concentrations of ARGs in hospital wastewater were higher or similar to that of general wastewater. Treatment reduced absolute concentrations of all the ARGs studied, but normalized concentrations (to 16S rRNA) of blaTEM, qnrS, and sul1 remained consistent or were elevated. This suggests that removal of bacteria was the primary means of ARG removal and indicates potential for selective pressure for antibiotic resistance in wastewater treatment. Absolute and relative ARG concentrations of most target ARGs in the receiving river were elevated downstream of the treatment facility, demonstrating that the treated wastewater was a point source of antibiotic resistance to that river.
 | ||
| Fig. 2 Cumulative concentration of five ARGs in hospital wastewater, treatment influent and effluent, and upstream and downstream sites in the receiving river. Adapted with permission from ref. 6: S. Rodriguez-Mozaz, S. Chamorro, E. Marti, B. Huerta, M. Gros, A. Sànchez-Melsió, C. M. Borrego, D. Barceló, J. L. Balcázar, Water Res., 2015, 69, 234. Copyright 2015 Elsevier. | ||
Application of wastewater solids to soil
Since removal of ARGs from wastewater correlates strongly with removal of bacteria, residual solids are the main vector for discharge of ARGs from the treatment process. While treatment of these solids can further reduce the quantity of ARGs, land application of the treated solids can release the remaining ARGs into the environment. Attenuation rates of these genes in soil, studied by Burch et al.,7 are important for assessing possible pathways to human exposure and developing practices to mitigate these risks.The attenuation of ARGs in soil was slow, with half-lives of 13–81 days (Fig. 3), compared to half-lives of under a week for various solids treatment technologies. This suggests that desired levels of ARG removal should be accomplished during the treatment process prior to land application. Attenuation rates were gene-specific, suggesting differing mechanisms, but the most persistent target was intI1, which is associated with multiple antibiotic resistance. Although mass loading rate of treated solids did not affect attenuation rates, nonetheless loading may still be important for preventing accumulation of ARGs. The persistence of these genes in soils means that various exposure events including tilling, high-wind, and runoff need to be evaluated for associated health risks.
 | ||
| Fig. 3 Range of ARG and intI1 half-lives following application of treated wastewater solids to soil. Reprinted with permission from ref. 7: T. R. Burch, M. J. Sadowsky, T. M. LaPara, Environ. Sci. Technol., 2014, 48, 5620. Copyright 2014 American Chemical Society. | ||
Natural transformation of ARGs
Once ARGs are in the environment, there are several mechanisms for spread of these genes. While models exist for conjugation and transduction, there has not been a mathematical model for natural transformation, which is the uptake of extracellular DNA by competent cells. Lu et al.8 proposed such a model, which accounts for time-dependent concentrations of transforming DNA and competent cells. This is an important step for understanding the fate and transport of ARGs and for assessing the relative importance of extracellular ARGs in the spread of antibiotic resistance.The authors developed a model for natural transformation frequency (example predictions shown in Fig. 4) and verified it using motile and non-motile strains of Azotobacter vinelandii, a soil bacteria known to be naturally competent, non-specific in DNA uptake. Within the range of conditions studied, transforming DNA was the limiting factor, and increasing concentrations of cells resulted in reduced transformation frequency (normalized by viable cells). Available transforming DNA was quickly transformed, with transformation frequency plateauing within 30 minutes. Interestingly, motility appeared to reduce transformation frequency. The authors hypothesized that motility decreased contact time between competent cells and transforming DNA. While environmental conditions may differ sufficiently from this study to yield different natural transformation patterns, this work, together with existing conjugation and transduction models, enables a comprehensive model for gene transfer.
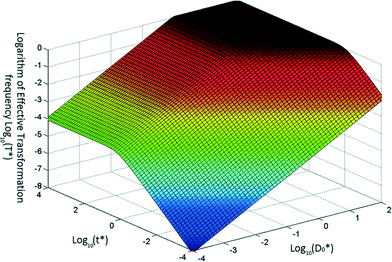 | ||
| Fig. 4 Typical variation of transformation frequency as a function of dimensionless transferable DNA number concentration and dimensionless time. Reproduced from ref. 8. | ||
Outlook
These papers illustrate the pathways through which ARGs enter the environment from municipal wastewater and add to the growing body of knowledge about attenuation rates and potential for uptake in the environment. A crucial part of solving the ARG puzzle is closing the loop: establishing how antibiotic resistance genes in aquatic and soil environments affect antibiotic resistance levels in the human population. This will enable risk assessment, identification of strategic points for reducing the spread of ARGs, and determination of the degree of removal necessary at these points to mitigate this risk.References
- L. L. Ling, T. Schneider, A. J. Peoples, A. L. Spoering, I. Engels, B. P. Conion, A. Mueller, T. F. Schäberle, D. E. Hughes, S. Epstein, M. Jones, L. Lazarides, V. A. Steadman, D. R. Cohen, C. R. Felix, K. A. Fetterman, W. P. Millett, A. G. Nitti, A. M. Zullo, C. Chen and K. Lewis, Nature, 2015, 517, 455–459 CrossRef CAS PubMed.
- D. Nichols, N. Cahoon, E. M. Trakhtenberg, L. Pham, A. Mehta, A. Belanger, T. Kanigan, K. Lewis and S. S. Epstein, Appl. Environ. Microbiol., 2010, 76, 2445–2450 CrossRef CAS PubMed.
- T. M. LaPara, T. R. Burch, P. J. McNamara, D. T. Tan, M. Yan and J. J. Eichmiller, Environ. Sci. Technol., 2011, 45, 9543–9549 CrossRef CAS PubMed.
- A. Pruden, Environ. Sci. Technol., 2014, 48, 5–14 CrossRef CAS PubMed.
- S. Jechalke, M. Broszat, F. Lang, C. Siebe, K. Smalla and E. Grohmann, Front. Microbiol., 2015, 6, 1–10 Search PubMed.
- S. Rodriguez-Mozaz, S. Chamorro, E. Marti, B. Huerta, M. Gros, A. Sànchez-Melsió, C. M. Borrego, D. Barceló and J. L. Balcázar, Water Res., 2015, 69, 234–242 CrossRef CAS PubMed.
- T. R. Burch, M. J. Sadowsky and T. M. LaPara, Environ. Sci. Technol., 2014, 48, 5620–5627 CrossRef CAS PubMed.
- N. Lu, A. Massoudieh, X. Liang, T. Kamai, J. L. Zilles, T. H. Nguyen and T. R. Ginn, Environ. Sci.: Water Res. Technol., 2015, 1 10.1039/C5EW00023H.
| This journal is © The Royal Society of Chemistry 2015 |
