A fixed bed column study for the removal of Pb2+ ions by watermelon rind
R.
Lakshmipathy
a and
N. C.
Sarada
*b
aCentre for Material Science, KCG College of Technology, Karapakkam, Chennai, India-600097. E-mail: lakshmipathy.vit@gmail.com
bEnvironmental and Analytical Chemistry division, School of Advanced Sciences, VIT University, Vellore, India-632014. E-mail: ncsarada@vit.ac.in; Fax: +914162243092; Tel: +91 416 2202358
First published on 26th January 2015
Abstract
The present study reports the feasibility of removing Pb2+ ions from aqueous solution using watermelon rind (WR) as a low cost adsorbent. Fixed bed column studies were employed to study the removal efficiency of Pb2+ ions by varying the column parameters such as flow rate, bed height and initial metal ion concentration. The results showed that breakthrough and exhaustion time increases with a decrease in flow rate, inlet concentration, and an increasing bed height. The breakthrough curves obtained were analyzed with Adams–Bohart, Thomas and Yoon–Nelson models. On comparison of the R2 values, both the Thomas and Yoon–Nelson models were found to have a better fit than the Adams–Bohart model and these two models can be used to predict the adsorption of Pb2+ ions in a fixed bed column. Desorption of Pb2+ ions on WR was repeated for three cycles in 0.1 M HCl solution. The loading capacity of WR was compared with other adsorbents and was found to be high. These results show that watermelon rind, a non-hazardous agro waste, can be successfully employed for the elimination of Pb2+ ions from aqueous solution.
Water impactThe majority of adsorption studies have been carried out in batch modes which are limited to a laboratory process and generally not applicable to most of the long term treatment processes. Continuous column studies are the most valuable and economical treatment processes used in practice for the removal of heavy metal ions from industrial effluents. Activated carbon is a commonly used sorbent for the removal of inorganic and organic pollutants from waste water. In spite of its effectiveness in the removal of pollutants, high activation cost limits its use as an economical adsorbent. Hence, the present study reports the use of a low cost adsorbent such as watermelon rind for the removal of Pb2+ ions from aqueous solution. |
1 Introduction
The increasing trend of industrialization in recent decades has resulted in severe environmental contaminations. Heavy metal ions are distinguished from other toxic pollutants, due to their non-biodegradability which causes severe physiological or neurological damage to human and aquatic life.1 Various treatment methodologies include precipitation, oxidation/reduction, ion-exchange, membrane filtration, electrochemical reduction, and adsorption.2 Each process has its own advantage and disadvantage; however adsorption produces high quality treated water with low cost.3,4Activated carbon is a commonly used sorbent for the removal of inorganic and organic pollutants from waste water. In spite of its effectiveness in the removal of pollutants, high activation cost limits its use as an economical adsorbent. Many waste by-products from the food industry have been evaluated for the removal of heavy metals from waste water as low cost economical sorbents.5–8 Agricultural by-products are rich in functional groups like pectin, lignin and cellulose which can easily facilitate the binding of metal ions.9 The majority of adsorption studies have been carried out in batch modes which are limited to a laboratory process and generally not applicable to most of the long term treatment processes.10 Continuous column studies are the most valuable and economical treatment processes used in practice.11,12 Hence, the study of the removal of heavy metals in continuous flow using agricultural by-products is desirable.
Watermelon (Citrullus lanatus), being the largest and heaviest fruit, is one of the most abundant and cheapest fruit available in India with 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons produced every year. Watermelon production occupies 6–7% of overall fruit production and is high during the summer because of its tropical nature. In watermelon, the red flesh present inside is sweet, edible and used for juices and salads but the outer rind is considered as waste and has no commercial value.13 Watermelon rind (WR) consists of phenols, citrulline, cellulose, proteins and caroteniods.14–16 These polymers are rich in functional groups like hydroxyl (cellulose) and carboxylic (pectin) and can easily bind metal. Lead (Pb2+) is one of the prominent pollutants among the heavy metals which cause damage to the kidney, nervous system and liver, and can cause still births and sterility.17,18 Hence, it is environmentally desirable to remove Pb2+ from waste water and industrial effluents.
000 tons produced every year. Watermelon production occupies 6–7% of overall fruit production and is high during the summer because of its tropical nature. In watermelon, the red flesh present inside is sweet, edible and used for juices and salads but the outer rind is considered as waste and has no commercial value.13 Watermelon rind (WR) consists of phenols, citrulline, cellulose, proteins and caroteniods.14–16 These polymers are rich in functional groups like hydroxyl (cellulose) and carboxylic (pectin) and can easily bind metal. Lead (Pb2+) is one of the prominent pollutants among the heavy metals which cause damage to the kidney, nervous system and liver, and can cause still births and sterility.17,18 Hence, it is environmentally desirable to remove Pb2+ from waste water and industrial effluents.
The present study reports the removal of Pb2+ ions from aqueous solution through continuous column studies using WR, a green and economical sorbent. The influence of several operational parameters (flow rate, bed depth and influent concentration) has been studied and the experimental data were fit to various models that describe the breakthrough curves.
2 Materials and methods
2.1 Preparation of adsorbent
Watermelon rinds (WRs) were obtained from a local fruit market and washed under tap water several times followed by washing with double distilled water. After thorough washing the WR was cut in to small pieces and dried under sun light for 7 days to remove all the moisture content present. Later, the dried WR pieces were washed with hot water (70 °C) to remove any soluble matter present and dried in an oven at 85 °C for 48 h. The oven dried WR was powdered using conventional mixture and sieved between a 500–1000 BSS mesh. The sieved WR particles were stored in an air tight container and used for further column experiments.2.2 Column adsorption studies
Continuous column adsorption experiments were performed in a laboratory glass column with an internal diameter of 1 cm and a length of 15 cm. Initially the column was packed with WR (0.354 g cm−3) and a synthetic Pb2+ ion solution was fed into the column from the top at a desired flow rate using a peristaltic pump. The samples were collected at the exit of the column at different time intervals and the concentration of the eluted samples was determined using a Flame atomic absorption spectrophotometer (AAS). To study the effect of flow rate on the adsorption of Pb2+ ions onto WR, experiments were conducted at three different flow rates: 1, 2 and 3 ml min−1. The study of the effect of bed height on the column adsorption process was conducted at three different bed heights: 1, 3 and 5 cm, keeping other parameters constant. After optimizing the flow rate and bed height, the effect of the initial concentration of metal ion was studied at 500, 750 and 1000 mg L−1. The flow to the column was continued until there was no adsorption i.e. the Pb2+ concentration of influent and effluent remained unchanged.19 In order to ensure the accuracy and reproducibility of all the data, all the column adsorption experiments were conducted in triplicate, and the mean values were used in the data analysis. The percentage standard deviation was found to be not more than 3%.2.3 Column data analysis
The performances of the packed bed column studies are obtained in the form of breakthrough curves. The time for the breakthrough and the shape of the curve are very important characteristics for determining the operation and dynamic response of a sorption column.20 The breakthrough point of an S shaped curve is considered to be when the effluent concentration (Ct) from the column reaches about 0.1% of the influent concentration. The column is considered to be saturated or exhausted at the point when the concentration of effluent reaches 95%. The breakthrough curve is generally expressed by Ct/Co as a function of time or volume of the effluent. The effluent volume can be calculated from the equation:| Veff = Qttotal | (1) |
Where Q is the volumetric flow rate and ttotal is the total flow time (min).
The total metal adsorbed, Mad (mg), in the fixed bed column can be calculated from the area under the curve multiplied by the flow rate eqn (2).
 | (2) |
Where Cad is the concentration of metal ion removal (mg L−1).
The total amount of metal ions entering the column (Mtotal) is calculated from the following equation:
 | (3) |
And the total metal removal percentage can be obtained from eqn (4):
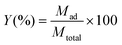 | (4) |
2.4 Column breakthrough curve modeling
The successive operation of a lab scale column towards industrial applications can be well explained by simple mathematical models.21,22 The breakthrough curves obtained for flow rate, bed height and initial metal ion concentration were predicted with well known mathematical models such as the Adams–Bohart, Thomas and Yoon–Nelson models.The Adams–Bohart model23 is one of the most widely used models for the prediction of column breakthrough curves. According to this model, the equilibrium is not instantaneous and the rate of sorption is proportional to the fraction of sorption capacity.24,25 This model is used to explain the initial part of the breakthrough curve and the model equation is expressed as
 | (5) |
One of the most general and widely used models for describing the performance theory of the sorption process in a fixed bed column is the Thomas model.26 This model assumes the plug flow behavior in the bed and the equation is expressed as
 | (6) |
Yoon–Nelson developed a relatively simple model for the adsorption of vapours or gases in activated coal.27 This model assumes that the rate of decrease in the probability of adsorption for each adsorbate molecule is proportional to the probability of sorbate sorption and the probability of sorbate breakthrough on the sorbent. The linear form of the equation is:
 | (7) |
3 Results and discussion
3.1 Characterization of adsorbent
The physico-chemical parameters of WR are summarized and represented in Table 1. The total acidic and basic sites of WR were determined using the traditional Boehm titration method. The acidic sites of WR were found to be high in number compared to the basic sites, suggesting that WR is capable of binding cations. The FTIR spectra of native watermelon rind (Fig. 1) displayed a number of peaks pertaining to different functional groups. The broad and intense peak at around 3371 cm−1 corresponds to –OH (hydroxyl) stretching vibrations of cellulose and pectin, and the peaks at 2917 cm−1 are attributed to –CH stretching vibrations of methyl groups. A peak at 1734 cm−1 corresponds to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of carboxylic acid groups or esters, and asymmetric and symmetric vibrations of ionic carboxylic groups (–COO−), respectively, appeared at 1633, and 1423 cm−1. These results suggest that WR is rich in metal ion binding functional groups such as hydroxyl, carboxyl and carbonyl groups. The SEM analysis was carried out to study the surface morphology of WR (Fig. 2). It is observed from the image that the surface was porous in nature. In general, porous surface materials act as very good adsorbents for sequestration of heavy metal ions.
O stretching of carboxylic acid groups or esters, and asymmetric and symmetric vibrations of ionic carboxylic groups (–COO−), respectively, appeared at 1633, and 1423 cm−1. These results suggest that WR is rich in metal ion binding functional groups such as hydroxyl, carboxyl and carbonyl groups. The SEM analysis was carried out to study the surface morphology of WR (Fig. 2). It is observed from the image that the surface was porous in nature. In general, porous surface materials act as very good adsorbents for sequestration of heavy metal ions.
| Parameter | WR |
|---|---|
| Point zero charge (pHpzc) | 4.9 |
| Moisture (%) | 13 |
| Density (g cc−1) | 0.35 |
| Acidic sites (mmol g−1) | 2.46 |
| Basic sites (mmol g−1) | 0.71 |
| Total cationic content (meq g−1) | 2.10 |
3.2 Fixed bed column studies
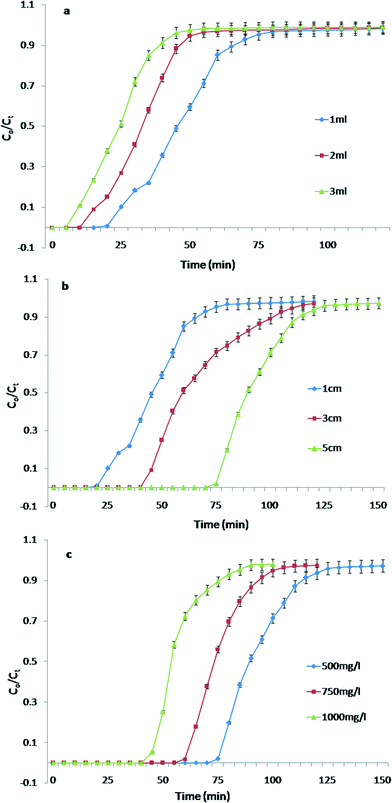
The breakthrough curves obtained are represented in Fig. 3a and the adsorption column data are summarized in Table 2. It was observed that with an increase in flow rate the breakthrough time decreases from 20 to 10 min. Similarly the saturation time of the column was also observed to decrease from 75 to 45 min. The removal efficiency of the WR substantially decreased with an increase in flow rate. This can be attributed to the fact that at a low flow rate, the residence time of the Pb2+ ions in the column increases and as result the Pb2+ ions have more time to diffuse into the pores of the WR through intraparticle diffusion resulting in a longer breakthrough time and saturation time.28
| Flow rate (ml) | Bed height (cm) | Initial metal concentration (mg L−1) | Breakthrough time (min) | Saturation time (min) | M ad (mg) | M total (mg) | Total % removal | EBCT (min) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 500 | 20 | 75 | 22.06 | 37.5 | 58.8 | 2.5 |
| 2 | 1 | 500 | 15 | 55 | 14.97 | 55 | 27.2 | 1.55 |
| 3 | 1 | 500 | 10 | 45 | 10.91 | 67.5 | 16.1 | 1.0 |
| 1 | 1 | 500 | 20 | 75 | 22.06 | 37.5 | 58.8 | 3 |
| 1 | 3 | 500 | 45 | 100 | 31.2 | 50 | 62.4 | 7.1 |
| 1 | 5 | 500 | 75 | 125 | 45.3 | 62.5 | 72.5 | 12.2 |
| 1 | 5 | 500 | 75 | 125 | 45.3 | 62.5 | 72.5 | — |
| 1 | 5 | 750 | 60 | 105 | 55.0 | 78.5 | 69.9 | — |
| 1 | 5 | 1000 | 45 | 90 | 54.0 | 90 | 61.0 | — |
3.3 Breakthrough curve modeling
The breakthrough curves obtained at different parameters can be used for predicting the efficacy of a particular design of column. Several mathematical models have been proposed for predicting the efficacy of lab scale column studies for the purpose of industrial applications. In the present study, Adams–Bohart, Thomas, Yoon–Nelson models are employed for the best fit model in predicting the dynamic behaviour of the columns.| Parameter | K AB (L mg−1 min) | N o (mg L−1) | R 2 | |
|---|---|---|---|---|
| Flow rate (ml) | 1 | 3.2 × 10−3 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 372 372 |
0.963 |
| 2 | 2.7 × 10−3 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128 128 |
0.979 | |
| 3 | 2.5 × 10−3 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 705 705 |
0.961 | |
| Bed height (cm) | 1 | 3.2 × 10−3 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 372 372 |
0.963 |
| 3 | 4.3 × 10−3 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 679 679 |
0.913 | |
| 5 | 7.6 × 10−3 | 9562 | 0.833 | |
| Initial metal concentration (mg L−1) | 500 | 7.6 × 10−3 | 9562 | 0.833 |
| 750 | 5.4 × 10−3 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 985 985 |
0.866 | |
| 1000 | 3.5 × 10−3 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 278 278 |
0.892 | |
The correlation coefficients obtained for the flow rate breakthrough curves were found to be high suggesting that the Adams–Bohart model is applicable for predicting the initial process of the present system. The correlation coefficients obtained for bed heights started to decrease with the increase in bed height and in the case of initial metal ion concentration, the correlation coefficient increases with the increase in initial metal ion concentration. These observations suggest that the Adams–Bohart model is not applicable to the present process at higher bed heights and initial metal ion concentrations.
| Parameter | K Th (ml min−1 mg) | q o (mg g−1) | R 2 | |
|---|---|---|---|---|
| Flow rate (ml) | 1 | 4.7 × 10−5 | 35.5 | 0.948 |
| 2 | 9.9 × 10−5 | 31.6 | 0.994 | |
| 3 | 1.5 × 10−4 | 27.3 | 0.998 | |
| Bed height (cm) | 1 | 1.1 × 10−5 | 35.5 | 0.948 |
| 3 | 1.0 × 10−5 | 41.2 | 0.971 | |
| 5 | 1.1 × 10−5 | 50.3 | 0.910 | |
| Initial metal concentration (mg L−1) | 500 | 1.5 × 10−5 | 50.3 | 0.910 |
| 750 | 1.5 × 10−5 | 63.9 | 0.925 | |
| 1000 | 1.4 × 10−5 | 59.4 | 0.920 | |
| Parameter | K YN (min−1) | τ (min) | R 2 | |
|---|---|---|---|---|
| Flow rate (ml) | 1 | 0.529 | 9.69 | 0.935 |
| 2 | 0.733 | 6.44 | 0.992 | |
| 3 | 0.723 | 4.76 | 0.997 | |
| Bed height (cm) | 1 | 0.529 | 9.69 | 0.935 |
| 3 | 0.483 | 12.65 | 0.972 | |
| 5 | 0.587 | 18.90 | 0.904 | |
| Initial metal concentration (mg L−1) | 500 | 0.587 | 18.90 | 0.904 |
| 750 | 0.739 | 15.66 | 0.885 | |
| 1000 | 0.633 | 11.63 | 0.905 | |
3.4 Desorption studies
Desorption and regeneration of the adsorbent in a fixed bed column is of importance in practical applications. In order to study the desorption and regeneration efficiency of WR, the fixed bed column containing Pb2+ ions loaded on the adsorbent was eluted with 0.1 M HCl as a desorbing agent. Since 0.1 M HCl showed good desorption efficiency in batch studies the same agent was used in the present study. It is observed from Fig. 4 that a 95% desorption efficiency was achieved within 15 min of run time. The maximum desorption and regeneration efficiency achieved with 0.1 M HCl for three repeated cycles was found to be 95%. These results suggest that WR is a potential adsorbent for the removal of heavy metal ions in a fixed bed column with repeated usage.3.5 Maximum loading capacity
The maximum loading capacity of WR towards Pb2+ ions in the fixed bed column was found to be 55.0 mg g−1. The loading capacity attained by the WR towards Pb2+ ions was compared with other adsorbents reported in literature and was found to be high (Table 6).| Adsorbent | Loading capacity (mg g−1) | Reference |
|---|---|---|
| a Fixed bed column study. | ||
| Ficus religiosa leaves | 37.4 | 30 |
| Mustard husk | 30.4 | 31 |
| Saw dust | 21.0 | 32 |
| Modified peanut husk | 29.1 | 32 |
| Sago waste | 46.6 | 33 |
| Papaya wood | 17.4 | 34 |
| Barley straws | 23.2 | 35 |
| Pomegranate peel | 13.8 | 36 |
| Banana peel | 41.4 | 37 |
| Watermelon rinda | 55.0 | This study |
4 Conclusion
Watermelon rind, an agro waste, was employed as an adsorbent for the removal of Pb2+ ions from aqueous solution. Fixed bed column studies were employed by varying the parameters such as flow rate, bed height and initial metal ion concentration. The column removal efficiency decreased with the increase in flow rate and the removal efficiency increased with the increase in bed height. The breakthrough curves obtained were successfully predicted with mathematical models such as the Adams–Bohart, Thomas and Yoon–Nelson models. It was found that the Thomas and Yoon–Nelson models fit well to the breakthrough curves and can be used in real time large scale industrial treatment processes. Successful desorption and regeneration of the adsorbent demonstrated the economical value of WR in the treatment of waste water.Notes and references
- S. Mohan and G. Sreelakshmi, J. Hazard. Mater., 2008, 153, 75 CrossRef CAS PubMed.
- O. Keskinkan, M. K. L. Goksu, A. Yuceer, M. Basibuyuk and C. F. Forster, Process Biochem., 2003, 39, 179 CrossRef CAS.
- E. Malkoc, Y. Nuhoglu and Y. Abali, Chem. Eng. J., 2006, 119, 61 CrossRef CAS PubMed.
- P. D. Saha, S. Chowdhury, S. Datta and S. K. Sanyal, Korean J. Chem. Eng., 2012, 29, 1086 CrossRef.
- S. Y. Quek and B. Al-Duri, Chem. Eng. Process., 2007, 46, 477 CrossRef CAS PubMed.
- S. Chowdhury and P. D. Saha, Korean J. Chem. Eng., 2013, 30, 369 CrossRef CAS PubMed.
- S. Inbaraj and N. Sulochana, Bioresour. Technol., 2004, 94, 49 CrossRef PubMed.
- P. D. Saha, S. Chakraborty and S. Chowdhury, Colloids Surf., B, 2012, 92, 262 CrossRef PubMed.
- M. Iqbal, A. Saeed and S. I. Zafar, J. Hazard. Mater., 2009, 164, 161 CrossRef CAS PubMed.
- W. Li, Q. Yue, P. Tu, Z. Ma, B. Gao, J. Li and X. Xu, Chem. Eng. J., 2011, 178, 197 CrossRef CAS PubMed.
- S. Chowdhury and P. D. Saha, Environ. Prog. Sustainable Energy, 2013, 32, 633 CrossRef CAS.
- S. Chowdhury and P. D. Saha, Environ. Sci. Pollut. Res., 2013, 20, 1050 CrossRef CAS PubMed.
- L. R. Veeranjaneya, Y. K. Kumar, L. P. Anjaneya and O. V. Sarathi, Process Biochem., 2008, 43, 748 CrossRef PubMed.
- A. M. Rimando and P. M. Perkins-Veazie, J. Chromatogr. A, 2005, 1078, 196 CrossRef CAS PubMed.
- A. Mort, Y. Zheng, F. Qiu, M. Nimtzc and G. Bell-Eunice, Carbohydr. Res., 2008, 343, 1212 CrossRef CAS PubMed.
- S. Y. Quek, N. K. Chok and P. Swedlund, Chem. Eng. Process., 2007, 46, 386 CrossRef CAS PubMed.
- A. G. El-Said, J. Am. Sci., 2010, 6, 143–150 Search PubMed.
- B. M. W. P. K. Amarasinghe and R. A. Williams, Chem. Eng. J., 2007, 132, 299 CrossRef CAS PubMed.
- S. S. Baral, N. Das, T. S. Ramulu, S. K. Sahoo, S. N. Das and G. R. Chaudhury, J. Hazard. Mater., 2009, 161, 1427 CrossRef CAS PubMed.
- A. A. Ahmad and B. H. Hameed, J. Hazard. Mater., 2010, 175, 298 CrossRef CAS PubMed.
- P. A. Kumar and S. Chakraborty, J. Hazard. Mater., 2009, 162, 1086 CrossRef CAS PubMed.
- V. Vinodhini and N. Das, Desalination, 2010, 264, 9 CrossRef CAS PubMed.
- G. Bohart and E. Q. Adams, J. Am. Chem. Soc., 1920, 42, 523 CrossRef.
- W. W. Eckenfelder Jr, Industrial Water Pollution Control, McGraw Hill, USA, 1989, p. 273 Search PubMed.
- M. Lehman, A. I. Zouboulis and K. A. Matis, Environ. Pollut., 2001, 113, 121 CrossRef.
- H. C. Thomas, J. Am. Chem. Soc., 1944, 66, 1466 Search PubMed.
- Y. H. Yoon and J. H. Nelson, Am. Ind. Hyg. Assoc. J., 1984, 45, 509 CrossRef CAS PubMed.
- G. Yan and T. Viraraghavan, Bioresour. Technol., 2011, 78, 243 CrossRef.
- Z. Aksu and F. Gonen, Process Biochem., 2004, 39, 599 CrossRef CAS.
- T. K. Naiyaa, A. K. Bhattacharya, S. Mandal and S. K. Das, J. Hazard. Mater., 2009, 163, 1254 CrossRef PubMed.
- A. K. Meena, K. Kadirvelu, G. K. Mishraa, C. Rajagopal and P. N. Nagar, J. Hazard. Mater., 2008, 150, 619 CrossRef CAS PubMed.
- Q. Li, J. Zhai, W. Zhang, M. Wang and J. Zhou, J. Hazard. Mater., 2007, 141, 163 CrossRef CAS PubMed.
- S. Y. Quek, D. A. Wase and C. F. Forster, Water SA, 1998, 24, 251 CAS.
- A. Saeed, M. W. Akhtar and M. Iqbal, Sep. Purif. Technol., 2005, 45, 25 CrossRef CAS PubMed.
- E. Pehlivan, T. Altun and S. Parlayýcý, J. Hazard. Mater., 2009, 164, 982 CrossRef CAS PubMed.
- G. Annadurai, R. S. Juang and D. J. Lee, J. Hazard. Mater., 2002, 92, 263 CrossRef CAS.
- E. S. Z. El-Ashtoukhy, N. K. Amin and O. Abdelwahab, Desalination, 2008, 223, 162 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |