Online fluorescence monitoring of RO fouling and integrity: analysis of two contrasting recycled water schemes
Sachin
Singh
a,
Rita K.
Henderson
a,
Andy
Baker
b,
Richard M.
Stuetz
a and
Stuart J.
Khan
*a
aUNSW Water Research Centre, School of Civil and Environmental Engineering, UNSW Australia, NSW 2052, Australia. E-mail: s.khan@unsw.edu.au
bConnected Waters Initiative Research Centre, UNSW Australia, NSW 2052, Australia
First published on 16th June 2015
Abstract
A fluorescence-based sensor was used to conduct real-time, online monitoring of reverse osmosis (RO) membranes at two Australian municipal advanced water recycling schemes. Trials monitored real-time changes in fluorescent organics, measured at λEx/Em = 350/430 nm (Peak C), in RO permeates as an indicator of water quality and membrane performance. The RO membranes in each plant were operated using contrasting conditions; the Water Reclamation and Management Scheme plant was operated under constant pressure and the Ground Water Replenishment Trials pilot plant under constant flux. The Peak C sensor detected differences between permeates from staged RO systems, changes in water quality due to an underperforming membrane suspected to have an integrity breach, and changes in water quality from increasing transmembrane pressure in fouled membranes. Peak C fluorescence had stronger correlation with transmembrane pressure and may have potential application in detecting the onset of membrane fouling. Interestingly, while RO EC signals indicated improving permeate quality, fluorescence revealed an increase in (Peak C) organics in the RO permeate. This demonstrated that the passage of fluorescent organics and ionic species are dissimilar, and monitoring EC alone is not an accurate surrogate measure to ensure on-going effective removal of organic chemicals.
Water impactThe work here presents online monitoring of reverse osmosis membranes at two contrasting recycled water schemes using a fluorescence-based sensor. Online monitoring is an integral component of ongoing membrane performance monitoring. Conventional techniques have been used successfully but have some associated limitations; there is therefore a need for sensitive and affordable technology. In this work, online monitoring of reverse osmosis membranes was conducted using fluorescence spectroscopy. The Peak C sensor detected differences between permeates from staged RO systems, changes in water quality due to an underperforming membrane suspected to have an integrity breach, and changes in water quality from increasing transmembrane pressure in fouled membranes. |
1. Introduction
Reverse Osmosis (RO) membranes have become an integral component of many advanced water treatment systems. In particular, the ability to produce high quality product water from variable wastewater sources has led to their adoption for a wide range of potable and non-potable water recycling applications.1 The intention for semi-permeable RO membranes is to allow the passage of some substances (primarily water), while rejecting others (the ‘contaminants’). However, a fine balance must be achieved to maintain both adequate water flux and contaminant rejection. Some of the parameters that influence this balance, such as membrane selection and operational pressures, can be controlled. However, long-term membrane performance, under variable operational conditions, can only be assured through regular monitoring.Online monitoring is an integral component of ongoing membrane performance monitoring. Conventional online monitoring techniques for RO include electrical conductivity (EC), total organic carbon (TOC) and, less commonly, particle counting.2,3 Each of these techniques have been used successfully but have some associated limitations; there is therefore a need for sensitive and affordable technology. Online EC sensors are relatively inexpensive and highly sensitive for measuring charged species but do not detect uncharged species. TOC is a more relevant parameter in light of current concerns with organic contaminants, and is the current ‘state of the art’ technology for online monitoring of organics rejection by RO. TOC does not differentiate between the organic matter fractions and thus provides very little information on organic matter characteristics.4 Particle counting lacks the required sensitivity due to the inherently low concentration of particles in RO permeates.
Fluorescent dissolved organic matter (fDOM) can be characterised and quantified using fluorescence excitation emission matrix (EEM) spectroscopy which is a three-dimensional map of emission ranges and intensities generated at a range of specified excitation wavelengths. The potential for fluorescence spectroscopy in monitoring recycled water systems has been discussed extensively.5 Fluorescence based studies on membrane systems have focused primarily on the characterisation of membrane foulants; including membrane bioreactors (MBR) for wastewater treatment,6–11 microfiltration (MF) and ultrafiltration (UF) membranes12–17 and nanofiltration (NF) membranes used in drinking water production.18 RO membrane performance has received very little scrutiny using fluorescence spectroscopy.19,20 Our earlier study was used to characterise fDOM in RO permeates, in order to identify the optimum EEM region for monitoring RO performance.19 Pype et al.20 compared EEM and conductivity characteristics for RO performance monitoring. In reuse applications, the reduction of a protein-like peak (λEx/Em = 276/329 nm) in RO concentrates has shown good correlation with the removal of pharmaceutical and personal care products (PPCPs).21 The authors of this study suggested that monitoring changes to this fluorescence peak may provide a rapid and inexpensive method for the quantitative estimation of PPCPs degradation under treatment plant conditions. More recently, the fate and transport of potential soluble foulants in an MBR-RO system was tracked using fluorescence spectroscopy.22 Although few studies on fluorescence characteristics of DOM in RO permeate have been reported, RO can remove significant levels of DOM so there are further potential applications of fluorescence spectroscopy for this treatment process.
Our preliminary investigations found the technique sufficiently sensitive to detect subtle changes in permeate quality pertaining to differences in direct feed quality within multiple staged RO systems.23 Parallel factor analysis (PARAFAC) of treatment processes at several advanced recycled water treatment plants (including RO) demonstrated that five common fluorescence components were present in four or more plants in that study and that the information derived from monitoring these components would be equivalent to monitoring full EEMs.24 The fluorescent component G3 (λEx/Em = 350/428 nm) which was described as a wastewater/nutrient enrichment tracer from that study, matched closely with the fluorescent regions commonly described as ‘Peak C’ (λEx/Em = 320–350/410–430 nm). The relevance of these regions to water quality was first identified by Coble,25 who related it to humic-like dissolved natural organic matter in surface water. Further investigations revealed the Peak C region as the most suitable for detecting changes in RO permeate quality from membrane underperformance, due to well-resolved signals and sensitivity in that region.19
This paper presents the results of a fluorescence-based, online monitoring trial at two advanced water recycling facilities using RO membranes. The aims of this study were to: 1) establish an online log rejection profile for fluorescence; and 2) compare online RO permeate fluorescence data from two contrasting RO plants. The two plants in this study were the Water Reclamation and Management Scheme (WRAMS) and Beenyup Groundwater Replenishment Trials (GWRT), which had been part of a broader fluorescence-based, grab-sampling study.24 The RO membranes in the WRAMS plant were operated under constant pressure and the GWRT under constant flux. The RO membranes at both plants exhibited symptoms of underperformance, with integrity loss and membrane fouling as possible causes, and added real world considerations to the assessment of fluorescence spectroscopy as a potential online monitoring tool for RO membrane performance.
2. Materials and methods
2.1 Location descriptions
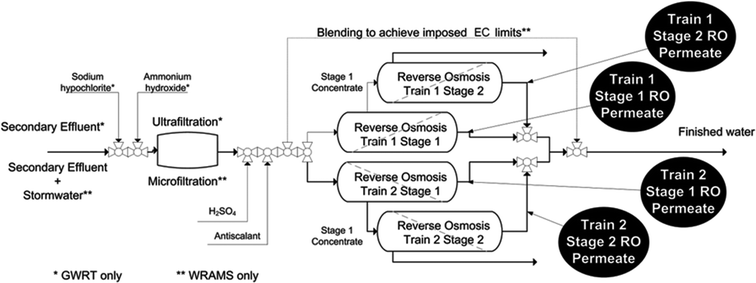
Online fluorescence monitoring was trialled at two Australian water recycling sites, the Water Reclamation and Management Scheme (WRAMS) in New South Wales (NSW) and the Beenyup Groundwater Replenishment Trials (GWRT) in Western Australia (WA).A composite process flow diagram representing the two plants is presented in Fig. 1.
2.2 Equipment, data collection and interpretation
Three separate monitoring trials were conducted. Trial 1 was located at the WRAMS plant and involved monitoring RO feed and permeate fluorescence. Trials 2 and 3 were conducted at WRAMS and GWRT respectively, where only the RO permeate fluorescence was measured.Fluorescence measurements were taken using a stainless steel Cyclops 7 Peak C sensor (Turner Designs, California, USA). The sensor uses a light emitting diode (LED) to excite at λ = 350 nm ± 20 nm (full width half maximum or FWHM) and measures emission at λ = 430 nm ± 30 nm (FWHM). This was interfaced with a DT80 Series 2 data logger (Datataker, Vic, Australia) which also powered the probe (12 V). The sensor was set to maximum attenuation (Gain = 100) for RO permeate measurements, with the signal collected at 1 minute intervals and averaged over every 5 minutes. The RO feed fluorescence was measured using a second Cyclops 7 Peak C sensor at a gain setting of 10. A flow through cap was used in conjunction with polypropylene tubing to divert the RO permeate stream to the probe. Typical flow-rates varied between 40–80 mL min−1, which was shown in preliminary testing to have no observable impact on fluorescence measurements. Electrical conductivity and pH for RO permeates at WRAMS were measured with a HACH HQ14d portable meter (Biolab, NSW, Australia) at a data collection interval of 15 minutes. Existing online sensors at GWRT were used to collect data for TOC, pH and EC at 10 minute intervals. The EC sensors measured the Stage 1 and Stage 2 permeates from each train while the TOC and pH probe measured the combined permeate from both trains.
Each sampling point was monitored simultaneously, with a side-stream diverted from the sampling point, first to the flow through cap of the fluorescence probe and then to the pH and EC probes.
Sampling points at the WRAMS site consisted of a single Stage 1 and Stage 2 module and the combined Stage 1 + Stage 2 permeate stream from each train. At the GWRT, permeates were sampled from the combined Stage 1 (15 modules), combined Stage 2 (8 modules) and the combined (Stage 1 + Stage 2; all modules) permeate from each train. Approximately 24 hours was targeted for monitoring duration at each sampling point but this varied due to clean-in-place (CIP) and shutdown events, as well as accessibility to the plants.
Extra caution was taken during the CIP events as the exact tolerance level of the sensor to caustic and acidic environments has not yet been fully explored. However, we have successfully analysed water in the laboratory within a pH range from 2 to 10. There were three separate trials. The sensor was running continuously for a maximum of 260 hours (11 days) in each trial with only the hose inlet switched to the different permeate modules and trains. Data from non-operational periods of the membranes has been excluded. No indication of fouling of the probe surface was detected during this period, and no deterioration of probe performance was observed during these experiments.
The log rejection is typically calculated using as:26
| LRV = log(Cf) − log(Cp) | (1) |
For the purposes of this study, the LRV was calculated as:
| LRV = log(Vf) − log(Vp) | (2) |
3. Results and discussion
3.1. Peak C membrane rejection
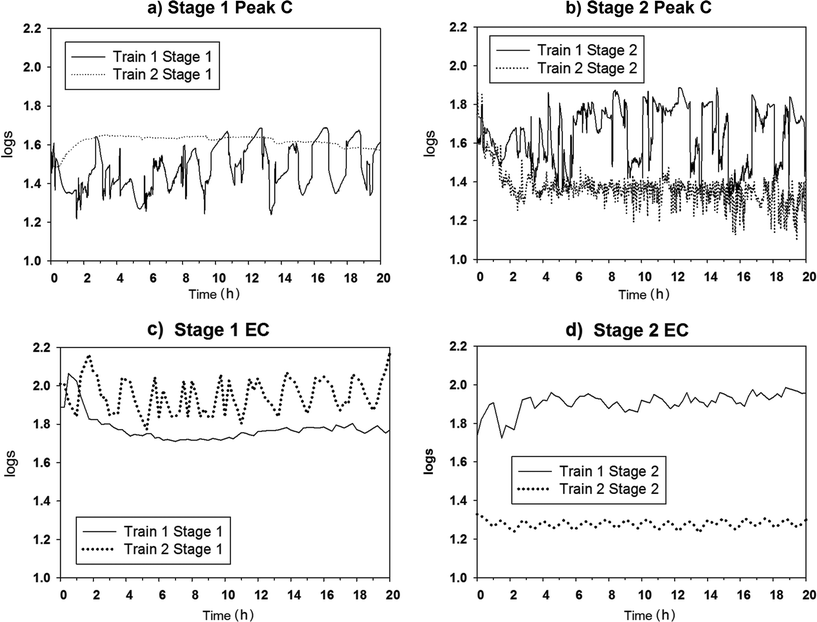
Trial 1 at WRAMS established Peak C and EC profiles for the RO feed and permeate. The rejection profiles over the monitoring period are illustrated in Fig. 2. The log rejection value (mean ± relative standard deviation) by Train 1 Stage 1 membranes was 1.51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log ± 8% for Peak C and 1.82
log ± 8% for Peak C and 1.82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log ± 4% for EC. Rejection by Train 2 Stage 1 membranes was 1.61
log ± 4% for EC. Rejection by Train 2 Stage 1 membranes was 1.61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log ± 2% (Peak C) and 1.91
log ± 2% (Peak C) and 1.91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log ± 5% (EC). Rejection by Stage 2 membranes were as follows, Train 1: 1.71
log ± 5% (EC). Rejection by Stage 2 membranes were as follows, Train 1: 1.71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log ± 9% (Peak C), 1.89
log ± 9% (Peak C), 1.89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log ± 3% (EC) and Train 2: 1.43
log ± 3% (EC) and Train 2: 1.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log ± 7% (Peak C) and 1.32
log ± 7% (Peak C) and 1.32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log ± 2% (EC).
log ± 2% (EC).
 | ||
| Fig. 2 Log rejection values (LRV) for membranes at WRAMS (a) Stage 1-Peak C (b) Stage 2-Peak C (c) Stage 1-EC (d) Stage 2-EC. | ||
Train 2 Stage 2 rejection was lower that of Train 1 Stage 2, indicative of an underperformance of Train 2 Stage 2 membranes in comparison to Train 1. The EC rejection difference of 0.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log between the Stage 2 membrane trains was noticeably larger than the Peak C rejection difference of 0.30
log between the Stage 2 membrane trains was noticeably larger than the Peak C rejection difference of 0.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log. While this implies that EC was more sensitive to this particular membrane underperformance, it also reveals that the passage of fluorescent organics and ionic species are dissimilar and that monitoring EC is unlikely to be an accurate surrogate measure to ensure on-going effective removal of organic chemicals.
log. While this implies that EC was more sensitive to this particular membrane underperformance, it also reveals that the passage of fluorescent organics and ionic species are dissimilar and that monitoring EC is unlikely to be an accurate surrogate measure to ensure on-going effective removal of organic chemicals.
The data presented in this study is uncorrected for pH, temperature and the inner filtering effect (IFE), which are known to impact fluorescence measurements. IFE occurs when the emitted light is absorbed by other molecular species present in the concentrated solution, thus giving an underestimation of the concentration of fluorescent intensity.27 IFE explains why previously reported grab sampling data obtained from the same sites19 contrasts with the results obtained in the current study. Results from the grab sampling study, which were diluted before analysis, demonstrate a Peak C rejection above 2 LRV (>99%), whereas in the online trials at WRAMS, Peak C rejection was below 2 LRV. The maximum light path length of the Cyclops sensor was approximately 10 mm, however the path length can be significantly reduced in concentrated solutions.28
The effects of temperature and pH on DOM have been well publicised,29–31 and a number of strategies have been proposed, including thermal correction methods.32 Generally, strategies used in grab sampling analysis, including dilution of samples, are impractical for routine online monitoring at water recycling plants. Correction with UV absorption may be possible using dual sensors but the cost and feasibility of such an enterprise requires further investigation. Temperature and pH correction are a possibility but would also require extra sensors and an associated program or software to automatically apply correction factors. Although these limitations can affect fluorescence intensities, the uncorrected data presented in this study can still be used effectively for monitoring deviations over time in the fluorescence intensities.
3.2. Case studies in RO membrane underperformance
Further monitoring of RO permeates at the GWRT and WRAMS plants showed unusual patterns in two RO permeate profiles which highlighted membrane underperformance issues. These case studies are discussed in sections 3.2.1 and 3.2.2.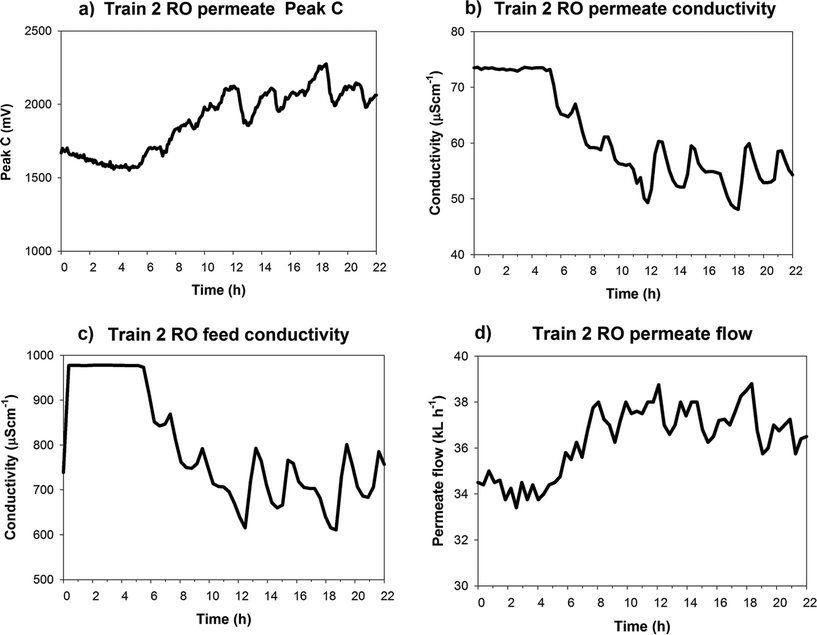 | ||
| Fig. 3 WRAMS Train 2 Stage 2 RO (a) permeate Peak C fluorescence (b) permeate electrical conductivity (c) feed conductivity (d) permeate flow. | ||
There are two possible explanations for the opposing trends observed between RO permeate EC and Peak C measurements. Firstly, the increase in permeate Peak C fluorescence may be in response to changes in RO feed Peak C fluorescence. The feedwater conductivity changed drastically within a short time. As the WRAMS scheme treats a blend of stormwater and biologically treated sewage, and an increase in stormwater composition may have increased the fluorescent DOM in the feedwater. As simultaneous fluorescence data for RO feed was not collected during this monitoring period, it could not be verified whether the changes to Peak C in permeate were in direct response to changes in the RO feed Peak C.
Results shown here are from Train 2 Stage 2 RO membranes. Train 1 membranes (Stage 1 + 2) and Train 2 Stage 1 were also monitored sequentially during this monitoring trial (results not shown). The RO permeate EC measured from all Trains/Stages in this study showed either weak (Pearson's r = −0.29 to −0.36, α = 0.05), or statistically insignificant correlation with Peak C fluorescence, with the exception of Train 2 Stage 2 RO membranes (Pearson's r = −0.96, α = 0.05). Thus it is unlikely that under normal operating conditions EC and Peak C would exhibit such strongly opposing trends.
A second possibility was that the membrane integrity was breached, which is plausible as lower LRVs were observed in Train 2 Stage 2 membranes compared to Train 1 Stage 2 membranes (Fig. 2).
The opposing Peak C and EC trends from Train 2 Stage 2 permeates (Fig. 3) can be explained by the solution-diffusion model (eqn (3)).33
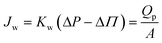 | (3) |
According to the solution-diffusion model, no water flux occurs until the applied pressure is greater than the osmotic pressure of the solution, and once the osmotic pressure is exceeded, the water flux will increase linearly with increasing applied pressure.33 Osmotic pressure of the solution is a function of feed, concentrate and permeate concentrations. The decreasing feed EC in Train 2 Stage 2 is due to decreasing concentration of solute ions in the RO feed and should result in a decrease in osmotic pressure. The WRAMS plant is operated under constant pressure conditions, thus a decrease in osmotic pressure would increase the difference between applied pressure and osmotic pressure, and increase the water flux through the membrane. Train 2 permeate flow showed the same incrementing pattern as Peak C (Fig. 3d). As the RO membranes at this plant were operated under constant pressure, it can be inferred that the increasing permeate flow (and membrane water flux) was related to the decreasing feed EC.
In an intact membrane, the flux of solute should remain reasonably constant over a range of applied pressure, as predicted by eqn (4):33
| Js = K(CA2 − CA3) | (4) |
Therefore when the applied pressure increases and water flux increases the solute concentration in the permeate should appear to decrease and rejection improve as more water molecules would pass though the membrane relative to solute.
Despite allowing for differential rejections of ionic substances (conductivity) and organic components (Peak C fluorescence), the increase in permeate flow or flux should not cause a significant increase in concentration of solutes. If the Train 2 Stage 2 membranes are impaired as the data suggests, then an increase in flux would force more solute to pass from the feed stream into the RO permeate unrestricted at the breach location and change the concentration of solutes in the RO permeate.
This was observed in the Peak C permeate profile which showed a signal increase as RO feed EC decreased (and flux increased), thus supporting the hypothesis of an integrity breach.
More importantly these results demonstrate the limitations of relying solely on EC as an RO permeate quality indicator. RO EC signals were observed to decrease, indicating improving permeate quality, while fluorescence revealed that there was in fact an increase in (Peak C fluorescent) organics in the RO permeate.
The TOC profile appeared to increase from 0.043 mg L−1 to 0.045 mg L−1 respectively and the mean profile values were 0.04 mg L−1 ± 2% (Fig. 4b). As TOC was measured on the combined permeate from both trains, any effects on TOC due to Train 2 Stage 1 RO modules was significantly reduced. Train 2 feed EC had increased from 1320 μS cm−1 to 1340 μS cm−1 (data not shown) while Train 2 permeate EC increased from 48 μS cm−1 to 53 μS cm−1 (mean = 51 μS cm−1 ± 5%) (Fig. 4c).
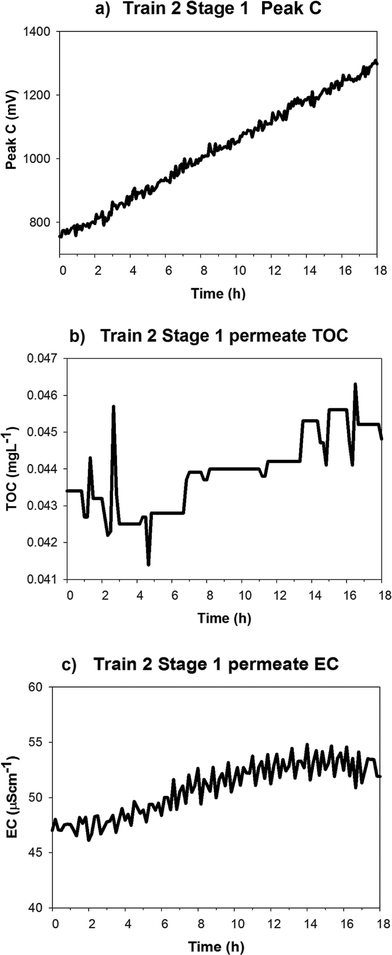
The GWRT plant was operated under constant flux which meant that flux did not exhibit large changes. Train 2 Stage 1 TMP increased from 366 kPa to 372 kPa over a sixteen hour period before shutdown was initiated and the specific flux appeared to decline over this same period (Fig. 5). A linear increase in the Peak C measurements was observed in Train 2 Stage 1 permeates, which corresponded with the TMP increase (Fig. 5a).
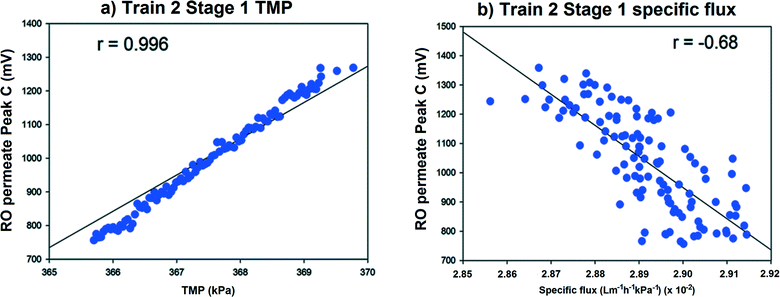 | ||
| Fig. 5 Relationship between Train 2 Stage 1 RO a) TMP and b) specific flux with Peak C fluorescence at GWRT plant. | ||
A strong correlation was observed (Pearson's r = 0.996) between increasing TMP and Peak C in Train 2 Stage 1 membranes (Fig. 5a). The Peak C signal had increased to double its original value, while in the other Stage 1 membrane the Peak C signal had a very small change in response to TMP increase. This suggests that the observed increase in Peak C in the permeate was not caused by the TMP increase but vice versa. Specific flux was negatively correlated (Pearson's r = −0.68) to Peak C permeate fluorescence (Fig. 5b) and this also implied that decreases in specific flux may be related to increasing permeate Peak C fluorescence. No statistically significant correlations were observed (at α = 0.05) between TMP and specific flux with RO permeate TOC and EC signals.
A major cause of flux decline and increasing TMP is membrane fouling.34–36 Hoek and Elimelech identified cake enhanced concentration polarisaton (CECP) as a source of flux decline in RO membranes.37 This phenomena occurs when colloidal particles are present in the feed solution. These particles form the concentration polarisation layer and cake layer on the membrane surface. The cake layer hinders solute back diffusion, increases osmotic pressure and promotes flux decline.37
The RO membranes used in the current GWRT plant were different to those used at the pilot plant and had not performed to expectations, requiring frequent cleaning due to increasing TMP requirements. The cause of this was suspected to be from membrane fouling.38
A linear increase in Peak C fluorescence of Train 2 Stage 1 permeate was observed and this corresponded to the increase in TMP. This rapid increase may be due to decreased rejection caused by membrane fouling. The Peak C sensor operates in the Peak C fluorescence region, and fluorophores in this optical region are recognised as constituents of membrane foulants.39–41 As such, it appears plausible that monitoring Peak C may provide an effective means of predicting degrees and rates of organic fouling on membranes. The results obtained in this study suggest that Peak C may indeed be a sensitive indicator of membrane fouling potential compared to EC and TOC, and could have potential application in early indication of organic fouling.
Membrane fouling has been reported to increase the rejection of hydrophobic non-ionic organic contaminants in RO and NF membranes under constant pressure conditions.42 In the same study, a decrease in rejection was observed for hydrophilic non-ionic organics, possibly due to the decrease in surface charge from membrane fouling. Ng et al.43 found that colloidal fouling decreased permeate flux and rejection of low molecular weight organics.
3.4 Advantages and limitations
The fluorescence sensor was sufficiently sensitive to detect the subtle differences between RO permeates and respond to changes in the membrane performance. In particular, anomalous Peak C profiles were recorded for membranes that appeared not to be functioning optimally at both plants in this study. The TOC and EC values of the RO permeates from the two plants were within the range reported at other RO plants,44,45 and are thus not atypical of expected RO permeate quality. However, this cannot be used to conclude that the Peak C fluorescence reported here will also be typical to that measured at other RO plants. It is as yet unknown what proportion of DOM is fluorescent, what proportion of fluorescent DOM is constituted by Peak C, and how variable these are in different feedwater types. No consistent correlations of Peak C permeate fluorescence with EC and TOC were observed, which suggests that the rejection of these by RO are dissimilar and cannot be predicted by a single parameter. As such it is recommended that a pilot study be undertaken before implementing fluorescence RO monitoring in advanced water treatment facilities.The sensor ran continuously for 11 days, however further testing would be required to assess their long term robustness for ongoing RO process monitoring.
The results from GWRT and WRAMS indicate that monitoring the permeate at the operational stages within an RO system rather than just the combined permeate can increase opportunities for detecting underperformance issues as this would eliminate the masking of specific RO stage problems from dilution when the permeates are combined. Placing multiple sensors at strategic locations, while useful, can be an expensive enterprise. Measuring each stage permeate may be difficult to implement in practice, especially in large RO installations.
Currently, there are several fluorescence measuring systems available which can be used for online monitoring. These are relatively inexpensive and can be interfaced readily to supervisory control and data acquisition (SCADA) systems. The Cyclops 7 fluorescence sensor and data logger in this study were purchased for AU$6000 while the estimated cost of the online TOC analyser in use at the GWRT was approximately AU$40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000,38 making fluorescence sensors economically competitive.
000,38 making fluorescence sensors economically competitive.
Conclusions
Fluorescence spectroscopy has the potential to be a powerful online monitoring tool for RO performance and permeate quality. The results showcase the first successful fluorescence-based online monitoring trial of RO systems at full-scale advanced water recycling plants.The Cyclops 7 in situ Peak C (excitation at λ = 350 nm and emission at λ = 430 ± 30 nm probe was able to measure and detect subtle changes to fDOM concentrations in RO permeates at various operational stages and identify underperforming membranes. Membrane rejection (as LRV) for Peak C was lower than that observed for EC.
The sensitivity of fluorescence was highlighted as the sensor showed an increase in fluorescent organics from an underperforming membrane suspected to have an integrity breach, while EC results showed an improvement in permeate water quality, indicating that EC is not an ideal surrogate measure for organic species (in particular for uncharged organic species). The enhanced Peak C signal from the breached membrane was detected by the fluorescence sensor and could be differentiated from other permeates thus proving the sensitivity of fluorescence to detect integrity loss in RO membranes.
Increases in Stage 1 permeate Peak C fluorescence was linked to increasing TMP (Pearson's r = 0.996) at the GWRT plant. This may have been due to organic fouling on the membrane as a decrease in flux was also observed. This highlights the potential of the Peak C sensor to be used for early indication of organic fouling potential in membranes.
The results from both plants indicate Peak C fluorescence is quite sensitive to RO membrane performance. The sensitivity of the fluorescence sensor to detect subtle changes in (fluorescent) organic matter in the RO permeate is maximised when individual modules or specific arrays of operational stages are monitored rather than the final combined permeate stream. The fluorescence sensor can be easily integrated into SCADA systems at recycled water treatment facilities either with the data logger or independently as an analog device.
While results obtained, in terms of log removal, were low in this study; technology in this area is rapidly improving and this research provides further issues for consideration in the development of new generation fluorescence instruments for monitoring water treatment processes.
Acknowledgements
This sampling program was funded by the Australian Research Council Linkage Projects (LP0776347) with additional contributions from Sydney Water Corporation, Sydney Olympic Park Authority (SOPA), Gold Coast City Council, Melbourne Water, Yarra Valley Water, City West Water, South East Water and Water Corporation of Western Australia. The authors thank Palenque Blair, Kevin Martin, Daniel Bisgrove, Adam Henderson, Paul Skelsy, Glen McGregor (Water Corporation); Michael James, Ralph Wardell (United KG) and Andrzej Listowski (SOPA) for their assistance in facilitating the monitoring trials.References
- L. Malaeb and G. M. Ayoub, Desalination, 2011, 267, 1–8 CrossRef CAS PubMed.
- S. Adham, P. Gagliardo, D. Smith, D. Ross, K. Gramith and R. Trussell, Desalination, 1998, 119, 143–150 CrossRef CAS.
- M. Kumar, S. Adham and J. DeCarolis, Desalination, 2007, 214, 138–149 CrossRef CAS PubMed.
- R. K. Henderson, R. M. Stuetz and S. J. Khan, Water Sci. Technol., 2010, 62, 2747–2753 CrossRef CAS PubMed.
- R. K. Henderson, A. Baker, K. R. Murphy, A. Hambly, R. M. Stuetz and S. J. Khan, Water Res., 2009, 43, 863–881 CrossRef CAS PubMed.
- K. Kimura, T. Naruse and Y. Watanabe, Water Res., 2009, 43, 1033–1039 CrossRef CAS PubMed.
- Z. Wang, Z. Wu and S. Tang, Water Res., 2009, 43, 1533–1540 CrossRef CAS PubMed.
- Y. An, Z. Wang, Z. Wu, D. Yang and Q. Zhou, Chem. Eng. J., 2009, 155, 709–715 CrossRef CAS PubMed.
- S. Tang, Z. Wang, Z. Wu and Q. Zhou, J. Hazard. Mater., 2010, 178, 377–384 CrossRef CAS PubMed.
- T. Jiang, M. D. Kennedy, V. D. Schepper, S. N. Nam, I. Nopens, P. A. Vanrolleghem and G. Amy, Environ. Sci. Technol., 2010, 44, 6642–6648 CrossRef CAS PubMed.
- Y. Wang, C. Zhong, D. Dan Huang, Y. Wang and J. Zhu, Bioresour. Technol., 2013, 136, 488–495 CrossRef CAS PubMed.
- N. Lee, G. Amy and J. P. Croue', Water Res., 2006, 40, 2357–2368 CrossRef CAS PubMed.
- R. H. Peiris, H. Budman, C. Moresoli and R. L. Legge, J. Membr. Sci., 2010, 357, 62–72 CrossRef CAS PubMed.
- S. Zhou, Y. Shao, N. Gao, L. Li, J. Deng, C. Tan and M. Zhu, Sci. Total Environ., 2014, 470–471, 201–207 CrossRef CAS PubMed.
- F. Qu, H. Liang, J. Zhou, J. Nan, S. Shao, J. Zhang and G. Li, J. Membr. Sci., 2014, 449, 58–66 CrossRef CAS PubMed.
- K. Kimura, K. Tanaka and Y. Watanabe, Water Res., 2014, 49, 434–443 CrossRef CAS PubMed.
- F. Chen, S. Peldszus, R. H. Peiris, A. S. Ruhl, R. Mehrez, M. Jekel, R. L. Legge and P. M. Huck, Water Res., 2014, 48, 508–518 CrossRef CAS PubMed.
- B. R. H. Peiris, C. Halle, J. Haberkamp, R. L. Legge, S. Peldszus, C. Moresoli, H. Budman, G. Amy, M. Jekel and P. M. Huck, Water Sci. Technol.: Water Supply, 2008, 8, 459–465 CrossRef CAS.
- S. Singh, R. K. Henderson, A. Baker, R. M. Stuetz and S. J. Khan, J. Membr. Sci., 2012, 421–422, 180–189 CrossRef CAS PubMed.
- M.-L. Pype, D. Patureau, N. Wery, Y. Poussade and W. Gernjak, J. Membr. Sci., 2013, 48, 205–211 CrossRef PubMed.
- S. B. Abdelmelek, J. Greaves, K. P. Ishida, W. J. Cooper and W. Song, Environ. Sci. Technol., 2011, 45, 3665–3671 CrossRef PubMed.
- B. Wu, T. Kitade, T. H. Chong, T. Uemura and A. G. Fane, Desalination, 2013, 311, 37–45 CrossRef CAS PubMed.
- S. Singh, R. K. Henderson, A. Baker, S. J. Khan and R. M. Stuetz, Water Sci. Technol., 2009, 60, 2017–2023 CrossRef CAS PubMed.
- K. R. Murphy, A. Hambly, S. Singh, R. K. Henderson, A. Baker, R. Stuetz and S. J. Khan, Environ. Sci. Technol., 2011, 45, 2909–2916 CrossRef CAS PubMed.
- P. G. Coble, Mar. Chem., 1996, 51, 325–346 CrossRef CAS.
- A. Bennett, Drinking water: Pathogen removal from water - technologies and techniques, Filtr. Sep., 2008, 45(10), 14–16 CrossRef CAS.
- K. R. Murphy, K. D. Butler, R. G. M. Spencer, C. A. Stedmon, J. Boehme and G. R. Aiken, Environ. Sci. Technol., 2010, 44, 9405–9412 CrossRef CAS PubMed.
- What volume do your instruments measure?, http://www.turnerdesigns.com/component/content/article/155-general-fluorometer-faqs/836-volume-measured, (accessed 9 June, 2015).
- A. Baker, Water Res., 2005, 39, 4405–4412 CrossRef CAS PubMed.
- B. D. Downing, B. A. Pellerin, B. A. Bergamaschi, J. F. Saraceno and T. E. C. Kraus, Limnol. Oceanogr.: Methods, 2012, 10, 767–775 CrossRef CAS.
- A. Baker, S. Elliott and J. R. Lead, Chemosphere, 2007, 67, 2035–2043 CrossRef CAS PubMed.
- C. J. Watras, P. C. Hanson, T. L. Stacy, K. M. Morrison, J. Mather, Y.-H. Hu and P. Milewski, Limnol. Oceanogr.: Methods, 2011, 9, 296–301 CrossRef CAS.
- J. Kucera, Reverse Osmosis: Industrial applications and processes, Scrivener Publishing LLC, John Wiley & Sons, Inc, Hoboken, New Jersey, 2010 Search PubMed.
- H.-A. Kim, J.-H. Choi and S. Takizawa, Sep. Purif. Technol., 2007, 56, 354–362 CrossRef CAS PubMed.
- S. R. Suwarno, X. Chen, T. H. Chong, V. L. Puspitasari, D. McDougald, Y. Cohen, S. A. Rice and A. G. Fane, J. Membr. Sci., 2012, 405–406, 219–232 CrossRef CAS PubMed.
- P. Xu, C. Bellona and J. E. Drewes, J. Membr. Sci., 2010, 353, 111–121 CrossRef CAS PubMed.
- E. M. V. Hoek and M. Elimelech, Environ. Sci. Technol., 2003, 37, 5581–5588 CrossRef CAS.
- K. Martin , personal communication.
- H. C. Kim and B. A. Dempsey, J. Membr. Sci., 2013, 428, 190–197 CrossRef CAS PubMed.
- J. Shao, L. Zhao, X. Chen and Y. He, J. Membr. Sci., 2013, 435, 38–45 CrossRef CAS PubMed.
- S. Lee, B. Kwon, M. Sun and J. Cho, Desalination, 2005, 173, 131–142 CrossRef CAS PubMed.
- P. Xu, J. E. Drewes, T. U. Kim, C. Bellona and G. Amy, J. Membr. Sci., 2006, 279, 165–175 CrossRef CAS PubMed.
- H. Y. Ng, Desalination, 2005, 174, 211–217 CrossRef CAS PubMed.
- J.-J. Qin, M. H. Oo, M. N. Wai and K. A. Kekre, Sep. Purif. Technol., 2005, 46, 125–128 CrossRef CAS PubMed.
- C. Bele, Y. Kumar, T. Walker, Y. Poussade and V. Zavlanos, Water Sci. Technol., 2010, 62, 1560–1566 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |