Research highlights: detecting, characterizing and quantifying the presence and impact of carbon nanomaterials in environmental systems
John M.
Pettibone
and
Stacey M.
Louie
*
Materials Measurement Science Division, National Institute of Standards and Technology, Gaithersburg, MD 20899, USA. E-mail: stacey.louie@nist.gov
First published on 17th July 2015
Abstract
Here, we highlight articles examining different aspects contributing to the fate and role of carbon nanomaterials in environmental systems by developing new insight through measurement methodologies or systematic approaches. The first study examined the role of dissolved organic matter on the colloidal stability of bare and functionalized fullerene and used tools that provide data on the change in size and structure of aggregates with changing media composition. The origin of the aggregates in aqueous media observed is further discussed from other computational and experimental work from other groups. Another study focuses on the development of new mass spectrometry methods for improved detection and quantification of these species in complex media, which provides methods to better assess current methods for detection and water processing. The last study examines the role of carbon nanomaterials in soils and sediments, which provides data on the roles different carbon nanomaterials have in the bioavailability of contaminants.
Introduction
With the continual development of commercial and industrial products containing carbon nanomaterials, the exposure risk for species with known toxicity will likely increase.1 Fullerenes are a subclass of carbon nanomaterials that have distinct stoichiometry (and properties), which are being incorporated into consumer products and used in biomedical applications with direct human exposure as well as exploited for electronic and catalytic applications. Because of their broad use and natural occurrence, understanding the properties controlling structure, stability, and sorption capacity and an ability to detect and characterize these species in complex media are important for predicting environmental fate and developing water treatment procedures.2Colloidal stability of bare and functionalized fullerene aggregates and the origin of their stability
Haftka et al.,3 compared the colloidal stability of bare fullerenes (C60), which are hydrophobic, and functionalized fullerenes, exhibiting phenyl and methyl ester groups, as a function of solution parameters with a suite of measurement techniques. The methods for generating suspensions of C60 aggregates result in similar size distributions, and these ensembles have distinct properties from their molecular precursors. The authors demonstrated the utility of flow cytometry to monitor the changes in agglomerate size and granularity in salt solutions. Unlike the unfunctionalized fullerenes, increased stability was observed for the functionalized derivatives (Fig. 1). The bare C60 exhibited increased stability in the presence of Leonardite humic acid in NaCl solution, but fast settling occurred in the presence of divalent cations. Overall, the work demonstrated that composition of the organic matter and functionalization of the fullerenes control the interaction between these species and significantly contribute to their colloidal stability under environmentally relevant conditions. However, an understanding of the weighted contributions of aromaticity and hydrophobicity of the dissolved organic matter (DOM) on the interaction with the carbon surfaces is necessary to better predict colloidal stability, as suggested by this work and others.4The interactions between DOM and the bare fullerene aggregates are affected by the surface properties. The mechanisms leading to C60 solubility in water for distinct preparation methods were reportedly not well described. Prylutskyy et al.5 presented experimental work aimed at elucidating the mechanism by comparing two common methods: (i) extended mixing and sonication in water and (ii) solvent exchange from toluene. Both methods resulted in similar size aggregates that exhibited similar spectroscopic signatures with Fourier transform infrared spectroscopy and were similar to other results in the literature. The generation of the hydroxyl groups on unfunctionalized fullerenes was reported to be independent of the process by which suspensions are made (solvent exchange, sonication, extended stirring). The authors suggest that the origin of the colloidal stability is from these species, and similarities in size in the absence of other sorbents may be expected for any preparation. Other groups have contemporaneously examined their generation and stability computationally.6,7 However, the interaction with environmental media constituents or other transformation processes may significantly alter their behavior, and the authors suggest more work is necessary.
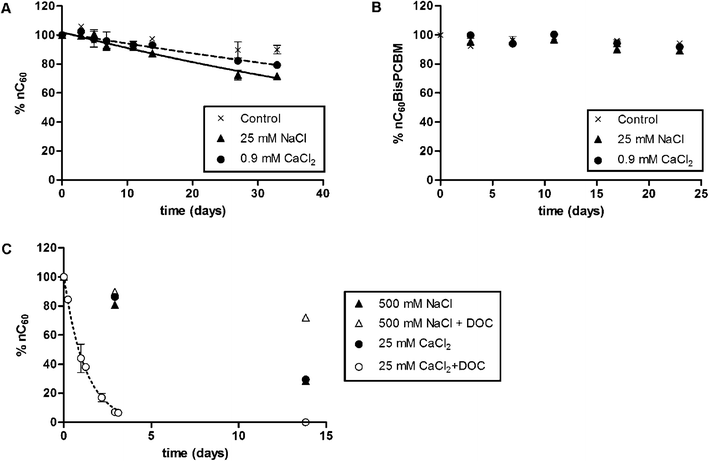 | ||
| Fig. 1 Relative stability of (A) aggregated C60 and (B) functionalized C60 in the presence of typical freshwater ionic strength conditions. (C) The stability of the unfunctionalized C60 aggregates in the presence of humic substances and monovalent and divalent cations, which exhibited decreased colloidal stability in the presence of Ca2+ and was similar to previous results for less hydrophobic dissolved organic carbon (DOC). Figure reprinted with permission from Haftka et al.3 Copyright 2015 Royal Society of Chemistry. | ||
Advancement of methods for the detection of carbon nanoparticles in complex media
The further development of detection methods is needed for monitoring and characterizing engineered nanomaterials in waste streams, environmental systems or other complex media. The use of mass spectrometry for the detection and identification of fullerenes has been well demonstrated, but with the further implementation of functionalized carbon species, new methods for extraction, processing and quantification will likely be needed.The unambiguous detection of this class of material remains a challenge in environmental media, but Emke et al.8 report the development of methods employing atmospheric pressure photoionization (APPI) coupled with high resolution mass spectrometry to demonstrate detection of functionalized C60 and C70 species in sewage waters. The inclusion of a front end stationary phase with more specificity and elimination of commonly used solvents (acetonitrile or methanol) were reported to improve detection and assignment of fullerenes compared to electrospray ionization (ESI) techniques. The use of pure toluene in the column and substitution of ESI for APPI resulted in lower adduct formation compared to ESI methods, as observed in Fig. 2. Furthermore, higher reported sensitivities were achieved, and 13C/12C ratios were consistent with their theoretically predicted values. The applicability of the developed methods for field detection was also demonstrated by the same group, which collected field soil and sediment samples to validate the ability to detect and quantify fullerenes in environmental matrices.9 Overall, the characterization of bare and functionalized fullerenes extracted from both aqueous and particulate matter was demonstrated with high sensitivity based on the removal of commonly used organic solvents and incorporation of APPI, which may provide a tool for more accurate quantification of these species in complex media.
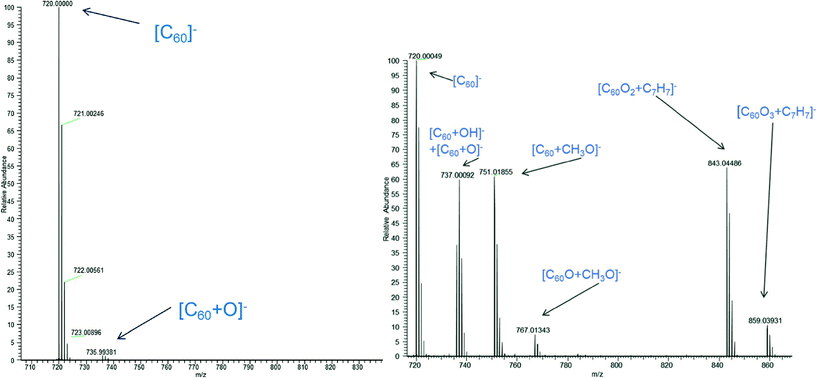 | ||
| Fig. 2 Mass spectra of C60 obtained from (left panel) pure toluene and the APPI-MS system and (right panel) post column infusion of methanol employing ESI-MS in negative mode. The suppression of the oxidized ion and other adducts observed in the ESI-MS spectrum demonstrate the reported improvement and sensitivity for the current measurement methods. Figure reprinted with permission from Emke et al.8 Copyright 2015 Royal Society of Chemistry. | ||
Effects of carbon nanomaterials in sediments and soils
As an environmental contaminant, one sink for hydrophobic carbon species, such as fullerenes, is sediments and soils.1 The toxicity of carbon nanomaterials (CNs) to soil microorganisms is currently an area of interest. However, the role specific CNs play in bioavailability of other contaminants in these systems is not well understood.Oyelami and Semple10 examined the catabolic activity of a polycyclic aromatic hydrocarbon (PAH) in the presence of different loadings of CN, which included C60, multi-walled carbon nanotubes (MWCNTs), and fullerene soot (FS). With increased loadings of CN into soil, differences in the length of the lag phase were observed, but fullerene-containing soils showed the shortest lag phases. Also, concentration dependence for the CN was observed, but the amount of mineralization was highest for fullerene at the highest loadings. Fullerene was the only CN to not show a statistically significant reduction in the catabolic activity of PAH with increased loading, which is suggestive of minimal impact on the biodegradation of PAH in the presence of fullerenes (Fig. 3). The authors suggest the decreased surface area of the fullerene may lead to increased bioaccessible PAH on the fullerene compared to MWCNTs or especially the FS, which has interstitial spaces for slow release of sorbed species.
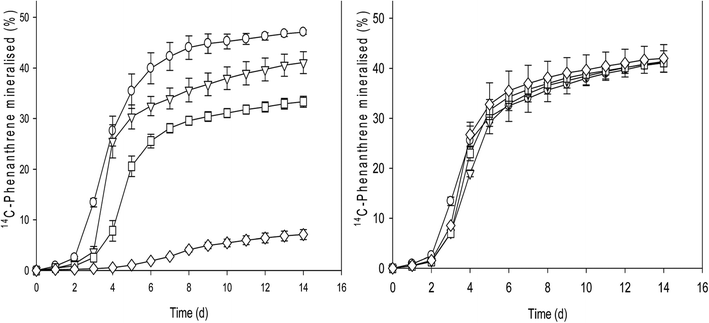 | ||
| Fig. 3 Catabolism of 14C-phenanthrene by indigenous microorganisms after the addition of (left panel) FS and (right panel) fullerene at contact time 50 days. The increasing concentration of fullerene does not significantly change the extent of mineralization measured, which is in contrast to the FS species demonstrating significant decrease from 0.1% (triangles) to 1% (diamonds) loadings. Figure reprinted with permission from Oyelami and Semple.10 Copyright 2015 Royal Society of Chemistry. | ||
Overall, the work demonstrates disparate effects on microbial activity in the presence of each CN. Further work investigating the role of functionalized CNs and their long term effects on similar microorganisms is suggested.
References
- B. Nowack and T. D. Bucheli, Environ. Pollut., 2007, 150, 5 CrossRef CAS PubMed.
- N. O. McHedlov-Petrossyan, Chem. Rev., 2013, 113, 5149 CrossRef CAS PubMed.
- J. J.-H. Haftka, P. S. Bauerlein, E. Emke, N. Lammertse, D. Belokhovstova, B. Hilvering, P. de Voogt and T. L. ter Laak, Environ. Sci.: Nano, 2015, 2, 280 RSC.
- K. L. Chen and M. Elimelech, J. Colloid Interface Sci., 2007, 309, 126 CrossRef CAS PubMed.
- Y. I. Prylutskyy, V. I. Petrenko, O. I. Ivankov, O. A. Kyzyma, L. A. Bulavin, O. O. Litsis, M. P. Evstigneev, V. V. Cherepanov, A. G. Naumovets and U. Ritter, Langmuir, 2014, 30, 3967 CrossRef CAS PubMed.
- J. I. Choi, S. D. Snow, J.-H. Kim and S. S. Jang, Environ. Sci. Technol., 2015, 49, 1529 CrossRef CAS PubMed.
- Y. S. Djikaev and E. Ruckenstein, J. Phys. Chem. Lett., 2015, 6, 1761 CrossRef CAS.
- E. Emke, J. Sanchis, M. Farre, P. S. Bauerlein and P. de Voogt, Environ. Sci.: Nano, 2015, 2, 167 RSC.
- J. Sanchís, L. F. S. Oliveira, F. B. de Leão, M. Farré and D. Barceló, Sci. Total Environ., 2015, 505, 172 CrossRef PubMed.
- A. O. Oyelami and K. T. Semple, Environ. Sci.: Processes Impacts, 2015, 17, 1302 CAS.
| This journal is © The Royal Society of Chemistry 2015 |
