Cellulose nanomaterials: life cycle risk assessment, and environmental health and safety roadmap
Jo Anne
Shatkin
*a and
Baram
Kim
b
aVireo Advisors, PO Box 51368, Boston, Massachusetts 02205, USA. E-mail: jashatkin@gmail.com
bIndependent, Somerville, Massachusetts, USA
First published on 30th July 2015
Abstract
Cellulose nanomaterials (CNs) derived from wood fibers are renewable materials with wide applicability for use in consumer products as bio-based composite materials and have the potential to replace petroleum-based materials in many existing and novel applications. Because their nanoscale features may impart novel chemical properties and behaviors, it is necessary to address the environmental and safety aspects of CNs to ensure safety in commercial applications, before wide introduction into society. NANO LCRA, a proposed life cycle risk assessment framework, was used for pre-commercial screening of selected applications of CN as a method for systematically identifying and assessing potential risks of CN from occupational, consumer and environmental exposures throughout the product life cycle. The analysis identifies potential exposure scenarios, evaluates toxicity and assesses the adequacy of available data to characterize risk, highlighting data needs and gaps that must be filled to reduce current uncertainty about CN safety. The analysis revealed that occupational inhalation exposure associated with handling CN as a dry powder was the highest priority data gap including the challenge of quantitative measurement for exposure assessment, followed by gaps in knowledge about the toxicity of CN in consumer use products, such as packaging, particularly for food contact. The NANO LCRA findings were then organized into a roadmap for filling key data gaps to allow safety and sustainability assessment that prioritizes data needs according to risk significance to ensure that uncertainty about the CN safety does not interfere with the commercialization of products to market.
Nano impactThis applied research employs a novel approach to risk analysis for an important emerging class, cellulose nanomaterials (CNs). While CNs are widely judged as low environmental impact, there remain significant measurement and knowledge gaps that prohibit a risk conclusion. Our broad analysis of potential fate/exposure pathways across the product life cycle for occupational, environmental and consumer scenarios integrates existing knowledge with risk prioritization and uncertainty. The analysis focuses research to address outstanding nano-specific concerns about safety and sustainability in advance of commercial launch and environmental introduction. The innovation in our approach is a high level analysis to prioritize filling research gaps across the product life cycle, such that safe introduction and use is guided by the analysis of evidence. |
1. Introduction
Cellulose nanomaterials (CNs) are an important group of materials nearing commercialization in many economic sectors, markets and products. Cellulose, the most abundant organic material on earth, is widely used in manufacturing, and is ubiquitous in nature. Mechanical and chemical methods generate CNs from cellulose, with novel physical and chemical properties and nanoscale dimensions, raising concern over whether they also may exhibit different biological and environmental behaviors from conventional cellulosic materials. As this report describes, the few existing CN studies create a dataset that is inadequate for either commercial or regulatory demonstration. Here we describe a screening level risk analysis of CN across the product life cycle. This evaluation identifies: critical potential health and environmental concerns; current areas of uncertainty over biological and ecological effects; and data needs to inform the key uncertainties for safe and sustainable commercialization. These are prioritized into a roadmap.CN is a nanomaterial (NM) derived from a diversity of sources of cellulosic biomass, including those from the agricultural, forest and recycling industries, as well as bacteria and tunicates. CNs may be typically isolated as whiskers or crystals (cellulose nanocrystals or CNC), or as cellulose nanofiber (CNF) forms, by chemically or mechanically treating cellulose.1 The work presented here, however, primarily focuses on the use of wood pulp from the forest industry because its applications are most likely to be produced on a large-scale in the near term.
As consumers and industry look toward sustainable and renewable resources, CN holds promise in replacing other resource-intensive materials, such as plastics that come from fossil fuels. Manufacturers can harness CNs' particular characteristics, including high-tensile strength, color/transparency, light weight and biodegradability, for existing and novel applications. CN is proposed for myriad uses, including in paper and packaging, composite polymers,2 insulation, aerogels, paints, cosmetics, water and air filtration, and recyclable electronics.3 Other potential uses are those that are being developed for the biomedical industry, such as sensors and scaffolding for tissue engineering.4,5 Most production is at pilot scale today, which creates an important opportunity to address safety concerns prior to commercialization, informing safer product design and manufacturing.
For the market to be assured of safety and to overcome its reluctance to adopt any new product, particularly those produced using manufactured NMs, technology sponsors must be proactive about managing safety and concerns due to uncertainty over potential risks. Research to explore the environmental health and safety aspects of NMs, thus far, has sometimes demonstrated differences from conventional non-nanoscale substances, due to the particulate nature, small size, and in cases, novel properties. Regulatory authorities, producers, industry, customers, and consumers still need more information, however, about the potential health and environmental impacts of products that are produced using NMs. The challenges of measuring NMs in biological and environmental media, particularly with organic materials, require expertise to conduct the necessary physical and chemical characterizations, exposure and release studies, and toxicity evaluations.
Investigations into the safety of CN have focused largely on short term bioassays and the inhalation route of exposure.6 Cellulose nanocrystals produced by a proprietary sulfate reduction method has been the focus of detailed environmental and human toxicity evaluations.7,8 Occupational exposure to cellulose nanofibrils was investigated in a simulated experiment, along with a focused in vivo/in vitro study.9 These and the few additional references on toxicity of CN form a thin basis for assessing potential health and safety risks in the context of diverse commercial applications. Current uncertainty about the safety of nanomaterials hinders their use in commercial applications. A more comprehensive and systematic analysis of risks was sought to support the development of an environmental, health and safety (EHS) roadmap for CN, as it is being investigated in a wide variety of applications with potentially significant tonnages in production10 and uses in a diversity of economic sectors. As manufacturers are bringing pilot scale production plants online, potential adopters of CN need demonstration of safety, as do regulatory authorities charged with oversight of the products in consumer goods, food and agriculture, transportation, medical applications, and cosmetics, among others.
Recent calls for the adoption of a life cycle approach to risk analysis have focused on the need to address the breadth of potential concerns about nanoscale materials11,12 but are not matched by the current state of practice. These calls in part reflect the limitations of past approaches to chemical and other emerging technological issues, such as in biotechnology, for which it is generally recognized that early assessments to identify and address potential health and safety concerns could have led to more sustainable development. NANO LCRA is proposed here as an approach to screen nanomaterials by combining risk analysis and life cycle thinking early in the development process,13,14 responds to the need for a systematic approach across broad fields of inquiry.
Conducting risk analysis across the product life cycle is a little explored field and remains a research effort. Life cycle risk analysis (NANO LCRA) is a foresighting modeling tool to broadly identify and screen for potential impacts of new materials and applications well in advance of commercial development, as a method of reducing unforeseen consequences of new technologies. Conventional risk analysis and life cycle analysis approaches for nanomaterials are currently fraught with methodological issues due to the need for novel metrics and also require significant data development, which is neither feasible nor appropriate for early stage technologies. The research value of NANO LCRA is that the analysis identifies the most significant health and environmental data issues and gaps from a toxicity and exposure (risk) perspective, resulting in a targeted, prioritized and focused research plan (a ‘roadmap’) designed to fill them.
Purpose of roadmap
The roadmap is the output of the NANO LCRA analysis. It represents a summarized form of the findings from the data gap analysis and prioritization. It is structured so that research priorities address outstanding knowledge gaps about safety affecting commercialization and are ordered in terms of greatest potential risk, as estimated in the analysis. In the absence of formal regulatory guidance or standards for NMs, the roadmap provides direction to producers, investors and governmental researchers seeking to safely commercialize CN and the products that are produced with it. This type of transparency about what is known and needs to be known casts a more objective light on the state of knowledge and research priorities about safety for pre-commercial NMs.1.1. Risk and life cycle screening versus quantitative research
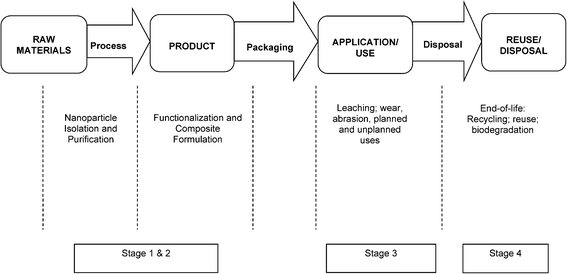
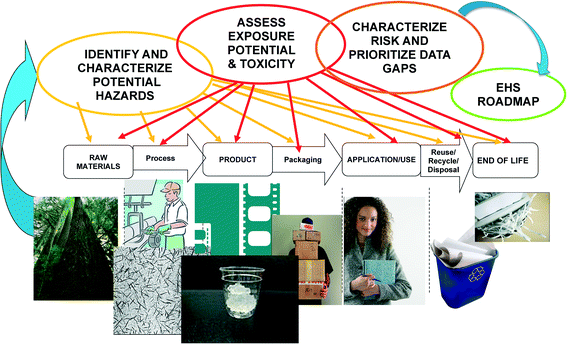
Novel applications of chemical substances typically require assessment of safety which analyzes the potential of an adverse effect (hazard) as a result of contact with a substance (exposure), or source of harm, to exposed populations or the environment. Risk is the product of hazard and exposure, meaning that both factors are required for a health risk to exist. Risk is characterized by some level of uncertainty, reflecting a possibility of harm, not a certainty. When risk levels are acceptable (i.e. low), safe use of substances may occur. For nanomaterials, assessing risk is challenging due to uncertainties about hazard and exposure, in part derived from size and surface properties, which can differ from conventional substances. Further, the current challenges in measurement science on the nanoscale pose hurdles in the accurate assessment of toxicity and exposure. In this context, risk may not be quantifiable, and rather is assessed in terms of potential magnitude or significance. Similarly, for the product life cycle, data gaps prohibit quantitative assessment. The value of screening for risks across the life cycle is to narrow future research investments into key stages of greater potential impact.Briefly, NANO LCRA builds on the tenets of traditional risk assessment, and characterizes the potential risks of a NM across the dimensions of the life cycle, from the raw material stage through the end-of-life or disposal/reuse,14 rather than at a static time point for the particular material. Fig. 1 schematically depicts the NANO LCRA framework for risk assessment. The NANO LCRA evaluates NM transformations across applications and focuses on risks in real-world scenarios that incorporate occupational, consumer and environmental pathways of exposure. The NANO LCRA, therefore, provides a more realistic conceptual model of exposure and risk potential, and provides what traditional risk analysis can not: analysis of risk potential without years of methods and data development for nanoscale materials. The NANO LCRA develops scenarios, events that could occur and that might result in NM exposure. The event might be an expected event, such as a machine cleanout, which could emit NM, or it might be an unexpected event, such as an accidental NM release. Relative risk significance of the scenarios then is determined, following the methodology in the next section.
 | ||
| Fig. 1 The NANO LCRA framework showing the steps in complexity of risk assessment of a NM over the life cycle. The blue arrow to the left indicates the iterative, adaptive nature of the framework. | ||
NANO LCRA is implemented at critical phases of new product/technology development to inform risk management and safety testing early in the process. It informs product design and safety data development incrementally, ‘stage-gate’-like fostering sustainable product design in real time. The NANO LCRA is intended to be conducted iteratively to provide the opportunity to identify and characterize potential safety risks early in the product development cycle, and to focus EHS research and risk management practices on the key uncertainties, knowledge gaps, and exposure pathways. As newer information becomes available, which is expected to happen for emerging NMs, the NANO LCRA can be revised in its next iteration to more accurately reflect the risks associated with NMs as technologies advance toward commercialization. The NANO LCRA framework is designed as a screening-level tool to be used for early evaluation in NM risk management and, to rapidly identify data needs, which then informs additional testing and risk characterization in real time, in the form of a research roadmap.
2. Methodology: NANO LCRA for cellulose nanomaterials
The NANO LCRA methodology analyses the best available data under uncertainty for management of risks related to novel materials. The NANO LCRA for CN characterizes CN risk by evaluating potential hazard, exposure, and toxicity to identify potential risks and data gaps for five CN product applications across the product life cycle. The assessment develops and analyzes scenarios where a NM is released and may contact a human or ecological receptor. By assessing the data available to evaluate these scenarios and their potential significance, priorities for risk management and for data collection were established. In future iterations, filled data gaps are used to further refine risk estimates.2.1. Hazard Identification
At the outset of the assessment, uses were identified through a systematic review and market analysis.3,10 Thirty three potential applications of CN into products were identified in the market analysis, including packaging and paper strengthening, building material applications, flexible electronics, and others; of these, five applications were selected for inclusion in this NANO LCRA to represent a diversity of potential use scenarios: barrier films (paper); barrier coatings (packaging); paint and coating additives; aerogels; and cosmetic additives. While informed by market analysis, the product applications included in the NANO LCRA were included based on their potential for exposure and commercialization potential in the near term, and not necessarily based on projected production volume.The life cycle for each application was mapped in four stages from raw materials processing of cellulose through the CN product end-of-life.15 For each life cycle stage, possible scenarios that could lead to exposure were identified, then evaluated by incorporating potential hazard and exposure for an occupational, consumer, or environmental receptor. This process was repeated for each stage until the product end-of-life, for each of the five applications. The different combinations of application, receptor, hazard, and exposure, assessed across the life cycle led to the development of 59 discrete scenarios that were then ranked to determine overall relative risk. Space precludes listing all 59 scenarios, however the top 22 are shown in Table 4. The Exposure Assessment explains the ranking process used. Fig. 2 shows the product life cycle, comprising the four stages described below.
The NANO LCRA evaluated the potential hazards for each of the three receptor categories (occupational, consumer, and ecological) categories, at each life cycle stage, for each application. Knowledge of the processes and procedural steps (if any) allowed for identification of potential hazards at each stage, whether borne from direct exposure to free CN (in concentrated powder or wet slurry) or a less direct exposure. In one step of the production process, a worker may have direct exposure to CN particles after handling dry CN during packaging, wherein the potential hazard is inhalation of airborne CN particles, released to the air after handling. Another possible scenario during manufacture of a CN-containing product (Stage 2), may involve a slurry of CN being mixed with other materials to create the desired product. The employee performing this step may accidentally spill the slurry on their clothes or skin, which is the potential hazard. Development of these scenarios in the Hazard Identification for each Stage formed the basis of the exposure ranking step.
2.2. Exposure assessment
There are many possible scenarios within each life cycle stage that could occur where a human or ecological receptor would be exposed to CN. In the NANO LCRA, each of the 59 scenarios depicts one potential hazard, one exposure pathway, and one receptor for a given life cycle stage. In other words, it depicts a planned or unplanned event that could result in a receptor coming into contact with CN. As an example, Table 1 identifies select scenarios in the life cycle for CN in food packaging. It presents the relevant possible scenarios for each stage, including the identified hazard (from Hazard Identification), the receptor and the exposure pathway. The following narrative traces a series of potential scenarios, focusing specifically on those involved with the barrier coating on food packaging application, to illustrate the life cycle exposure assessment for CN. The scenario numbers are included in brackets here and are found in Table 1. Starting with the raw material, the cellulose is received by the plant and is processed by acid hydrolysis, which could spill [A.1.1.]. Workers may have direct contact with a CN slurry via dermal exposure [A.1.3]. During the freeze-drying or packaging processes, a worker may accidently inhale CN powder [A.1.2] at a CN production facility. The CN products at a production facility would most likely be packaged as dry powder or aqueous suspension. So, potentially, a worker could come into contact with the CN during any of these previous steps.| Scenario number | Scenario description | Life cycle stage | Potential hazards | Receptor | Exposure pathway |
|---|---|---|---|---|---|
| A.1.1 | Accidental acid spill during production, on the production floor, accidental dermal contact with facility employees | Production | Acid | Occupational | Dermal |
| A.1.2 | Processed material is dried and then accidentally inhaled by a facility employee | Production | Inhalation contact with CN particles | Occupational | Inhalation |
| A.1.3 | Accidental spill of liquid/wet nano cellulose at facility, accidental dermal contact with facility employees | Production | CN particles | Occupational | Dermal |
| B.1.4 | Production of coating using liquid nano cellulose, contacting facility employees via inhalation | Manufacturing | CN particles in the lungs, becoming wet and potential internal exposure | Occupational | Inhalation |
| B.1.5 | Facility employee touches not-yet-dried coating, intentional dermal contact with film material and CN particles | Manufacturing | Dermal contact with CN particles | Occupational | Dermal |
| B.1.6 | CN released to waste water enters ambient waters. | Manufacturing | Release from manufacturing | Environmental | Aquatic |
| B.3.1 | CN in packaging migrates to food; accidental ingestion of CN particles by consumer | Application/use | Ingestion of CN particles in matrix | Consumer | Ingestion |
| B.3.3 | Food heated in microwave while still in packaging (coating), CN in packaging migrates to accidental ingestion of CN particles | Application/use | Ingestion of CN particles in matrix | Consumer | Ingestion |
| B.4.1 | Discarded with petroleum-based plastic material and sent to a recycling center, processed as plastic and handled by facility employees | Post-consumer | Dermal contact of CN particles in matrix | Occupational | Dermal |
| B.4.2 | Discarded with petroleum-based plastic material and sent to a recycling center, processed as plastic and product particles inhaled by facility employees | Post-consumer | Inhalation of CN particles in matrix | Occupational | Inhalation |
| B.5.1 | Material disposed of in landfill to biodegrade; intentional contact with environmental | End-of-life | Environmental contact with CN particles | Environmental | Environmental |
| B.5.2 | Material disposed of at a composting facility; intentional contact with environment and potential contact with future soil use (e.g. food production) | End-of-life | Environmental contact with CN particles, and potential uptake by plants grown for human consumption | Environmental | Environmental |
During Stage 2, the CN could be coated (and might be shipped to another site), or it could be mixed in with other materials to create a CN-containing product. For example, in the production of a barrier coating on food packaging, a food package manufacturing facility may receive the CN as wet slurry. While handling the wet slurry, a worker may come into contact with airborne wet CN particles [B.1.4]. This stage could include many intermediary manufacturing steps before a final product is packaged and shipped. Here, environmental exposure might occur, for example if CN is released with wastewater from manufacturing. Once the consumer buys the coated food package (Stage 3), he/she may then heat the packaged food in a microwave causing the coating to heat and interact with the food, and the consumer to accidentally ingest the CNs [B.3.1]. After using the food packaging, the consumer may dispose of it with the municipal trash, from where it is later transported to a landfill to biodegrade [B.5.1], or it is recycled, and handled by a recycling worker [B.4.2]. The end-of-life fate and transport disposition of the NM at the landfill would depend partly on whether the underlying soil adsorbs the CN particles, a leachate plume mobilizes the CN particles, or the CN particles are discharged in leachate to surface waters nearby where aquatic species could ingest or absorb them. The extent of biodegradation is important in assessing CN exposure in these scenarios.
The exposure assessment process evaluates the potential for biological and environmental exposure to NMs at each stage of the product life cycle by ranking scenarios on the following four dimensions of hazard and exposure:
1) Hazard potential, which relates mainly to the significance of the potential for direct contact, such as unbound dry CNC crystals, versus a gel solution containing CNC in water; or exposure to dust vs. solid composite material containing CN. Differences by exposure route are factored in.
2) Magnitude, which indicates the relative size of the exposure by percentage of NM in the scenario.
3) Likelihood, which assesses and scores intentional or usual events relative to unanticipated ones, such as a spill.
4) Frequency, which estimates how often an exposure is expected, with routine exposures ranking higher in frequency than an accidental or infrequent ones.
The scenario activities generated from the Hazard Identification phase were evaluated on the basis of a relative rating, ‘high’, ‘medium’, ‘low’ for each of these four variables, generally represented by the criteria in Table 2. Summing across the four variables generated an overall score for a scenario. The overall scores were then used to rank all scenarios with the greatest potential for exposure and to further assess those in the risk characterization.
| Hazard | Magnitude | Likelihood | Frequency | |
|---|---|---|---|---|
| Low | Covalently bound particles in substrate | Exposure is to article where one component is >1% NM | Direct contact mitigated | Infrequent – exposure possible <10 times per year |
| Medium | Particles potentially releaseable from substrate | Exposure to material >1% to <10% | Unintentional – exposure possible based on activity | Incidental – use 10–50 times per year |
| High | Dried particles in powder form/direct contact exposure | Exposure to material is greater than 10% of mixture | Intentional – repeat exposure during normal use | Regular – greater than 50 times per year |
Following the example of the food packaging application, four scenarios ranked in the top tier on the basis of the scores in Table 2: B.5.1; B.3.1; B.1.4; and B.4.2. B.5.1 is an environmental release scenario, while B.3.1 is a consumer release scenario – not intentional but still possible, and due to the nature of consumer use packaging, fairly frequent. Scenarios B.1.4 and B.4.2 are examples of post-use occupational exposure, scenarios not likely to be identified without consideration of the product life cycle.
2.3. Physico-chemical characterization
Nanoscale materials are often categorized as having unique properties as a function of small particle size.16–18 The properties may relate to toxicity and environmental behavior, and are generally recommended to be reported in toxicity testing evaluations.19–21 The available information is evaluated in the results in Section 3.2.4. Toxicology review and gap analysis
This first iteration of the NANO LCRA included a literature review of CN to assess current knowledge about potential hazards by key exposure pathways, with regard to absorption, distribution, metabolism and excretion (ADME), as well as potential short- and long-term toxicity hazards, for mammalian and non-mammalian endpoints. We reviewed the available literature to characterize toxicological hazard and identify knowledge gaps. The data were culled from published literature on CNF, CNC, and other forms of cellulose through March 2015. When possible, we focused on wood-based CN, but in categories where the data were deficient, we included studies on CN from other CN sources and data from studies using microscale and conventional forms of cellulose. The relevant information categories used to characterize risk included physico-chemical data; delayed, immediate, or chronic human health effects of both short- and long-term exposures; effects of environmental exposure including ecotoxicity, bioaccumulative potential, mobility in soil; and environmental persistence for persistent, bioaccumulative and toxic (PBT) and/or for very persistent and very bioaccumulative (vPvB) assessment.To identify current data gaps specific to CN, a comparative survey, or gap analysis, of recent literature was performed by Vireo Advisors on behalf of the P3Nano partnership. The gap analysis was conducted to evaluate the adequacy of available data for completing Safety Data Sheets for CNC and CNF in compliance with the United Nations Globally Harmonized System of Classification and Labeling of Chemicals (GHS).22 The GHS includes 16 categories of safety information for reporting physical, health, and environmental hazards of a chemical substance, some of these categories are optional according to the US Occupational Safety and Health Administration (OSHA) Hazard Communication Standard. While a separate hazard class does not currently exist for nanomaterials, a gap analysis using the SDS categories of information facilitated the identification of knowledge gaps, building on the toxicological review described above.
2.5. Risk characterization
The results obtained in the Hazard Identification, Exposure Assessment, and the Toxicological Evaluation are integrated to develop the risk characterization. The risk characterization, in essence, evaluated scenarios based on available information analysis, then assessed the adequacy of the available information in terms of understanding and managing risks. Next, the key gaps and uncertainties identified through this step of risk analysis guided the development of the EHS roadmap.3. Results
This section discusses the findings of the NANO LCRA, the literature review, and the data gap analysis. These findings form the basis for the roadmap in Section 5.3.1. Hazard Identification and physico-chemical characteristics of CNC and CNF
Researchers have linked more than a dozen different physico-chemical characteristics of nanoparticles to differences in biological activity,19 yet, to date, no directly reproducible relationships, including particle size, between a specific property and biological effects are agreed to be predictive. As these relationships are further explored, the current recommendations are for measurement of several key properties. One limitation to this effort, however, is poor measurement science, including lack of standardized property reporting, which limits the comparability across studies. Consideration of the influence of CN physico-chemical properties on biological effects can aid in developing safer-by-design principles and our understanding of predictive toxicological outcomes.Physico-chemical properties relevant to NMs include, but are not limited to: size and size distribution; diameter (hydrodynamic and aerodynamic); aspect ratio (relationship of length to diameter for non-spherical particles); surface charge (zeta potential); morphology; crystallinity; surface chemistry; surface area; and aggregation level. For CN, these properties vary according to the extraction methods that are employed. Chemical extraction processes for CNC, such as sulfuric acid hydrolysis and 2,2,6,6-tetramethylpiperidinyl-1-oxyl (TEMPO)-mediated oxidation, affect the surface chemistries of the resultant CN,23 and the physiochemical and biological behavior. Physical methods for CNF such as grinding affect the level of branching, size and the particle agglomeration level.
Furthermore, surface charge can be manipulated to create overall anionic or cationic species, which may affect cellular uptake of CN24 and subsequent toxicity. The high aspect ratio shape of CN1 allows for its versatility in potential applications. The two forms of CNs, CNF and CNC, have both physical and chemical properties that are distinct from conventional cellulose, and from each other. For example, CNC is characterized by a greater degree of crystalline structure than CNF.1,23 CNC particles are typically rod or whisker-like structures (in dimensions of 50–500 nm length and a 3–5 nm1 width). Comparatively, CNF particles are typically fibrous and include amorphous regions (length typically 500–2000 nm and 4–20 nm1 width), still a nanoscale material, but not necessarily in three dimensions. Table 3 summarizes reported physico-chemical data for neat, unmodified CN and different surface modified CNs.
| CN material | Surface chemical modification | Surface charge | Length (nm) | Width (nm) | Aspect ratio (est.)a | Zeta potential (mV) | pH | Ref. |
|---|---|---|---|---|---|---|---|---|
| Measurements and ranges are approximate. Some original data sources include estimated standard errors.a Aspect ratio was calculated using length/width data reported by authors, unless expressly stated in publication. Aspect ratio for dimensional ranges was determined by midpoint estimates of each length and width.b Author-reported aspect ratio. | ||||||||
| CNF | None | Neutral | — | — | — | −8.43 | 7.2 | 26 |
| CNF | None | Neutral | — | — | — | −10.1 | 7.2 | 26 |
| CNC | None | — | 120–300 | 10–20 | 42 | −31.3 | 7 | 24 |
| CNC | Carboxylated | Anionic | 137 | 15 | 9 | −28.3 | 7.2 | 26 |
| CNC | Taurine | Anionic | 124 | 10 | 12 | −28.2 | 7.2 | 26 |
| CNC | Sulfonated | Anionic | 107 | 5 | 21 | −38 | 7.2 | 26 |
| CNC | Ethoxyethanol | Neutral | 110 | 10 | 11 | −26.9 | 7.2 | 26 |
| CNC | Hexanediamine | Neutral | 129 | 9 | 14 | −29 | 7.2 | 26 |
| CNC | Ethylenediamine | Neutral | 123 | 9 | 14 | −17.7 | 7.2 | 26 |
| CNC | Glycidyltrimethylammonium chloride | Cationic | 107 | 5 | 21 | 5.1 | 7.2 | 26 |
| CNC | Rhodamine B | ? | 125 | 11 | 11 | −27.8 | 7.2 | 26 |
| CNF | Carboxylated | Anionic | — | — | — | −41.1 | 7.2 | 26 |
| CNC | Rhodamine B isothiocyanate | Cationic | 120–300 | 10–20 | 42 | 8.7 | 7 | 24 |
| CNC | Fluoroscein isothiocyanate | Anionic | 120–300 | 10–20 | 42 | −46.4 | 7 | 24 |
| CNC | Sulfated | — | — | — | −37.5 | 7.8 | 27 | |
| CNC | Unsulfated | — | — | 2.5b | −32.4 | 7.4 | 27 | |
| CNC | Sulfated | 105 | 10 | 12b | −40.1 | 7.3 | 27 | |
| CNF | Carboxylated | 165 | 11 | 14b | −48.3 | 7.2 | 27 | |
| CNF | None | — | — | — | −10.7 | 3.7 | 27 | |
| CNC | Sulfated | Anionic | 130 | 5.9 | 23 | — | — | 23 |
CN's high aspect and sometimes fibrous nature has been associated in the literature with potential inhalation hazards from comparative materials such as asbestos and carbon nanotubes.25 Countering the idea that morphology and dimensional characteristics directly affect toxicity, Harper and colleagues26 reported that surface characteristics, more so than aspect ratio, influenced CN toxicity to zebrafish embryos.
The dimensional characteristics of CN have been linked to its thermal properties, an area of research focus. Nanoparticles may have lower thermal stability due to their greater surface to volume ratio. CN particle surface area- to-volume ratio, which increases as particle diameter decreases, may explain free radical production in vitro.27 As thermal stability decreased (measured by decomposition temperature), free radical production, measured by electron spin resonance, increased.27
In an occupational setting, physical storage of dry CN as a powder is also a consideration for material safety. Conventional cellulose dust is combustible at 240 °C,28 and an experimental deflagration index (Kst) of 22929 demonstrates that tested CN also meets the GHS classification of a Strong Explosive.30 Because CNs have higher surface areas, and possibly higher reactivity than conventional forms of cellulose, CNs may pose an even greater safety hazard, as has been demonstrated for comparative ignition studies on micro and nano forms of metal oxides.31 Cellulose, in conventional form, is considered highly flammable. Flammability studies of CNF, CNC-sulfated, and CNC-neutralized forms demonstrated that, under experimental conditions, CNF had not only had the highest thermal stability (onset temperature = 320 °C), but it also had the highest flammability measures (measured by microscale combustion calorimetry, Total Heat Release = 8.2 kJ g−1; Heat Release Capacity = 154 J g−1 K−1).32 CNC, with sulfate groups on the surface, exhibited the lowest thermal stability (onset temperature = 175 °C) and the lowest flammability measure (Total Heat Release = 4.5 kJ g−1; Heat Release Capacity = 60 J g−1 K−1).32 Refer to Fox et al.33 for further published work on comparative studies of modified and unmodified nanofibrillated cellulose thermal analyses.
3.2. NANO LCRA – ranking risk of scenarios
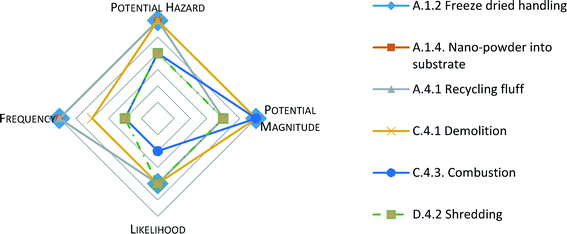
The Hazard Assessment identified 59 discrete scenarios from the five applications, while considering worker, consumer and environmental exposures. A scenario requires that exposure pathways are complete, from CN release in environmental media to receptor contact. Here the hazard identification and exposure assessment are jointly evaluated.Priority scenarios were defined as those having an overall score of ‘8’ or more, out of 12 possible points. By that designation, 22 out of 59 scenarios were in the priority category. Four scenarios were rated highest with a score of 11; one scenario scored ‘10’; nine scenarios were scored ‘9’ and the remaining eight scenarios totaled a score of 8. The top four scenarios with the highest priority ranking of 11 involved occupational exposures, shown in Fig. 3, as spiderplots, which are useful for identifying the data gaps, and establishing priorities based on the gaps. 13 of the priority scenarios included occupational exposures through various pathways that might occur across the product life cycle, many of which would involve article production and manufacture (Stages 1 and 2). Table 4 shows the highest priority scenarios with a score of 8 or above, listing each variable's relative rank. The individual ranking is not as informative as the highest overall rank, which indicates a significant potential exposure risk based on the four dimensions.
| Scenario number | Scenario description | Life cycle stage | Potential hazards | Receptor | Exposure pathway | Potential hazard | Potential magnitude | Likelihood | Frequency | Overall rank |
|---|---|---|---|---|---|---|---|---|---|---|
| A.1.2 | Processed material is dried and then accidentally inhaled by a facility employee | Production | Inhalation contact with CN particles | Occupational | Inhalation | 3 | 3 | 2 | 3 | 11 |
| A.1.4 | Application of dry nano cellulose to create film product, contacting facility employees via inhalation | Manufacturing | Inhalation contact with CN particles | Occupational | Inhalation | 3 | 3 | 2 | 3 | 11 |
| A.1.7 | Mixing dry CN material with other materials to manufacture a product and powder inhaled by employee | Manufacturing | Inhalation contact with CN particles | Occupational | Inhalation | 3 | 3 | 2 | 3 | 11 |
| C.1.5 | Facility employee contacts CN particles, incidental inhalation with airborne CN particles | Manufacturing | Inhalation contact with CN particles | Occupational | Inhalation | 3 | 3 | 2 | 3 | 11 |
| E.5.1 | Release to environment via waste water | Post-consumer/end-of-life | Physical impacts on receiving waters | Environmental | Environmental | 2 | 2 | 3 | 3 | 10 |
| E.5.2 | Degradation in landfill | Post-consumer/end-of-life | Degradation of CN particles | Environmental | Environmental | 2 | 2 | 2 | 3 | 9 |
| A.1.5 | Stored CN becomes unstable and explodes | Manufacturing | CN particles/energetic release | Occupational | Direct contact | 3 | 3 | 2 | 1 | 9 |
| A.4.1 | Paper shredded at a recycling facility (dry); accidental contact with facility employee via inhalation | Post-consumer/end-of-life | Inhalation of CN particles and matrix | Occupational | Inhalation | 2 | 2 | 2 | 3 | 9 |
| B.5.1 | Material disposed of in landfill to biodegrade; intentional contact with environment | Post-consumer/end-of-life | Environmental contact with CN particles | Environmental | Environmental | 1 | 2 | 3 | 3 | 9 |
| C.3.2 | Spray application, accidental inhalation | Application/use | Inhalation of wet CN in matrix | Consumer | Inhalation | 2 | 3 | 2 | 2 | 9 |
| B.3.1 | Migration to food; ingestion of CN particles by consumer | Application/use | Ingestion of CN particles in matrix | Consumer | Ingestion | 2 | 2 | 2 | 3 | 9 |
| E.1.5 | Mixing wet CN material with other materials to create product | Manufacturing | Dermal contact with CN particles | Occupational | Dermal | 1 | 3 | 2 | 3 | 9 |
| E.3.2 | Cosmetics application to consumer skin | Application/use | Inhalation of CN in matrix as a powder | Consumer | Inhalation | 2 | 2 | 2 | 3 | 9 |
| E.4.1 | Wash into residential sewer system | Post-consumer/end-of-life | Degradation of CN particles | Environmental | Environmental | 2 | 2 | 2 | 3 | 9 |
| A.1.1 | Accidental acid spill during production, on the production floor, accidental dermal contact with facility employees | Production | Acid | Occupational | Dermal | 3 | 3 | 1 | 1 | 8 |
| A.4.2 | Paper pulped at a recycling facility (wet); accidental dermal contact with facility employee | Post-consumer/end-of-life | Dermal contact with CN particles in matrix | Occupational | Dermal | 2 | 1 | 2 | 3 | 8 |
| B.1.4 | Production of coating using liquid CN, contacting facility employees via inhalation | Manufacturing | CN particles in the lungs, becoming wet and potential internal exposure | Occupational | Inhalation | 2 | 2 | 2 | 2 | 8 |
| B.4.2 | Discarded with petroleum-based plastic material and sent to a recycling center, processed as plastic and product particles inhaled by facility employees | Post-consumer/end-of-life | Inhalation of CN particles in matrix | Occupational | Inhalation | 2 | 2 | 2 | 2 | 8 |
| C.4.3 | Building fire | Post-consumer/end-of-life | Combusted material, smoke/emissions containing CN or vapor from combusted CN | Occupational | Inhalation | 2 | 3 | 2 | 1 | 8 |
| D.5.1 | Longevity of biodegradable product | Post-consumer/end-of-life | Degradation of CN particles | Environmental | Environmental | 2 | 2 | 2 | 2 | 8 |
| C.3.4 | Kid eating wet paint or dry chips/dust | Application/use | Ingestion of wet CN in matrix | Consumer | Ingestion | 2 | 2 | 2 | 2 | 8 |
| D.4.1 | Deconstruction/demolition, airborne dust/particles inhaled by workers | Post-consumer/end-of-life | Inhale airborne dry CN particles | Occupational | Inhalation | 2 | 2 | 2 | 2 | 8 |
One of the NANO LCRA Hazard Identification and Exposure Assessment's top-ranking scenarios involved occupational exposure to a CN product during its production stage, where a facility employee handled a tray-sized ‘cake’ of CN after freeze-drying processing and could inhale airborne CN particles. The worker removes the cake from the tray and transfers it into bags with other CN powder (see Fig. 3 and Table 4 [A.1.2]). Similarly, this raises concern about one's exposure to CN during packaging of spray-dried CN material, where inhalation contact rates ‘high’ because of the CN exposure directness and the assumed number of respirable CN particles. Here, a worker would potentially be exposed to unbound CN particles. Frequency was also ranked high in this scenario, since in an occupational setting, a worker would likely be exposed regularly to the dry CN while handling it. The likelihood ranking however, was assessed ‘medium’ due to the anticipated probability of this incidental inhalation exposure to be somewhat likely, relative to other exposures evaluated in the NANO LCRA. An overall rank for the scenario was determined by ranking hazard potential, magnitude, and frequency high at ‘3’, while ranking likelihood medium at ‘2’, for an overall ranking of ‘11’.
Among the top priorities, eleven scenarios involved the inhalation exposure pathways, nine of these also involved occupational pathways. Given these findings, while simultaneously considering the overall scenario relative rank, the NANO LCRA revealed that the highest priority scenarios were those associated with occupational exposures in the production and manufacturing stages of the product life cycle. Workers could be directly exposed to aerosolized airborne CN material from various occupational activities. The highest ranking consumer exposure was found to be incidental CN ingestion from food packaging, because if migration occurred, this would be a direct pathway, via food consumption. Several environmental pathways also ranked in the top 22 scenarios, because of the potential for CN release to wastewater, sewer, or landfill. The exposure assessment highlights the life cycle stages, receptors, and exposure pathways that should be prioritized for further information gathering. Scenario A.1.5 (Table 4), which reflected material safety, also ranked relatively high among the priority scenarios, as the next section further explains.
The US National Institute of Occupational Safety and Health (NIOSH), in partnership with the United States Department of Agriculture (USDA) Forest Product Laboratory (FPL), has conducted on-site field studies at the FPL Cellulose Nanomaterial Pilot Plant and at the University of Maine. NIOSH researchers' initial investigation at the FPL pilot plant34 evaluated potential worker exposure to CN resulting from various production activities, using cesium-tagged CNC, in post-synthesis and post-purification stages. NIOSH found that airborne cesium concentrations were highest in filter-based samples collected during centrifugation, whereas concentrations found in freeze-drying samples were nearly three orders of magnitude less. Incidentally, FPL no longer operates the centrifuge for CN clarification due to process improvement. While cesium detection through elemental analysis was used as a proxy for CN exposure, and could broaden exposure pattern knowledge, it is not possible to quantify CNC exposure levels from cesium concentration observations. NIOSH also reported difficulty in confirming detection by way of electron microscopy of CNC deposited onto cellulose-based filters. This report demonstrated that there is a lack of validated methods to measure CN exposure. Further, few occupational exposure limits specific to nanoparticles exist. Thus, the available data do not shift the scenario rankings.
3.3. Toxicology: literature review and gap analysis
Conventional cellulose is an abundant and naturally occurring material that is used widely in food preparation and packaging, hence, a priori one would not anticipate CN toxicity. In fact, one form of CNC is listed on Canada's Domestic Substances List on the basis of testing described below.8 However, because of the nanoscale dimensions, toxicity needs to be more closely evaluated, particularly because differences in production methods may change physico-chemical properties and consequently, behavior. The available health and environmental studies of cellulose CNC and CNF toxicity were reviewed to inform the NANO LCRA, identify gaps in the priority scenarios, and compare to regulatory information requirements. The following subsections summarize these data by endpoint; Table 5 presents the data, which are mostly for wood-derived cellulose, but there are some noted exceptions.| Toxicity endpoints | Systemic endpoints | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relevant pathway | Exp. duraton | Material (generals) | Material (specific) | Ref. | Cytotoxicity/cell viability | Oxidative stress | Immunotoxicity | Genotoxicity | Neurotoxicity | Carcinogenicity | Reproductive effects | Mortality | Gross toxicity | Pathology | Body wt./wt. gain | Pulmonary | Cardiovascular/hemologic | Sensitization/irritation | Neurological/behavioral |
General overview of knowledge gaps of health effects and endpoints. Not intended to be comprehensive. Red-colored symbol indicates response reported. N/A = not available. MCC = microcrystalline cellulose. Symbols: ◆ in vivo;  in vitro; ● acellular. in vitro; ● acellular. |
|||||||||||||||||||
| Inhalation | Short-term | CNF | Var. prep | [37] |

|
||||||||||||||
| CNC | NCC™ | [34] | ◆ | ◆ | ◆ | ||||||||||||||
| CNC | Gel/powder | [38] |

|

|

|
||||||||||||||
| CNC | [40] |

|

|

|
|||||||||||||||
| CNC | [41] |

|

|
||||||||||||||||
| Cellulose | Various | [43] | ◆ | ||||||||||||||||
| Cellulose | MCC | [48] |

|
||||||||||||||||
| Cellulose | Dust | [35] |

|

|
|||||||||||||||
| Cellulose | MCC | [50] |

|

|
|||||||||||||||
| Long-term | CNF | [N/A] | |||||||||||||||||
| CNF | [N/A] | ||||||||||||||||||
| Cellulose | Fiber/dust | [51] |

|
||||||||||||||||
| Ingestion | Short-term | CNF | N/A | ||||||||||||||||
| CNC | NCC™ | [8] | ◆ | ||||||||||||||||
| Cellulose | Variuos | [43] | ◆ | ||||||||||||||||
| Long-term | CNF | N/A | |||||||||||||||||
| CNC | NCC™ | [8] | ◆ | ◆ | ◆ | ||||||||||||||
| Cellulose | MCC/bulk | [44] | ◆ |

|

|
◆ | ◆ | ||||||||||||
| Dermal | Short-term | CNF | N/A | ||||||||||||||||
| CNC | NCC™ | [8] | ◆ | ||||||||||||||||
| CNC | NCC™ | [8] | ◆ | ||||||||||||||||
| CNC | NCC™ | [8] | ◆ | ||||||||||||||||
| Cellulose | MCC | [44] | ◆ | ||||||||||||||||
| Cellulose | Various | [43] | ◆ | ||||||||||||||||
| Long-term | CNF/CNC/cellulose | N/A | |||||||||||||||||
| Eye contact | Short-term | CNF | N/A | ||||||||||||||||
| CNC | N/A | ||||||||||||||||||
| Cellulose | MCC | [44] |

|
||||||||||||||||
| Long-term | CNF/CNC/cellulose | N/A | |||||||||||||||||
| Systemic effects | Short-term | CNF | [9] |

|
|||||||||||||||
| CNF | Var. prep | [37] |

|

|

|
||||||||||||||
| CNF | [44] |

|
|||||||||||||||||
| CNC | [42] |

|
|||||||||||||||||
| CNC | NCC™ | [8] |

|
||||||||||||||||
| CNC | NCC™ | [8] |

|
||||||||||||||||
| CNC | [46] |

|
|||||||||||||||||
| Long-term | CNF | N/A | |||||||||||||||||
| CNC | N/A | ||||||||||||||||||
| Cellulose | MCC | [44] | ◆ | ||||||||||||||||
| Bio-durability | Short-term | CNF | Var. prep | [27] | ● | ||||||||||||||
| CNC | Var. prep | [27] | ● | ||||||||||||||||
| Cellulose | MCC | [27] | ● | ||||||||||||||||
| Long-term | CNF | Var. prep | [27] | ● | |||||||||||||||
| CNC | Var. prep | [27] | ● | ||||||||||||||||
| Cellulose | MCC | [27] | ● | ||||||||||||||||
| Bio-persistence | Short-term | CNF | N/A | ||||||||||||||||
| CNC | N/A | ||||||||||||||||||
| Cellulose | MCC | [48] |

|
||||||||||||||||
| Long-term | CNF/CNC/cellulose | N/A | |||||||||||||||||
Most studies investigated short-term exposures to CN, mainly focusing on the inhalation routes. Similarly to most poorly soluble particles and fibers, cellulose dust, when inhaled, can cause irritation,35 so there is reason to anticipate that CNs in air may also be irritants. However, key differences in particle size are anticipated between inhalation of cellulose particles/fibers and inhalation of nanoscale cellulose dust. Smaller particles tend to be inhaled deeper in the lung than larger particles, which tend to be trapped in the upper airways and be cleared.36 The high aspect ratio of CNC and CNF raises concern about fibrotic behavior in the lung, and whether exposures to CN are potentially of greater impact than to cellulose dust. In one study on CNF in mice37 researchers observed slight adverse immune effects in vivo, but the authors also reported that bacterial presence in the CNF sample might have been a confounding and limiting factor in their conclusions. In a recent conference presentation, Finnish researchers reported two of four types of CNF activated an inflammatory response after 24 hours pharyngeal aspiration exposure to 10 and 40 μg per mouse, but no long term effects (after 28 days) were noted. One material also demonstrated inflammatory effects in vitro.38
For CNC, two animal studies and two cellular assays were reported. In one in vivo study,8 researchers reported no adverse effects for 14 days after rats were exposed to CNC for 4 hours, measured by rat mortality, gross toxicity, and behavioral effects after acute exposure to up to 0.26 mg L−1 of CNC. This relatively low concentration was used because researchers were unable to obtain higher aerosol levels in the exposure chamber due to the highly viscous CNC material's clogging of the equipment. A second murine study reported an increase in immune responses and oxidative stress 24 hours after a single exposure to CNC powder or solution (10 weight% suspension and freeze-dried powder form) by pharyngeal aspiration.39 In this study, the 50 mg exposure to the material occurred instantly. Interpretation of these findings is challenging, since the exposures may have created ‘dust overload’ conditions in the lungs, and particularly because the experimental design did not include time for lung clearance before response was measured. A repeat exposure study to 1000 fibers ml−1 with conventional cellulose in rats revealed a decrease in initial inflammatory responses after day 1, despite repeat exposures over three weeks.35
Contrary to overt toxicity effects, sublethal responses may be early markers of disease states, but they also may resolve some time after the exposure. Data from two similar in vitro investigations using a novel human 3D cell co-culture model to represent a human airway barrier, varying in dose and exposure method, generally suggest no adverse effects in regard to lung cell morphology, oxidative stress, or immunological effects.40,41 Cell viability results, however, differed between the two investigations, with one resulting in diminished cell viability in the apical layer of CNC-exposed cells.41 CNC exposure in vitro to human monocyte-derived macrophages did not elicit an immune response, or an inflammatory effect by measuring cytokines TNF-alpha and IL-1beta.42
These studies suggest that further work is needed to discern the extent to which cellulose nanomaterials may affect the lungs after it is inhaled, and to establish relative potency, particularly for longer term exposures and more realistic exposure levels.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 supported by dose–response testing with conventional cellulose. Data for short-term oral and dermal exposures were not found for CNF, nor were studies found that involved eye contact exposure for either CNC or CNF.
43 supported by dose–response testing with conventional cellulose. Data for short-term oral and dermal exposures were not found for CNF, nor were studies found that involved eye contact exposure for either CNC or CNF.
One in vivo study of CNC, a 28 day repeated ingestion dose experimental design in accordance with OECD TG 407, was the only subchronic study; researchers reported no toxicity was observed at any dose.8 The measured parameters of neurological effects, body weight, and food consumption were not found to be statistically different from the control animals, and a no-observable effect-level (NOEL) was determined to be greater than 2000 mg per kg per day. Similar results were observed in a 1963 study of oral exposure to cellulose, microcrystalline cellulose (MCC), and MCC gel in rats. This long-term feeding study, which the Joint Expert Committee on Food Additives of the World Health Organization (WHO) cites,44 reported no adverse effects from these forms of cellulose at 30 percent of the rats' diets for 72 weeks.
Our review indicates a deficiency of published data, in vitro and in vivo, on other health endpoints associated with chronic effects, as well as carcinogenicity, neurotoxicity, and reproductive effects. For carcinogenicity (and carcinogenic potential) and reproductive toxicity, no data on CNF or CNC were available. A similar dearth of data characterized the information on these endpoints for conventional celluloses, where only one study assessed tumorigenesis in cellulose and MCC and found no significant effects in rats, from the 72 week oral study published in 1963.44 To date, very few data exist in the peer-reviewed literature on neurotoxicity of CN. However, researchers in Finland employed an in vivo nematode model with a primitive nervous system, C. elegans, to study neurotoxic and behavioral endpoints.23 They report that CNF did not cause adverse neurologic effects.49
The available data indicate that a variety of CN forms and biological endpoints have been investigated with differing variables such as physico-chemical properties, length of exposure, cell types and organisms, and dose metrics. These influencing variables and their effects were not uniform across studies making it difficult to assert a unifying weight-of-evidence conclusion based on the data for any particular exposure route or material. The prime example here is the inconclusive nature of the existing lung assays evaluating inhalation exposure. With the exception of one 28 day in vivo ingestion study,8 no studies were available on longer-term exposures (chronic and subchronic). Further, specific quantitative exposure data for likely routes of exposure were not found.50,51
Harper and colleagues26 conducted several assays using zebrafish embryos and an in vivo developmental toxicity model to assess impacts on growth and reproduction as well as to assess relative toxicity. Test results varied according to dose, surface functionalization, synthesis method, morphology, and form (CNC and CNF). They report that CNC was relatively non-toxic in zebrafish embryos until extremely high dose levels (above 2000 mg L−1), after confirmed uptake of CNC.26 They also found that CNF, similar to CNC, also demonstrated little to no toxicity in zebrafish embryos below doses of 250 mg L−1.26 Zebrafish embryo toxicity from exposure to homogenized CNF occurred only at the highest tested concentration of 250 mg L−1. No effects or mortality were observed at this level from exposure to CNF prepared by TEMPO or sulfonated acid hydrolysis methods. These levels are significantly higher than anticipated environmental concentrations in ambient waters.
Limited ecotoxicological data have been published for CNF relative to CNC, but some data on sublethal effects of CNF have been investigated in bacterial organisms. Vartiainen et al.9 conducted an ecological assessment, including a kinetic V. fischeri luminescence assay exposure to mechanically ground and spray-dried CNF to determine sublethal toxicity. The researchers reported a NOEC of 300 μg mL−1 after 30 minute exposure to the bacterial organism. Exposure to CNF inhibited luminescence by 23 percent at the highest dose of 2500 μg mL−1. The NOEC derived from that assay was much greater than anticipated ambient concentrations (0.24 μg mL−1, see above). Kovacs et al. assessed CNC in a 15 minute V. fischeri luminescence assay, and reported a 25 percent inhibiting concentration (IC25) of >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg mL−1, indicating no sublethal effects at very high concentrations.7 CNF effects on microalga C. vulgaris was investigated by Pereira et al.,52 who found that an organism's exposure to cotton-derived CNF affected cell growth and viability.
000 μg mL−1, indicating no sublethal effects at very high concentrations.7 CNF effects on microalga C. vulgaris was investigated by Pereira et al.,52 who found that an organism's exposure to cotton-derived CNF affected cell growth and viability.
3.4. NANO LCRA risk characterization
Here, The NANO LCRA characterizes the data taken from the previous steps: Hazard Identification; Exposure Assessment; and Toxicological Data Review. The Risk Characterization prioritizes specific scenarios across life cycle stages for further investigation. From the available data in the current literature, the toxicological data review indicated low ecological toxicity, which varied by form and surface substitution. For both human and ecological receptors, there are missing or conflicting data among endpoints. These gaps increase the priority of some exposure scenarios in terms of data gathering, since they were already areas of uncertainty. For example, in the scenario ranking, occupational inhalation exposures ranked highest because of the frequency, potential magnitude and the directness of potential hazard. In the integration of the ranking with knowledge and gaps, the available data suggest that CN inhalation may be hazardous. Thus, occupational exposure assessment rises to the top priority in the roadmap.A primary concern in evaluating the potential exposure risks is an occupational users' inhalation of pure dried CN material during specific production and manufacturing tasks. Cellulose dust is a known irritant,35 and some studies indicate CNC and CNF may cause inflammation if inhaled. The studies are difficult to interpret in the context of real-world exposures, and do not indicate safe or unsafe level of exposure.
The exposure assessment LCRA findings revealed that more than half of the ‘priority’ scenarios involved occupational exposure from different routes over the CN life cycle, with several implicating the inhalation exposure route. The available data from the literature indicate a main concern for CN exposure is inhalation of dry CN or CN-containing particles that are dispersed in the air. These findings collectively support the prioritization of inhalation exposure investigation especially to workers in a production and manufacturing environment, for further experimental study and refinement of data. The findings also indicate where mitigation steps should be executed to reduce potential exposure. Studies are needed to assess exposure levels in the workplace, and to assess the toxicity from inhaled CNC and CNF under realistic exposure conditions (lower levels and longer term exposures and recovery periods). This requires better methods for aerosolizing CN. In addition, we found that workplace exposure studies were limited because of the challenge of positive detection of CN in air (background). Discerning CN from other organic matter also present in air is an important measurement need for exposure testing. Thus, while the top research priority is to assess worker exposure to CN, confirmatory detection methods are first needed to inform the exposure assessment, becoming the top priority of the roadmap.
Other priority exposure pathways include direct contact exposures where ingestion of CN might occur, for example during consumer use of CN containing food contact materials. Consumer exposure and toxicity information represent significant data gaps. That said, several forms of cellulose are already in use in food packaging, and in cases, as food ingredients e.g., cellulose microcrystals (CMC, E466). The available data do not allow characterization of risks from ingestion, representing an important gap to understand whether there are differences between CN and conventional forms of cellulose by ingestion. It would be relatively straightforward to compare the uptake of different forms of CN to current food additives to assess whether there are differences in uptake or absorption, distribution, metabolism and excretion.
Further, regarding material safety our findings from evaluating potential hazards discretely, suggests a ‘high’ potential explosion hazard of dry CN in storage (Table 4 [A.1.5]). OSHA, NIOSH, and others identify the potential for surface-active small particles to combust in the presence of an ignition source, and highlight the need to plan for safety in storage of dry CN.
Finally, while there are a number of data gaps for ecological and environmental exposures, the available information suggest low to no hazard from environmental exposure to CN. Thus, while it is important to fill the knowledge gaps prior to commercialization, particularly because there may be associated environmental benefits with using a bio-based renewable material for some current technologies, environmental scenarios ranked lower than consumer exposure routes when factoring in toxicity in the NANO LCRA, and are represented as such in the roadmap.
4. Discussion
4.1. Data gaps
Currently, CN exposure concentrations are difficult to ascertain and are missing in the literature. Particle counting has been used to study other nanoparticles in the air.56 However, for CN, particle measurement is challenging because filter media and many background sources of particles are also cellulosic. Only one published study in the peer-reviewed literature9 attempted to quantify CN particle exposure during mechanical production practices. Further, particle counting equipment is better calibrated for spherical particles than for high aspect ratio materials.In work completed by NIOSH,34 air samples ranging from 11.6 μg m−3 to non-detectible (cesium-tagged CNC) suggest exposure levels are well below the OSHA Time Weighted Average (TWA) exposure level of 15 mg m−3 for conventional cellulose dust, as well as the TWA for respirable particles of 5 mg m−3, perhaps by a factor of 1000. However, it remains unclear whether these occupational exposure limits for conventional cellulose particles are adequately protective for CN exposure. There is a large margin of exposure based on the current sampling, which needs to be verified through additional monitoring that can distinguish CN from other airborne particles. In particular, NIOSH highlights concerns about inhalation due to the size, shape and surface area of nanoparticles generally and CN specifically, and suggests caution under uncertainty, including several specific strategies to avoid exposure.
No published studies were identified that examined exposure to CN during manufacture of other products, such as paper or polymer packages and films, or during activities such as cutting that could release CN to the air from a matrix. Occupational exposure from professional use of products, for example, installation of aerogel foam insulation, may also result in inhalation exposure and should be investigated. However, this exposure is likely of a lower magnitude than during manufacturing of CN products.
The review of the available information demonstrated that the characterization and reporting of physico-chemical data differed on key variables that could affect the bioactivity of the CN. Differences between CNF and CNC, and the preparation methods used in isolating those forms can influence the effects found in the studies in vivo and in vitro, as can surface modifications. Some data on CNF indicative of explosivity and flammability33 – material safety aspects—are available, but are yet to be reproduced in the literature, particularly for other forms of CN.
Most of the studies reviewed in the toxicological literature relevant to human health effects investigated short-term exposures rather than longer-term exposures. Therefore, there is a scarcity of chronic, subchronic and repeat exposure studies from which to draw sound conclusions. Currently, some data are available for the inhalation route in vivo and in vitro, but exposures via the dermal and oral route are mostly absent in the literature, especially for CNF. When evaluating the body of knowledge regarding specific human health endpoints, some data were found for inflammatory response (immunologic) and genotoxicity. However, other endpoints usually evaluated for human health, carcinogenicity, neurotoxicity, and reproductive effects, are missing in the published data. In addition, to date there are no published studies on the toxicokinetics of CN. While the available data do not suggest significant or novel hazards, there are key gaps which preclude safety demonstration. The conclusions from a recent review6 on effects of CNC exposures, reflect the findings presented here for CN, that more studies are necessary for elucidating seemingly discrepant effects. In addition, physico-chemical parameters especially influenced by CN preparation methods and modifications should be analyzed and reported.
The few existing studies on CN (CNC) either had too low or unrealistically high an exposure concentration, and therefore, it remains unclear whether nano-forms of cellulose exhibit different toxicological profile from that of conventional cellulose on the lung. A study to examine short-term lung toxicity by inhalation failed to meet the OECD test guidelines because the aerosolizing equipment clogged at a low concentration (0.26 mg L−1) below the standard test guideline of 5 mg L−1.8 Conversely, a recent 24 hour study by NIOSH39 relied on a technique called pharyngeal aspiration, where an anaesthetized mouse receives a bolus dose on the tongue is rapidly inhaled in extremely high lung overload conditions, so the observed inflammation that resulted is not informative for evaluating effects from occupational exposure, which typically occurs over time. A recent unpublished study at lower levels compared in vivo and in vitro assays following inhalation exposure to CNF in mice. While some preparations demonstrated inflammation 24 hours post exposure, none of the inflammatory responses persisted for 28 days. Past studies with cellulose dust also suggest inflammation resolves fairly quickly.35
Data on ecological impacts of CN include some gaps. Although Kovacs et al. 's study7 was comprehensive and used micro-scale cellulose for comparison to CNC effects on organisms, more ecotoxicological studies need to be conducted for other forms of CN. While the prevalence of CN and available biodegradation studies indicate low ecological risk, there are gaps, albeit lower priority ones. Priority scenarios for aquatic organisms relate to imminent commercial scale production and manufacturing processes, and any hazard posed by the realistic effluent, a mixture of CNC in acidic medium or byproducts, or surface modified CN. For instance, CNF exposure to microalgae cultured in natural Seine river water52 may be more indicative of behavior in realistic media, but data from such studies are currently insufficient.
Terrestrial and soil organism responses to CN exposure have not yet been studied, per se, although de Lima et al.46 present the results of an assay with onion (Allium cepa) root cells. Plant uptake otherwise has not been evaluated to date, nor have fate and transport studies been published that would establish if and how exposure to CNs would impact the environment. Adaptations of traditional meso- or micro- cosm studies for the study of NMs such as those being conducted at the Center for Environmental Implications of NanoTechnology (CEINT, Duke University), and Oregon State University in the US could provide critical information for assessing complex ecological impacts beyond the organism level, particularly if CN is used in products containing other NMs.
Many of the studies reviewed offered comparative data to different forms of CN and non-nano cellulose materials. However, most studies did not test the materials side-by-side with a suite of assays. These types of assessments would be helpful to discern which characteristics may influence bioactivity and toxicity. For instance, Mahmoud et al. showed that varying surface functionality and the zeta potential of CNC affected both uptake and cytotoxicity in vitro.24 As the volume of NM-enabled applications grow, the aggregate number of different forms, preparations, functionalizations and composites incorporating CN will also increase. As described, Harper's laboratory at Oregon State University has compared the toxicity of CNC and CNF generated from different processes and with differing surface characteristics in embryonic zebrafish assays,26 and researchers on the EU's 7th Framework Programme Scaling-Up Nanoparticles in Modern Papermaking (SUNPAP) project evaluated toxicity in a variety of standard OECD test methods using CNF prepared by different methods, and with and without biocides.37
Comprehensive studies would help determine potential for read-across, a method to predict endpoints, by first determining if one CN substance is sufficiently similar to other CN substances or non-nano forms that have already been well-tested and characterized. Conversely, if a CN substance is unique, i.e., physico-chemical characteristics, bioactivity, then further testing is warranted. While the gaps are ubiquitous across receptor categories, the hazard data in Section 3 indicate a low level of concern for many pathways.
4.2. Limitations
The analysis developed here relies on a ranking and prioritization of potential risks across three categories: occupational, consumer and environmental exposures. As such, it may prioritize short term and more immediate concerns over longer term issues, as is reflected in the roadmap in Section 5. As shown in Table 4, consumer, occupational and environmental hazards are among the top ranking scenarios. Thus, further consideration is needed to accurately prioritize research optimally for commercialization in the roadmap.The underlying assumptions used to rank hazard potential were guided by available data, and when data were missing, on the basis of conservative assumptions (e.g. assume CN can migrate from polymer matrix). In the absence of such data, and under uncertainty, it is appropriate to make assumptions that result in higher risk estimations that are protective of human and ecological populations.
The NANO LCRA analysis does not account for mitigation steps that a CN production or manufacturing facility would carry out, or for personal protective equipment that workers may use. Therefore, the rankings reflect conservative assessments of risk potential. Future iterations of the NANO LCRA will analyze available data about the actual implementation of these safety measures and revised exposure estimates, when in place, to more accurately assess real-world exposures to CNs.
The NANO LCRA scenario development and ranking identified the most significant situations to analyze based on the likely product applications and uses. In doing so, the knowledge base for assessing risks by these exposure routes and pathways was explored, which revealed a series of data gaps for both exposure and toxicity for all three receptor categories: occupational, consumer and environmental. The toxicity evaluation explored the knowledge base and identified gaps for inhalation, ingestion, dermal and environmental pathways. The combination of potential for direct exposure to free nanoparticles and indication of inhalation hazard creates a relatively greater concern about inhalation exposure versus environmental exposure pathways, where exposure levels would be lower, less direct, and the available data, albeit with gaps, suggest little to no environmental toxicity. The categories of uses where frequent exposure could bring about direct contact for consumers raise concern about oral exposure pathways, despite detailed understanding of the benefits of cellulose fiber in the diet, because of the nanoscale size. The data gaps for these exposures increased their priority over gaps in environmental exposure, because of the gaps in oral toxicity data. These priorities are reflected in the roadmap below.
5. EHS roadmap of cellulose nanomaterials
The findings from the NANO LCRA, including the toxicological reviews, identified key priorities, uncertainties and data gaps. The EHS roadmap details efforts to fill the most urgent data needs for ongoing and future EHS evaluative efforts on CN and is presented here in order of priority for EHS assessment and management.5.1. Measurement methods
Measurement needs are prioritized in the EHS roadmap because, without them, few other needed studies can be reliably conducted. This includes confirmatory detection techniques, quantitative measurements, and standardized protocols for sampling and analysis in environmental media, including air, water, and solid matrices. These methods should be reproducible, accurate, and easy to conduct in operating manufacturing environments. The most pressing need from top-ranking NANO LCRA Scenarios, A.1.2, A.1.4, A.1.7 and C.1.5, (Table 4) is for information about workplace exposure to CN in air in production environments, the ability to measure CN in air as a function of time, size and size distribution, and distinguish it from background is the first priority.Initial work may involve fluorescent tagging but post sampling detection is the goal. Next is to develop a sampling device to reliably collect and size segregate airborne CN, a challenge because of the high aspect ratio of the fibers. Following this, the third aim is to confirm CN collection with a detection device. The ultimate goal is a field-ready method that can be used by industrial hygienists in the field.
M1.1 Aerosolized dust. As discussed, dust exposure characterization is the highest priority, in order to assess exposure levels in the workplace and potential inhalation risk from CN production.
M1.2 Aqueous solutions. The next priority from the NANO LCRA (Table 4 [E.5.1]) is methods to measure the concentration of CN in process water, that will be discharged from facilities. Further, some producers will keep material in solution, and concentrations must be reliably characterized, including the level of aggregation. Required for effluent testing, as well as confirmatory presence of material in feedstock, methods are needed that can confirm the presence/absence of CN, and also measure concentration in process, waste, and ambient waters.
M1.3 Non-aqueous solutions. A related priority is for detection of CN in other liquids, such as biological fluids, and also in liquid composite materials.
M1.4 Solid matrices (e.g. composites, biological matrices). Further, since consumer exposures will be to composite products, not likely the raw materials, measurement techniques for CN in a variety of other materials is needed. For example, use of CN to reinforce food packaging requires migration testing, which relies on the detection of CN to measure the migration of CN from packaging matrices. These tie to NANO LCRA Scenario B.3.1 (Table 4).
5.2. Physico-chemical data
NANO LCRA Scenario A.1.5 (Table 4) highlights the priority data gap of the assessment of safety for nanomaterials requiring better characterization of key properties that may influence biological and environmental behavior. There is a strong link between bioactivity and environmental behavior of NMs to their surface properties moreso than mass.20 These measurements are not required only for dry and aqueous solutions, but also for as-delivered forms in toxicology experiments to measure dose. In characterizing and reporting these physico-chemical data, there is also a need to adopt a standard set of parameters that would facilitate toxicological assessment. Several groups are currently working on these measures, including MINChar,21 NanoImpactNet,57 International Organization for Standardization (ISO),58 and others. For CN, some additional physico-chemical characteristics are important to consider due to the behavior of the conventional cellulose.Measures include:
5.3. Human health safety studies
A variety of existing short-term (acute) toxicity test methods for testing chemicals were used to assess CNC and CNF, as were some novel assays, mostly in vitro. However, it is not clear whether the assays are appropriate for testing nanomaterials generally, or CN specifically. There is a need to confirm the methods work for the stated purpose, as some standard toxicity tests have been shown to give false results due to interference of the nanoparticles with test reagents or media.59–61 The OECD has been evaluating its standard test methods for their applicability to nanomaterials. These methods need to be reviewed and considered in terms of their ability to report on the safety of CN materials.Inhalation exposure studies are needed because all foreign particles are potentially hazardous to the lung, and there are few nano-specific requirements for workplace safety. There is a need to assess the effects of inhalation exposure in the workplace. The human health toxicity evaluation and gap analysis prioritize longer-term exposure studies to evaluate whether there is a more significant effect from nano-forms than more conventional forms when inhaled. Also, current studies are too brief to provide meaningful information about effects of CN on the lung as would occur during manufacturing. A key measurement requirement for this testing is a method to reliably aerosolize well characterized CNC and CNF up to a concentration of 5 mg L−1, in accordance with the OECD Test Guideline 403.
This step is critical because: 1) several forms of cellulose are already approved by the US Food and Drug Administration (FDA) and others for use as food additives and food contact substances, or have been affirmed as Generally Regarded As Safe (GRAS); 2) assessing whether novel nano-forms behave differently from approved forms has potential to open opportunities for food-contact applications. It would be best to compare a range of studies including oral feeding studies and those assessing absorption, distribution, metabolism and excretion (ADME) following ingestion, with data published in peer-reviewed literature; 3) this work is prioritized because further animal testing will not be required if differences in uptake among nano and approved forms (non-nano) are not observed; 4) a robust comparison of in vitro tests with in vivo (whole animal) bioassays will create an easier path forward for surface-functionalized CN and materials produced by alternate pathways to be assessed for safety. It will allow for bridging studies, so that animal testing need not be repeated for each surface modification.
5.4. Environmental safety
Although the NANO LCRA scenario analysis identified several priority environmental scenarios, CN is presumed to be a low hazard to the environment based on the toxicology review. However, more studies are required to demonstrate this. Based on the NANO LCRA, the following categories of information are prioritized.5.5 Develop and adopt standardized EHS methods
Finally, the development of standards for measuring and assessing the safety of cellulose nanomaterials will aid in commercialization of products. NIOSH and others have developed guidance for assessing worker exposure, and these generic guidelines are a useful starting framework. Some resources are specifically intended for persons conducting research, for example the Good Nano Guide,62 the California Nanosafety Consortium of Higher Education's Nanotoolkit,63 OSHA's Working Safely with Nanomaterials64 and NIOSH's Current strategies for engineering controls in nanomaterial production and downstream handling processes.65 The Technological Association of the Pulp and Paper Industry (TAPPI) is working on standards development and has hosted annual workshops on this topic as part of the TAPPI International Nanotechnology Conference since 2011. Below is a list of EHS standards that group has discussed.1) Sampling and Measurement Standards Development
a) Develop sampling protocols, including sample preparation
b) Develop test approaches for different materials and applications
2) Occupational Exposure Standards
a) Guidance for sampling and worker protection
3) Environmental Impact Standards
a) Guidance for monitoring environmental impacts
b) Measurement methods for air, water, other media
c) Decision tree analysis
4) Consumer Product Standards
a) Guidance for testing nano-enabled products
5) Sustainability Measurements
6. Conclusions
As an abundant bio-based material, cellulose nanomaterials hold potential for wide replacement of petroleum-derived substances, however to fulfill that potential, more robust demonstration of safety is needed than is currently available. The NANO LCRA analysis found several gaps in knowledge regarding CNC and CNF, affecting the ability to draw conclusions from the available data about the safety of cellulose nanomaterials. While few available data indicate health or environmental hazards, the available data do not allow a conclusion of safety or harm from exposure, at this time.The NANO LCRA prioritized EHS research needed to demonstrate safety on the basis of the immediacy of exposure, considering what is known about poorly soluble fibers and nanoscale particles, as well as studies of cellulose from other occupational environments. Improvements in measurement methods, and consistent testing of different forms (CNC, CNF and surface modified substances) will reduce uncertainty and allow detailed characterization of safety of these emerging nanomaterials. Based on the NANO LCRA, a roadmap was proposed to address critical data gaps and inform safety assessment for safe workplace handling, use in consumer products, and demonstration of benign environmental disposition.
The NANO LCRA for CN methodology and findings presented here facilitated early evaluation of CN safety issues and prioritization of exposures for further study based on a risk ranking that incorporates life cycle thinking for risk characterization from hazard identification to toxicity review, and exposure safety to human health and the environment, with the information that is currently available. The EHS roadmap for CN draws upon each of these analyses, providing a clearer path forward, with the objective of better characterizing risk and addressing EHS concerns. Understanding the key knowledge gaps and the rationale for the filling them in the near term lays the groundwork necessary in adopting a proactive, rather than reactive approach to ensuring safety of a product to workers, consumers, and the environment, across the life cycle of CN.
Caution should be exercised when interpreting the findings of CN studies, as physico-chemical characteristic differences between forms (CNC, CNF), as well as among variable preparation methods for the CNF (e.g. type and extent of homogenization, pretreatment with enzyme or TEMPO-mediated, biocides or not), likely influence the observed biological and ecological effects. The review of toxicological information for the NANO LCRA analysis underscores the importance of nomenclature, careful measurement and reporting of physico-chemical parameters unique to CN including surface charge, and agglomeration, and the metrology involved in their measurement.
Acknowledgements
The authors wish to acknowledge the Nanotechnology Team at the National Institute of Occupational Safety and Health, especially Charles Geraci, and Adrienne Eastlake, for their contributions. Theodore Wegner and Alan Rudie of the US Forest Service Forest Products Laboratory provided key insights into production and applications. Kimberly Ong is acknowledged for her analysis of data relative to the requirements of the GHS. This work was sponsored in part by the US Forest Service Forest Products Laboratory and by P3Nano, the public private partnership to advance the commercialization of nanocellulose.References
- R. J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, Chem. Soc. Rev., 2011, 40, 3941 RSC
.
- H. Wei, K. Rodriguez, S. Renneckar and P. J. Vikesland, Environ. Sci.: Nano, 2014, 1, 302–316 RSC
.
- J. A. Shatkin, T. H. Wegner, E. M. (Ted) Bilek and J. Cowie, Tappi J., 2014, 13, 9–16 CAS
.
- M. Jorfi and E. J. Foster, J. Appl. Polym. Sci., 2015, 132, 41719, DOI:10.1002/app.41719
.
-
J. K. Hong and M. Roman, in Production and Applications of Cellulose Nanomaterials, ed. M. T. Postek, R. J. Moon, A. W. Rudie and M. A. Bilodeau, TAPPI Press, Peachtree Corners, GA, 2013 Search PubMed
.
- M. Roman, Ind. Biotechnol., 2015, 11, 25–33 CrossRef CAS
.
- T. Kovacs, V. Naish, B. O'Connor, C. Blaise, F. Gagné, L. Hall, V. Trudeau and P. Martel, Nanotoxicology, 2010, 4, 255–270 CrossRef CAS PubMed
.
-
B. O'Connor, R. Berry and R. Goguen, in Nanotechnology Environmental Health and Safety, ed. M. S. Hull and D. M. Bowman, William Andrew Publishing, Oxford, 2nd edn, 2014, pp. 225–246 Search PubMed
.
- J. Vartiainen, T. Pöhler, K. Sirola, L. Pylkkänen, H. Alenius, J. Hokkinen, U. Tapper, P. Lahtinen, A. Kapanen, K. Putkisto, P. Hiekkataipale, P. Eronen, J. Ruokolainen and A. Laukkanen, Cellulose, 2011, 18, 775–786 CrossRef CAS
.
- J. Cowie, E. M. Bilek, T. H. Wegner and J. A. Shatkin, Tappi J., 2014, 13, 57–69 CAS
.
- National Science and Technology Council and Committee on Technology, National Nanotechnology Initiative Environmental, Health, and Safety Research Strategy, 2011.
- Royal Society and Royal Academy of Engineering, Nanoscience and nanotechnologies: opportunities and uncertainties, Royal Society : Royal Academy of Engineering, London, 2004.
- J. A. Shatkin, J. Ind. Ecol., 2008, 12, 278–281 CrossRef PubMed
.
-
J. A. Shatkin, in Nanotechnology: Health and Environmental Risks, CRC Press, Boca Raton, FL, 2nd edn, 2012, pp. 149–170 Search PubMed
.
- J. A. Shatkin, Environmental Health and Safety Roadmap for Cellulose Nanomaterials. Report to the US Forest Service, 2013 Search PubMed.
- European Commission and Directorate General for Health & Consumers, Guidance on the safety assessment of nanomaterials in cosmetics, European Commission, Brussels, 2012.
- European Food Safety Authority (EFSA) Scientific Committee, Scientific Opinion: The Potential Risks Arising from Nanoscience and Nanotechnologies on Food and Feed Safety, 2009.
- National Institute of Occupational Safety and Health (NIOSH), Approaches to Safe Nanotechnology: Managing the Health and Safety Concerns Associated with Engineered Nanomaterials, 2009.
- G. Oberdörster, A. Maynard, K. Donaldson, V. Castranova, J. Fitzpatrick, K. Ausman, J. Carter, B. Karn, W. Kreyling and D. Lai, Part. Fibre Toxicol., 2005, 2, 8 CrossRef PubMed
.
- G. Oberdörster, E. Oberdörster and J. Oberdörster, Environ. Health Perspect., 2005, 113, 823–839 CrossRef
.
- D. R. Boverhof and R. M. David, Anal. Bioanal. Chem., 2010, 396, 953–961 CrossRef CAS PubMed
.
-
United Nations, Globally Harmonized System of Classification and Labelling of Chemicals (GHS), United Nations, New York and Geneva, 5th Revised edn, 2013 Search PubMed
.
- I. A. Sacui, R. C. Nieuwendaal, D. J. Burnett, S. J. Stranick, M. Jorfi, C. Weder, E. J. Foster, R. T. Olsson and J. W. Gilman, ACS Appl. Mater. Interfaces, 2014, 6, 6127–6138 CAS
.
- K. A. Mahmoud, J. A. Mena, K.
B. Male, S. Hrapovic, A. Kamen and J. H. T. Luong, ACS Appl. Mater. Interfaces, 2010, 2, 2924–2932 CAS
.
- B. T. Chen, D. Schwegler-Berry, W. McKinney, S. Stone, J. L. Cumpston, S. Friend, D. W. Porter, V. Castranova and D. G. Frazer, Inhalation Toxicol., 2012, 24, 798–820 CrossRef CAS PubMed
.
- F. Sinche, J. Simonsen and S. Harper, Structural Basis for the Toxic Potential of Cellulose Nanocrystals, in The Toxicologist: Supplement to Toxicological Sciences, Society of Toxicology, 2013, vol. 132, issue 1, abstract no. 1752 Search PubMed.
- A. B. Stefaniak, M. S. Seehra, N. R. Fix and S. S. Leonard, Inhalation Toxicol., 2014, 26, 733–749 CrossRef CAS PubMed
.
- A. Raemy and J. Loeliger, Thermochim. Acta, 1985, 85, 347–352 CrossRef
.
- Occupational Safety and Health Administration (OSHA), Hazard Communication Guidance for Combustible Dusts, US Department of Labor OSHA 3371-08, Washington, D.C., 2009.
- National Fire Protection Agency (NFPA), NFPA 68 Standard on Explosion Protection by Deflagration Venting, 2007 Edition, Quincy, MA, 2007 Search PubMed.
- H.-C. Wu, R.-C. Chang and H.-C. Hsiao, J. Loss Prev. Process Ind., 2009, 22, 21–24 CrossRef CAS PubMed
.
- D. M. Fox, unpublished work.
- D. M. Fox, J. Lee, M. Zammarano, D. Katsoulis, D. V. Eldred, L. M. Haverhals, P. C. Trulove, H. C. De Long and J. W. Gilman, Carbohydr. Polym., 2012, 88, 847–858 CrossRef CAS PubMed
.
-
K. F. Martinez, A. Eastlake, A. Rudie and C. L. Geraci, in Production and Applications of Cellulose Nanomaterials, ed. M. T. Postek, R. J. Moon, A. Rudie and M. A. Bilodeau, TAPPI Press, Peachtree Corners, GA, 2013 Search PubMed
.
- R. T. Cullen, A. Searl, B. G. Miller, J. M. G. Davis and A. D. Jones, J. Appl. Toxicol., 2000, 20, 49–60 CrossRef CAS
.
- G. Oberdörster, V. Stone and K. Donaldson, Nanotoxicology, 2007, 1, 2–25 CrossRef PubMed
.
- V. Väänänen, E. Rydman, M. Ilves, K. Hannukainen, H. Norppa, A. von Wright, U. Honkalampi, I. Tsitko and J. Rouhiainen, Evaluation of the Suitability of the Developed Methodology for Nanoparticle Health and Safety Studies, 2012 Search PubMed.
- S. Vilske, M. Ilves, K. Aimonen, H. Lindberg, S. Personen, I. Wedin, M. Nuopponen, K. M. Savolainen, H. Norppa and H. Alenius, presented in part at the International Congress on Safety of Engineered Nanoparticles and Nanotechnologies (SENN 2015), Helsinki, April, 2015.
- N. Yanamala, M. T. Farcas, M. K. Hatfield, E. R. Kisin, V. E. Kagan, C. L. Geraci and A. A. Shvedova, ACS Sustainable Chem. Eng., 2014, 2, 1691–1698 CrossRef CAS
.
- C. Endes, O. Schmid, C. Kinnear, S. Mueller, S. Camarero-Espinosa, D. Vanhecke, E. J. Foster, A. Petri-Fink, B. Rothen-Rutishauser, C. Weder and M. J. D. Clift, Part. Fibre Toxicol., 2014, 11, 40 CrossRef PubMed
.
- M. J. D. Clift, E. J. Foster, D. Vanhecke, D. Studer, P. Wick, P. Gehr, B. Rothen-Rutishauser and C. Weder, Biomacromolecules, 2011, 12, 3666–3673 CrossRef CAS PubMed
.
- J. Catalán, M. Ilves, H. Järventaus, K.-S. Hannukainen, E. Kontturi, E. Vanhala, H. Alenius, K. M. Savolainen and H. Norppa, Environ. Mol. Mutagen., 2015, 56, 171–182 CrossRef PubMed
.
- National Institute of Occupational Safety and Health (NIOSH), The Registry of Toxic Effects of Chemical Substances (RTECS) - Cellulose, 2004.
- Joint FAO/WHO Expert Committee on Food Additives (JECFA), WHO Food Additives Series 40, 1998.
- M. Pitkänen, H. Kangas, O. Laitinen, A. Sneck, P. Lahtinen, M. S. Peresin and J. Niinimäki, Cellulose, 2014, 21, 3871–3886 CrossRef
.
- R. de Lima, L. O. Feitosa, C. R. Maruyama, M. A. Barga, P. C. Yamawaki, I. J. Vieira, E. M. Teixeira, L. H. C. Mattoso and L. F. Fraceto, Int. J. Nanomedicine, 2012, 3555 CrossRef CAS PubMed
.
- P. Borm, F. C. Klaessig, T. D. Landry, B. Moudgil, J. Pauluhn, K. Thomas, R. Trottier and S. Wood, Toxicol. Sci., 2006, 90, 23–32 CrossRef CAS PubMed
.
- H. Muhle, H. Ernst and B. Bellmann, Ann. Occup. Hyg., 1997, 41(Supplement 1), 184–188 CrossRef
.
- J. Rouhiainen, V. Väänänen, I. Tsitko and J. Kautto, Risk Assessment of Nanofibrillated Cellulose in Occupational Settings, 2012 Search PubMed.
- L. K. S. Nagato, M. G. F. Lourenço, R. A. Cadete, J. H. P. Leite-Júnior, V. L. G. Koatz, P. R. M. Rocco, D. S. Faffe and W. A. Zin, Respir. Physiol. Neurobiol., 2008, 164, 331–337 CrossRef CAS PubMed
.
- T. Kraus, A. Pfahlberg, P. Zöbelein, O. Gefeller and H. J. Raithel, Chest, 2004, 125, 731–736 CrossRef
.
- M. M. Pereira, L. Mouton, C. Yéprémian, A. Couté, J. Lo, J. M. Marconcini, L. O. Ladeira, N. R. Raposo, H. M. Brandão and R. Brayner, J. Nanobiotechnol., 2014, 12, 15 CrossRef PubMed
.
- C. Hohenthal, M. Ovaskainen, D. Bussini, P. Sadocco, T. Pajula, H. Lehtinen, J. Kautto and K. Salmenkivi, Final Assessment of Nano Enhanced New Products, 2012 Search PubMed.
- K. Kümmerer, J. Menz, T. Schubert and W. Thielemans, Chemosphere, 2011, 82, 1387–1392 CrossRef PubMed
.
- T. F. Fernandes, E. Trovatti, C. S. R. Freire, A. J. D. Silvestre, C. P. Neto, A. Gandini and P. Sadocco, Compos. Sci. Technol., 2011, 71, 1908–1913 CrossRef CAS PubMed
.
- M. Methner, L. Hodson, A. Dames and C. Geraci, J. Occup. Environ. Hyg., 2009, 7, 163–176 CrossRef PubMed
.
- H. Bouwmeester, I. Lynch, H. J. P. Marvin, K. A. Dawson, M. Berges, D. Braguer, H. J. Byrne, A. Casey, G. Chambers, M. J. D. Clift, G. Elia, T. F. Fernandes, L. B. Fjellsbø, P. Hatto, L. Juillerat, C. Klein, W. G. Kreyling, C. Nickel, M. Riediker and V. Stone, Nanotoxicology, 2010, 5, 1–11 CrossRef PubMed
.
- International Organization for Standardization (ISO), Nanomaterials -- Preparation of Material Safety Data Sheet (MSDS), 2012.
- K. J. Ong, T. J. MacCormack, R. J. Clark, J. D. Ede, V. A. Ortega, L. C. Felix, M. K. M. Dang, G. Ma, H. Fenniri, J. G. C. Veinot and G. G. Goss, PLoS One, 2014, 9, e90650 Search PubMed
.
- R. Guadagnini, B. Halamoda Kenzaoui, L. Walker, G. Pojana, Z. Magdolenova, D. Bilanicova, M. Saunders, L. Juillerat-Jeanneret, A. Marcomini, A. Huk, M. Dusinska, L. M. Fjellsbø, F. Marano and S. Boland, Nanotoxicology, 2015, Suppl. 1, 13–24 CrossRef PubMed
.
- A. Kroll, M. H. Pillukat, D. Hahn and J. Schnekenburger, Arch. Toxicol., 2012, 86, 1123–1136 CrossRef CAS PubMed
.
-
GoodNanoGuide, https://nanohub.org/groups/gng, (accessed February 2015) Search PubMed
.
- C. Scislowicz, C. Geraci, F. Parr, G. DeRose, H. Saebfar, H. Godwin, J. Bartlett, J. Brakensiek, J. Wong, K. McNamara and others, Calif. Nanosafety Consort. High. Educ., 2011 Search PubMed.
- OSHA, Nanotechnology: Working Safely with Nanomaterials, US Department of Labor OSHA FS-3634, Washington, D.C., 2013 Search PubMed
.
- NIOSH, Current Strategies for Engineering Controls in Nanomaterial Production and Downstream Handling Processes, US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2014–102, Cincinnati, OH, 2013 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |