Research highlights: comparing the biological response of nanoparticle solid solutions
Joseph W.
Bennett
*a,
Caley
Allen
b,
Sunipa
Pramanik
c,
Miranda J.
Gallagher
b,
Natalie V.
Hudson-Smith
 c,
Diamond
Jones
a,
Miriam O. P.
Krause
d and
Sara E.
Mason
a
c,
Diamond
Jones
a,
Miriam O. P.
Krause
d and
Sara E.
Mason
a
aDepartment of Chemistry, University of Iowa, Iowa City, IA 52242, USA. E-mail: joseph-bennett@uiowa.edu
bDepartment of Chemistry, Johns Hopkins University, Baltimore, MD 21218, USA
cDepartment of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA
dCenter for Sustainable Nanotechnology, Department of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA
First published on 22nd May 2017
Abstract
Scientific advances in the field of nanotechnology have led to the wide-scale use of engineered nanomaterials, resulting in an increased demand to understand the biological impact of these materials when released to the environment. This demand has led to an evolving field of science focused specifically on interactions at the nano–bio interface, where researchers investigate the biological responses of a wide range of organisms to engineered nanomaterials. The majority of investigations into the nano–bio interface are focused on the biological response of single-phase nanomaterials, yet engineered nanomaterials are often more complex than a single-phase nanomaterial. Many engineered nanomaterials can be described as solid solutions (or alloys) where multiple types of cations (and/or anions) are present in different ratios, and properties such as spin state, valence charge, and lattice constant can be tuned by changing the atomic composition. Research at the nano–bio interface must go beyond investigating the biological response of single-phase nanomaterials and include a systematic approach to predict how the biological interactions of nanomaterial solid solutions can be controlled via systematic changes in chemical composition. In this highlight, we focus on four publications that use a range of experimental methods to delineate the interactions of solid solutions composed of either Au metal or ZnO solid oxide on a variety of organisms. The first highlighted work tunes the composition of Au–Pt nanoparticles for antibacterial activity. The second article investigates the reprotoxicity of Au–Ag nanoparticles. The third highlighted work shows that Fe–ZnO nanoparticles demonstrate a reduced toxicity when compared to ZnO. Finally, the fourth study presents an in silico design strategy for cancer specific Fe–ZnO nanoparticles. Together, these four studies reveal the wide range of chemical compositions that are accessible in nanomaterial solid solutions and demonstrate that careful modifications in compositional phase space can result in selective nano–bio interactions.
1 Introduction
At the very heart of investigations at the nano–bio interface are the following questions: (a) how do complex nanomaterials interact with a diverse set of organisms over a variety of ever-changing environmental conditions, (b) how can one selectively control the interactions of complex nanomaterials with biological species, (c) how does exposure to complex nanomaterials affect different organisms, and (d) what transformations do nanomaterials undergo once exposed to the environment? To answer these questions, research at the nano–bio interface often takes a reductionist approach, where studies focus on the interactions of a single-phase nanomaterial across a limited range of cell lines and/or set of conditions in a controlled laboratory environment. These types of studies are useful in elucidating mechanistic insights about the interactions that can take place between single component model materials and complex biological systems, but engineered nanomaterials are often complex and composed of multiple constituent materials.In this highlight, we focus on how the compositional modification of nanomaterial solid solutions affects biological response. Solid solutions, also referred to as alloys, are complex materials that are created by adding two or more single-phase materials together such that the overall material is uniform, i.e. not phase separated. By changing the ratios of single-phase materials, often referred to as “end members”, the properties of the solid solution can be modified, and the process of adjusting the composition to optimize these properties is called compositional tuning. Compositional tuning in engineered nanomaterials has been shown to enhance the structural stability of thin films by modulating strain, to decrease the energetic barriers to structural and magnetic phase transitions in functional materials, and to optimize the carrier densities in photoactive semiconductors.
The four publications highlighted here analyze a range of nano–bio interactions that are a direct consequence of modifying the chemical composition of a single-phase nanomaterial to create a solid solution. In the first two articles, researchers find that they are able to create either bactericidal or reprotoxic nanoparticles by alloying Au with Pt or Ag, respectively. The last two articles report that introducing up to 10% Fe in ZnO nanomaterials can result in a way to chemically control nanoparticle dissolution; in one case the observed toxicity is cancer-cell specific. This means that the same general strategy to create a complex nanomaterial solid solution can lead to selective biological responses that are dependent upon the identity and ratios of the chemical constituents. We find that comparing multiple studies that employ the same types of engineered nanomaterials can strengthen our understanding of the diverse interactions that can take place at the nano–bio interface. They also allow us to devise new routes to manipulate the properties of solid solutions to allow for coherent chemical control at the nanoscale.
2 Tuning the composition of AuPt bimetallic nanoparticles for antibacterial application
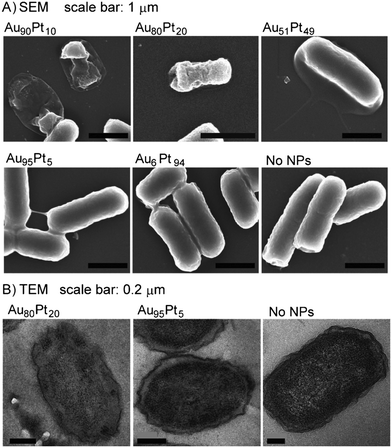
In this paper, Zhao et al. (Zhao et al., Angew. Chem., Int. Ed., 2014, 53, 8127, DOI: 10.1002/anie.201401035) report their work investigating the antibacterial properties of AuPt solid solutions, which they refer to as bimetallic nanoparticles (NPs). The authors here assess the antibiotic activities of bimetallic NPs (without any surface modifications) by combining two end member metallic NPs, which are each benign on their own. AuPt NPs are known for their catalytic activities, and the authors expected this to translate into effects on biological enzymatic systems in cells, making them potential antibiotic agents.A series of AuPt NPs with varying Au and Pt content were synthesized and characterized by inductively coupled plasma-optical emission spectroscopy (ICP-OES) and transmission electron microscopy (TEM). The NPs were screened for their antibacterial properties in two ways. First, by determining the minimal inhibitory concentration (MIC) on five clinically important pathogenic Gram-negative bacteria: Escherichia coli (E. coli), multidrug-resistant E. coli (MDR E. coli), Pseudomonas aeruginosa (P. a), Klebsiella pneumoniae (K. p), and Salmonella choleraesuis (S. c); and second, by assessing the minimal bactericidal concentration (MBC) on E. coli. MIC indicates the concentration at which a reagent inhibits the growth of a bacteria in a medium, whereas MBC is the concentration at which it kills the bacteria. Single-phase end-member Au and Pt NPs did not show any antibiotic activities in the MIC assay, while the bimetallic AuPt NPs with Pt content from 10–65% exhibited significant toxicity to all the bacteria. The authors reported the MICs to be 5 mg mL−1 against E. coli, MDR E. coli, P. a, and K. p, and 9 mg mL−1 against S. c. Additionally, the MBCs in the case of E. coli for AuPt NPs with Pt content of 10–65% were determined to be the same as their MICs, making them very strong bactericidal agents (MBC/MIC < 4 for effective bactericidal agents).
The article also reports further investigations into the mechanisms contributing to the antibacterial properties of AuPt NPs. Any effect of the NPs on the morphology and structural integrity of the (E. coli) cells was assessed using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The electron microscopy images indicated cell lysis in the case of Au90Pt10, Au80Pt20, and Au51Pt49 NPs (Fig. 1). An assay to determine any changes in inner membrane potential, using dye DiSC3, exhibited a decrease in membrane potential for cells that have been treated with Au90Pt10, Au80Pt20, and Au66Pt34 (these are the AuPt NPs that showed maximum antibacterial functions). Thus, the authors deduced that the antimicrobial properties could be attributed to the disruption in the inner cell membrane, caused by the NPs, resulting in changes in inner membrane potential. An increase in cellular ATP levels was observed as well. High ATP generation can be attributed to either an overexpression of Pck kinase, catalyzed by AuPt NPs (reported catalysts) or an accumulation of ATP in cells, due to inhibition in synthesis of ATP-consuming proteins by AuPt NPs, thus leading to bacterial death.
The authors concluded that the antibacterial properties of the AuPt NPs can be attributed to a decrease in inner cell membrane potential, due to inner membrane disruption caused by the NPs, as well as an elevation in the intracellular ATP levels. Interestingly, an increase in ATP production in cells is a mechanism employed by the human immune system to fight against infections. In order to assess whether these NPs are toxic to human cells, the authors performed a preliminary test to determine the viability of human umbilical vein endothelial cells in the presence of the NPs. Even after long-term incubation of the cells with the AuPt NPs (48 h and 72 h), there was no significant decrease in viability of the cells compared to the negative control. This led the authors to conclude that these NPs were selectively toxic to bacteria cells, making them good antibacterial agents.
3 Reprotoxicity of gold, silver, and gold–silver alloy nanoparticles on mammalian gametes
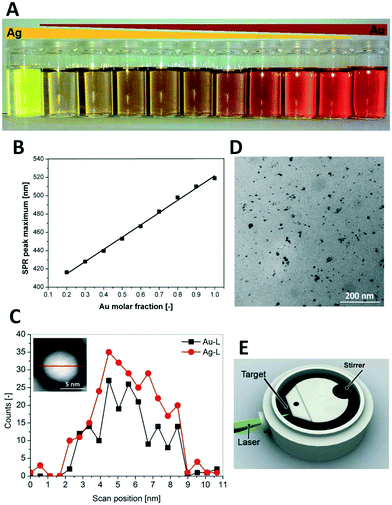
In a collaborative work between the Federal Research Institute of Animal Health and Center for Nanointegration Duisburg-Essen (CENIDE) in Germany, Tiedemann et al. (Tiedemann et al., Analyst, 2014, 139, 931, DOI: 10.1039/c3an01463k) examined the biological impact of tuning nanoparticle (NP) properties by utilizing an Au–Ag alloyed material. Pig ovaries and white boar spermatozoa were used to examine alloyed nanoparticle reprotoxicity, which is defined as the reproductive toxicity affecting gametes, fertility and offspring development.Pulsed laser ablation nanoparticle synthesis from Au–Ag alloyed foils, made by the Institute for Noble Metal and Metal Chemistry Schwäbisch-Gmünd, was intentionally selected for a straightforward NP synthesis method in order to remove potential toxicity from the organic ligands and precursors found in other solutions based NP synthesis methods. As SERS active NPs (Fig. 2) were synthesized, the Raman signal could be used in confocal microscopy to track to the location of accumulation of NPs.
The biological identity of a nanoparticle is defined here as the chemical composition of the surface when a nanoparticle interacts with a biological membrane. The biological identity of the silver, gold–silver alloy and gold nanoparticles was rendered equal when coated with a BSA protein corona prior to incubation with the gamete matrix. Despite similar biological identity, differences in nanoparticle composition were nevertheless correlated with differences in accumulation location as imaged by confocal microscopy. Gold was found inside the oocyte, silver in the cumulus and gold–silver alloys (20% Ag, 50% Ag, and 80% Ag NPs) in the cumulus.
When controlling for nanoparticle size, 6 nm 100% Ag, 6 nm 20% Ag, and 6 nm 50% Ag NPs had similar confocal microscopy results in oocyte cumulus uptake experiments, which could be due to the similar nanoparticle size or to the fact that all particles had a silver content above 20%. With respect to the Au–Ag nanoparticle solid solution series, composition was shown to determine oocyte reprotoxicity. Both 80% Ag NPs and 100% Ag NPs reduced oocyte maturation rates to a complete halt of maturation. The authors point to similar results in other biological models from previous literature, including piscine and avian oocytes.
When the authors tested a different membrane composition by switching gametes, they found that the net negative charge of the spermatozoa membrane blocked the negatively charged BSA coated nanoparticles from internalization. By comparing these results to those of citrate and PVP coated silver and gold nanoparticles, they conclude that the biological identity of the nanoparticle determines the interaction with gamete membranes and therefore spermatozoa reprotoxicity.
Nanoparticle impact on reprotoxicity is relevant to humans as well as the species studied here, and is key to guiding the synthesis and design of bimetallic nanoparticle solid solutions. In choosing an organism with established toxicity protocols, the well laid out procedure of this study could be followed up with a new hypothesis about a new set of nanoparticles. The authors’ goal to assess risk and inform regulation can be furthered with continued collaborative efforts bringing together experts in varied fields to assess nanoparticle biocompatibility.
4 Decreased dissolution of ZnO by iron doping yields nanoparticles with reduced toxicity in the rodent lung and zebrafish embryos
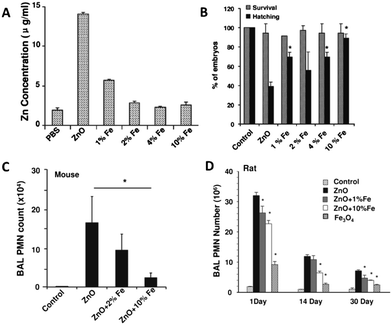
Xia et al. (Xia et al., ACS Nano, 2011, 5, 1223, DOI: 10.1021/nn1028482) explore a safe-by-design strategy of substituting up to 10% of the Zn in ZnO nanoparticles with Fe. (The authors refer to the Fe–ZnO solid solutions as doped and the ZnO end members as undoped.) Previous dissolution studies performed with inductively coupled plasma mass spectrometry (ICP-MS) have demonstrated that iron doping was inversely correlated with dissolution of zinc (George et al., ACS Nano, 2010, 4, 15, DOI: 10.1021/nn901503q; Xia et al., ACS Nano, 2008, 2, 2121, DOI: 10.1021/nn800511k). Because Zn2+ dissolution is an established mechanism of toxicity for nano-ZnO, doping ZnO nanoparticles with iron is explored as a strategy for the design of nanoparticles with a reduced environmental impact. Doped and undoped ZnO nanoparticle solid solutions were synthesized by flame spray pyrolysis (FSP) and characterized thoroughly by transmission electron microscopy (TEM), selected area electron diffraction (SAED), X-ray diffraction (XRD), N2 physisorption, and dynamic light scattering (DLS). Iron doping up to 10% was not found to disrupt the crystallinity of the ZnO particles although doping at (or greater than) 4% did result in a decrease of the zeta-potential of the nanoparticles.In order to study the potential changes in environmental impact of Fe doping of nano-ZnO, doped and undoped ZnO nanoparticles were evaluated in both in vitro and in vivo studies to determine their effects on a murine cell line, on zebrafish embryo hatching, and on pulmonary inflammation in both mice and rats. First, undoped and doped particles were screened for toxicity profiles on RAW 264.7 and BEAS-2B cells (murine and human cell lines, respectively). The assay demonstrated that Fe-doped particles induced less toxicological responses than undoped particles and the toxicological response declined with increasing iron doping percentage.
In the experiment on the hatching rates of zebrafish embryos, ZnO nanoparticles were shown to interfere with hatching without resulting in the death of embryos; however, prolonged delay of hatching ultimately results in embryo death by starvation. Hatching rates progressively increased with an increase in % Fe doping (Fig. 3). TEM micrographs of stained embryos show that upon exposure to ZnO, the outer layer of the embryo is not digested when compared to the unexposed embryos at the same time after exposure. This indicates that the dissolution of Zn2+ from undoped ZnO interferes with the enzyme involved with hatching (ZHE-1), preventing both digestion of the embryo's outer layer and hatching, while not directly harming the embryo.
Finally, in the study of pulmonary inflammation in rats and mice after aspiration of doped and undoped ZnO nanoparticles, the researchers observed a statistically significant decrease in pulmonary inflammation with ZnO doped with 10% iron when compared to undoped ZnO (only undoped, 2%, and 10% doped particles used in this portion of the study). Additionally, expression of heme oxygenase 1, used here as a biomarker of oxidative stress in the lungs, was decreased in 10% iron-doped ZnO nanoparticle exposure when compared to undoped ZnO particles. In rats, pulmonary inflammation was persistent and measured at 1 day, 14 days, and 30 days. Iron doping at 10% led to a significant reduction in pulmonary inflammation when compared to undoped particles at all time intervals, although all nanoparticle exposures resulted in significantly higher inflammation than in unexposed control rats.
In summary, Xia et al. demonstrated a viable method for reducing the environmental impact of ZnO nanoparticles by doping with variable amounts of iron and demonstrated the reduced negative biological impact in both in vitro and in vivo studies. To further explore the practicality of iron doping as a safe-by-design method, the authors recommend evaluation of these Fe-doped particles in their consumer uses, such as sunscreen, to determine if Fe-doping affects performance characteristics.
5 In silico design of optimal dissolution kinetics of Fe-doped ZnO nanoparticles results in cancer-specific toxicity in a preclinical rodent model
Previous studies have shown that engineered nanoparticles can potentially be used as anticancer therapeutic drugs. For example, the Zn2+ ions released by zinc oxide (ZnO) nanoparticles in aqueous solutions have an increased cytotoxicity towards cancer-specific cells. This may be due to the high metabolism of cancer cells that causes the pH of their surroundings to be more acidic, which offers an optimal environment for ZnO nanoparticles to readily dissolve. One problem with this scheme is that if the nanoparticles dissolve too quickly, they can also be toxic to normal cells. A way to control the rate of dissolution of Zn2+ from ZnO is to dope the ZnO with iron (Fe). The addition of Fe2+ to ZnO decreases the overall lattice constant of the solid solution, preventing the Zn from dissolving readily. In this study, Manshian et al. (Manshian et al., Adv. Healthcare Mater., 2017, 6, 1601379, DOI: 10.1002/adhm.201601379) tested the biological specificity of Fe-doped ZnO nanomaterials by exposing them to two types of cancer cells and two types of normal cells. They then tested the anticancer potential of the Fe–ZnO solid solution system on tumors in mice.Single-phase ZnO and 1–10% Fe-doped ZnO nanoparticle solid solutions were generated via flame spray pyrolysis and characterized using both X-ray diffraction (XRD) and Brunauer–Emmett–Teller (BET) techniques. These analyses showed that the incorporation of Fe decreased the unit cell volume and also led to a decrease in particle size. The authors then tested the interaction of the nanoparticles with two types of normal cells (murine mesenchymal stem (MSC) and human bronchial epithelial cells (BEAS-2B)) and two types of cancer cells (murine lung squamous carcinoma cells (KLN 205) and human cervical cancer cells (HeLa)) and found that the rate of dissolution was higher with the cancer cells.
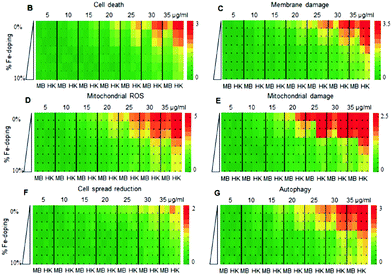
The team performed a computational analysis of full particle theoretical nanodescriptors to shed light on the correlation between observed toxicity and the nanoparticle properties that changed as the % Fe was adjusted. In this work, the nanodescriptors are attributes based solely on the structure and composition of the nanoparticle before the interaction experiments, so each Fe–ZnO solid solution will have a unique set of attributes that are based on the characterization techniques presented in the previous paragraph. The descriptors related to Zn dissolution were better correlated to the cancer cells and not the normal cells, a mechanistic specificity that would not have been possible without this analysis. In general, they found that the more Fe in ZnO, the less toxic the nanoparticles tended to be, while pure ZnO displayed toxicity across the assay (Fig. 4). A computational analysis of dosage and solid solution composition revealed that the optimal doping level of 2–3% would be toxic to cancer cells while leaving normal cells intact. The authors then tested the results of this analysis in a preclinical evaluation of antitumor response in mice.
In the preclinical evaluation, the researchers implanted DBA/2 mice with firefly luciferase-expressing KLN 205 cancer cells, and monitored the tumor growth. They then administered pure, 2% or 10% Fe-doped ZnO nanoparticles after the tumors were at least 50 mm3. The results of this work matched the in vivo trends in the previously highlighted work of Xia et al. (Xia et al., ACS Nano, 2011, 5, 1223, DOI: 10.1021/nn1028482): the dissolution of Zn2+ from ZnO was negatively correlated with the amount of Fe doping, meaning the pure ZnO was the most toxic and the 10% Fe-doped ZnO nanoparticles were the least toxic. The team were also able to evaluate the therapeutic efficacy of the nanoparticles; they determined that there was a delayed and slowed tumor growth in the mice that were treated with the 10% Fe-doped nanoparticles, and the tumors that were treated with 2% Fe-doped nanoparticles showed a near steady size for at least five weeks, when compared to the control mice. These findings illustrated a useful methodology for tuning the kinetics of dissolution that can be beneficial for future studies to exploit in novel medicinal approaches.
Acknowledgements
This highlight was initiated from the literature discussion of a biweekly trainee (student and research scientist) group meeting in the Center for Sustainable Nanotechnology, which is supported by the National Science Foundation under grant number CHE-1503408.| This journal is © The Royal Society of Chemistry 2017 |