ZnO nanowire-immobilized paper matrices for visible light-induced antibacterial activity against Escherichia coli
Indu
Chauhan
,
Sudiksha
Aggrawal
and
Paritosh
Mohanty
*
Department of Applied Science and Engineering, IIT Roorkee, Saharanpur Campus, Saharanpur, U.P.-247 001, India. E-mail: paritosh75@gmail.com; Fax: +91 132 271 4311; Tel: +91 132 271 4338
First published on 16th March 2015
Abstract
Paper matrices showing antibacterial activity were prepared by immobilizing ZnO nanowires (NWs) of diameter ca. 130–500 nm and length up to 2.5 μm on the surface of cellulose fibers by a single-step hydrothermal method. The antibacterial activity of the paper matrices has been investigated versus the visible light exposure time and ZnO contents (2.0 to 18.0 wt%). A complete inhibition of E. coli growth (i.e. 99.99%) was observed with the ZnO-immobilized paper matrices for 6 to 9 h exposure. A possible growth mechanism for the ZnO NW growth on the surface of the cellulose fibers has been proposed. The immobilization of ZnO NWs in paper matrices was characterized by field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), X-ray photoluminescence spectroscopy (XPS) and Fourier transform infrared (FTIR) spectroscopy.
Nano impactZnO nanowires (NWs) have been immobilized on the cellulose fiber surfaces of the paper matrices. The synthesis of the NWs and their immobilization have been carried out simultaneously by a newly developed hydrothermal synthesis method. The paper matrices have shown antibacterial activity against E. coli bacteria upon exposure to visible light. A complete deactivation of the E. coli was possible with a time frame of 6 to 9 h depending on the NW contents in the paper matrices. These paper matrices immobilized with the ZnO NWs could have potential biomedical applications and applications in packaging. |
Introduction
The recurrence of infectious diseases caused by various bacteria poses serious threat to human health.1 The commonly used materials with antibacterial activity to deactivate or kill these bacteria are mostly toxic for human beings2–6 and also unaffordable by economically weaker sections of the society in the developing countries. Thus, there is an utmost need to develop cheap, environmentally friendly antibacterial materials with low toxicity that can inhibit the bacterial contamination.7–10 Furthermore, the demand for the antibacterial materials is very high in the packaging industries, especially for health care and food packaging applications.11–13Over the past few decades, the use of various metal and metal oxide nanoparticles (NPs) such as Ag, TiO2, ZnO, CuO, etc. has received great attention because these materials can attack or target a broad range of bacterial species.1–15 However, the use of NPs in powdered form is limited as it may be difficult to separate the NPs from the products.16 This problem could be circumvented by immobilizing or incorporating these NPs on the surface of various substrates such as ceramics, glass, and polymers. Each substrate has its own benefits and disadvantages. Some of these substrates have finite resources, are expensive and are non-biodegradable. In some cases, improper disposal of substrates leads to environmental pollution.17–19
Paper being a natural biopolymer is a biodegradable, economical and abundant material that could become a very good alternative as a substrate for the development of antibacterial materials.10,20 In the past few years, cellulosic materials modified with various metal/metal oxide NPs have become an emerging research area that has attracted great attention especially as an antimicrobial material by preventing/inhibiting the growth of microorganisms.20–27 Other potential applications of these NP-modified cellulosic materials are photocatalysis, water purification, etc.28,29
Among the metal oxides, ZnO NPs (band gap energy in bulk Eg = 3.37 eV) have shown good photocatalytic, optical, magnetic, electrical, and photochemical properties and antimicrobial activity and thus have potential applications in gas sensors, solar cells, nanolasers, piezoelectric nanogenerators, optoelectronic devices, biodevices, biology, etc.30,31 Most importantly, the toxicity of ZnO to human cells is quite low and the toxicity does not increase significantly for the NPs compared to the conventional ZnO powders.10 Various methods such as ultrasound-assisted coating,21 dip coating,23,24 paper making technique,10,22 and layer-by-layer technique27 have been developed to decorate ZnO NPs in paper matrices. Ghule et al. reported the coating of ZnO NPs on paper surface by ultrasound-assisted coating method and studied its antibacterial activity against E. coli under UV light.21 Similarly, Baruah et al. and Jaisai et al. have demonstrated the antibacterial activity of the ZnO nanorod-coated paper matrices prepared by using the dip coating method.23,24 Recently, Martins et al. have shown the sunlight-induced antibacterial activity of the nanofibrillated cellulose (NFC)/ZnO nanocomposite-coated paper sheet against Gram-positive (S. aureus and B. cereus) and Gram-negative (K. pneumonia) bacteria, prepared by using a size press machine.1 Cheng et al. used a layer-by-layer technique to coat the paper sheet with carboxymethyl starch (CMS)-stabilized ZnO NPs and studied its antibacterial activity against meticillin-resistant S. aureus (MRSA) and A. baumannii.27 Very recently, Khatri et al. have demonstrated the use of a paper making technique to prepare the cellulose fiber sheets incorporated with saccharide-capped ZnO NPs which can also be used for antibody immobilization in addition to antimicrobial applications.10
In most of the above discussed methods, the nanostructures have been prepared/procured in the first step and the incorporation of the nanostructures was carried out in the second step.1,10,21,32 These methods possess various challenges, such as agglomeration of the nanostructures and retention %.32 The issue of agglomeration and retention % could be circumvented to some extent by using capping agents or surfactants along with the nanostructures. However, the use the capping agents or surfactants limits the applications as they stay at the surface of the nanostructures.33,34 Very recently, our group has developed a single-step in situ hydrothermal method to immobilize the TiO2 NPs on the cellulose fibers of paper matrices with high retention %.25,26 Both the synthesis of the nanostructures and their immobilization occur simultaneously. In the present research, a similar hydrothermal synthesis was performed to test the applicability of the method for the immobilization of ZnO nanostructures on the surface of the cellulose fibers. Furthermore, antibacterial activity of the ZnO nanowire-immobilized paper matrices has been investigated against E. coli under visible light.
Experimental
Materials
Zinc chloride (Rankem, RFCL Ltd., India), sodium carbonate (Himedia laboratories Pvt. Ltd., India), bleached softwood cellulose fibers (Star Paper Mill Ltd., India), ethanol (99.9% pure, Changshu Yangyuan Chemical, China), Luria-Bertani, LB media (Himedia laboratories Pvt. Ltd., India), and E. coli (MTCC no. 25 1698, Microbial Type Culture Collection and Gene Bank, India) were used as received without any further purification.Immobilization of ZnO NWs on cellulose fibers
For the preparation of ZnO-immobilized paper matrices, the cellulose fibers were hydrothermally treated with ZnCl2 and Na2CO3 at 140 °C for 14 h (Scheme 1), similar to our previous reports.25,26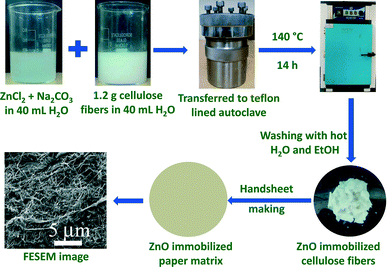 | ||
| Scheme 1 Schematic presentation for the immobilization of ZnO nanowires on the surface of the cellulose fiber of paper. | ||
In a typical experiment, x and y g of ZnCl2 and Na2CO3 were dissolved in 40 mL of distilled H2O (see Table 1 for the amounts of reagents used to immobilize the different contents of ZnO NWs on cellulose fibers). The whole suspension was stirred for 30 min. To this suspension, 1.2 g of bleached softwood cellulose fibers soaked in water (1.2 g of cellulose fibers soaked in 40 mL of distilled H2O for about 30 min) was added and again stirred for 30 min. The whole suspension was transferred into a 100 mL Teflon-lined autoclave and kept in an oven at 140 °C for 14 h and then cooled down to RT naturally. The cellulose fibers were collected by filtration and washed several times with ethanol and hot distilled H2O, respectively, to obtain the ZnO-immobilized cellulose fibers. Paper matrices were then prepared by the standard Handsheet making procedure25,26,32,36 using these ZnO-immobilized cellulose fibers. An identical hydrothermal synthesis was performed in the absence of cellulose fibers to compare the structure and microstructure of the obtained ZnO specimens.35
| Sample ID | ZnCl2 (x g) | Na2CO3 (y g) |
|---|---|---|
| Sample a | 0.0 | 0.0 |
| Sample b | 0.1 | 10 |
| Sample c | 0.2 | 20 |
| Sample d | 0.3 | 30 |
| Sample e | 0.6 | 60 |
Antibacterial activity of ZnO NW-immobilized paper matrices
Antibacterial activity of the ZnO-immobilized paper matrices was studied by inhibiting the growth of E. coli in the presence of visible light. The sterilized paper matrices (8 cm × 1 cm) were placed in bacterial solution with an initial bacterial concentration of 16.1 × 105 CFU mL−1 and were incubated at 36 °C for 3, 6 and 9 h under the exposure of a compact fluorescent lamp (0.276 J cm−2). At every 3 h duration (i.e. 3, 6 and 9 h), the treated bacterial solutions were serially diluted from which 100 μL was spread on LB media in petri-plates.20,25,26 The plates were then incubated at 36 °C for 24 h and the number of colonies was counted by the colony count method.20,25,26 The antibacterial efficacy of the prepared paper matrices was also calculated in terms of log reduction (LR) and percentage of bacterial cell reduction (R, %) using eqn (1) and (2), respectively: | (1) |
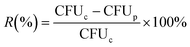 | (2) |
Characterization
The microstructure of the specimens was studied by FESEM (MIRA3 TESCAN) operated at a voltage of 2.0–10.0 kV. The crystallographic phase analysis of the prepared paper matrices was investigated by XRD (Rigaku Ultima IV) with CuKα radiation (λ = 0.15405 nm) at a scanning speed of 6° min−1 in the diffraction angle 2θ range of 10 to 80°. The presence of ZnO in the paper matrices and their interaction with the cellulose fibers of the paper matrices were studied by XPS (ULVAC-PHI, INC, Japan) and FTIR (PerkinElmer FTIR C91158 spectrophotometer) spectroscopy. The retention % of ZnO in the paper matrices was estimated by a standard combustion method. Subtracting the ash content of the blank paper matrix from the ZnO-immobilized paper matrices gives the value of the ZnO present in the specimen. The leaching of the ZnO from the paper matrices was investigated by inductively coupled plasma optical emission spectroscopy (ICP-OES; Prodigy SPEC, Teledyne Leeman Labs).Results and discussion
The amount (wt%) of ZnO retained in paper matrices was estimated by heating the paper matrices at 525 °C in air for 4 h. This is a standard method used by various research groups for determining the inorganic contents in the paper matrices.25,26,36–38 In the blank paper matrix (sample a), almost complete mass loss (~0.004 ash wt%) was observed. However, around 2.0, 7.0, 10.0 and 18 wt% mass were left after the combustion of samples b, c, d and e, respectively. Based on the content of the ZnO that remained in the paper matrices, the specimens were designated as 2.0Z-P, 7.0Z-P, 10.0Z-P and 18.0Z-P for the paper matrices immobilized with 2.0, 7.0, 10.0 and 18.0 wt% of ZnO, respectively. The retention % calculated from the difference of theoretical and experimentally observed ZnO contents has the values of 40.0, 70.0, 67.0 and 60.0% in the paper matrices 2.0Z-P, 7.0Z-P, 10.0Z-P and 18.0Z-P, respectively.The phase analysis of the ZnO NW- and ZnO-immobilized paper matrices was investigated by XRD (Fig. 1). The XRD pattern of the ZnO specimen synthesized in the absence of the cellulose fibers is shown in Fig. 1a. Sharp peaks were observed at the interplanar spacing, d, of 0.2832, 0.2609, 0.2488, 0.1919, 0.1632, 0.1484, 0.1385 and 0.1364 nm corresponding to the (100), (002), (101), (102), (110), (103), (112) and (201) reflections, respectively, of the hexagonal wurtzite crystal structure of ZnO. All the observed peaks fairly matched with the JCPDS file (79-205). Two broad characteristic diffraction peaks at d-values of 0.5823 and 0.3896 nm were also observed in the paper matrices with different ZnO contents (Fig. 1b–f), which could be indexed to (101) and (002) reflections of cellulose-I.25,26 In addition to that, broad and less intense diffraction peaks were observed in these specimens corresponding to the ZnO (Fig. 1c–f). The intensity of these peaks was increased with an increase in the ZnO contents. It is worth mentioning that all the peaks corresponding to the ZnO in these specimens were broadened and shifted toward lower 2θ values (higher d-values). This could be attributed to the formation of the nanostructures with a higher lattice strain and lattice distortion effects.39,40
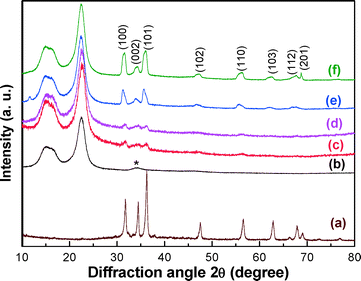 | ||
| Fig. 1 XRD patterns of (a) as-synthesized ZnO NWs, (b) 0.0Z-P, (c) 2.0Z-P, (d) 7.0Z-P, (e) 10.0Z-P and (f) 18.0Z-P [*CaCO3 as a filler in supplied raw cellulose fibers]. | ||
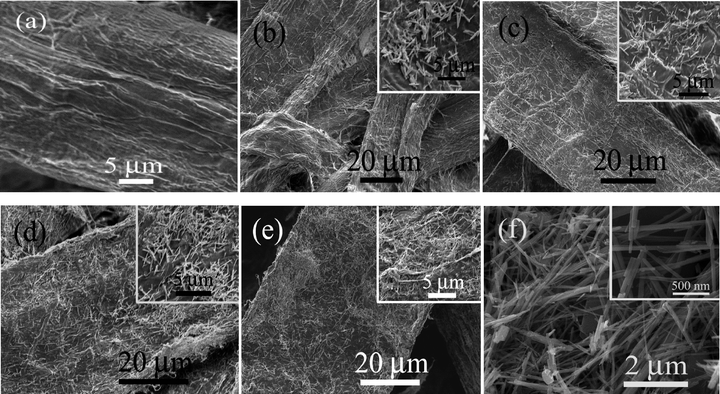
The microstructure of the specimens has been investigated by FESEM (Fig. 2). In all these specimens, ZnO NWs were formed and adhered to the surface of the cellulose fibers of the paper matrices. As expected, the population of the ZnO NWs increases with an increase in ZnO contents in the paper matrices as shown Fig. 2b–e. The formation of the NWs could be conceived due to the asymmetric crystal structure of ZnO with a longer c-axis. Moreover, the reactions were carried out in the presence of mineralizer Na2CO3 which facilitates the NW growth.35 It was further supported by the fact that ZnO NWs were observed when the experiment was carried out in the absence of the cellulose fibers for the synthesis of ZnO. It is interesting to note that with an increase in the ZnO contents, the diameters of the NWs decrease substantially from ca. 500 nm in the 2.0Z-P specimen to ca. 230, 150 and 130 nm for 7.0Z-P, 10.0Z-P and 18.0Z-P, respectively (Fig. 2b–e). This could be explained similar to the vapor–solid mechanism for the synthesis of the NWs.41–44 At lower Zn2+ concentration, less number of nuclei would be formed and the incoming ions would add to the width of the NWs. However, at a higher Zn2+ concentration, large numbers of nuclei would be formed due to the availability of the ions in the vicinity. These nuclei would grow in a faster rate and hence have a lesser chance of growth along the diameter.
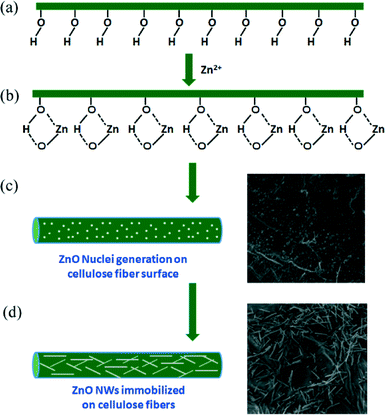
The proposed mechanism for the growth of ZnO NWs on the cellulose fiber surface is shown in Fig. 3. It can be comprehended that in the initial growth stage, the hydroxyl groups present on the surface of the cellulose fibers (Fig. 3a) interact with the Zn2+ ions or the Zn–OH through H-bonding interactions forming a Zn–O–C bond (Fig. 3b).25,26 Thus, the ZnO nuclei were formed on the surface of the cellulose fibers (Fig. 3c). This was further supported by the observation of ZnO NPs that adhered to the surface of the cellulose fibers at the beginning of the experiment as shown with the FESEM image in Fig. 3c, when the experiments were carried out only for 1 h instead of 14 h. As the reaction proceeds, ZnO NWs began to grow in one dimension (Fig. 3d). Furthermore, the Zn–O–C bonds could be formed not only in the nuclei but also in the whole NW surface that is exposed to the cellulose surface, making the NW immobilization possible. This was supported by the fact that the NWs did not grow vertically on the cellulose surface, but rather the NWs grow parallel to the fiber surface as can be seen in the FESEM images of all these specimens (Fig. 2b–e).
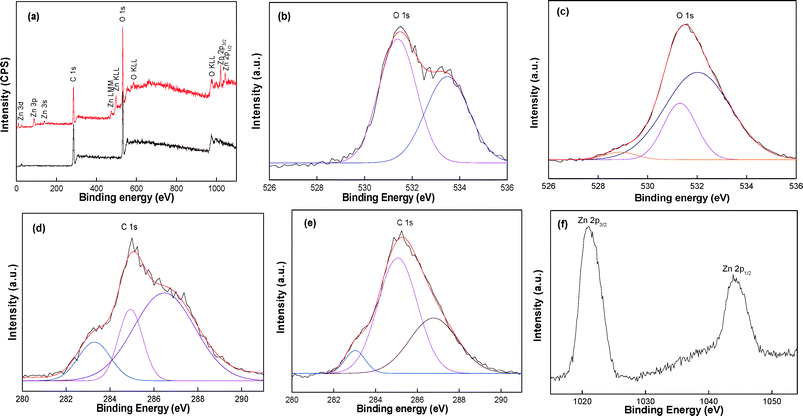
The interaction of ZnO NWs with cellulose fibers was studied by XPS as shown in Fig. 4. In the wide scan, 0.0Z-P shows two major peaks with binding energies of 286.2 and 531.3 eV corresponding to the C 1s and O 1s of cellulose, respectively (Fig. 4a).25,26 However, in 10.0Z-P, in addition to the C 1s and O 1s peaks, few other peaks due to the Zn were also observed. The peaks at binding energies of 1021.2 and 1044.61 eV were indexed to Zn 2p3/2 and Zn 2p1/2, respectively (Fig. 4a and f).45–47
In both specimens, three peaks were observed for the C 1s (Fig. 4d and e).25,26 Two peaks were observed in the high-resolution spectra of O 1s at 531.0 and 533.4 eV (Fig. 4b) in the 0.0Z-P sample.25,26 These two peaks are assigned to the oxygen in the hydroxyl groups and glycosidic linkage, respectively. On the other hand, in the high-resolution spectra of 10.0Z-P, three peaks were observed at 528.9, 531.3, and 532.05 eV (Fig. 4c). The additional peak at 528.9 eV could be attributed to the O 1s from ZnO. The intensity of the peak due to the hydroxyl group of the cellulose at 531.0 eV was decreased in the 10.0Z-P. As proposed in the mechanism, this indicates the decrease in the hydroxyl groups in the specimen and this could be due to the formation of the covalent bonds between the Zn–O–C. A similar change in the intensity of the hydroxyl O 1s peak was earlier observed for the TiO2 immobilization.26 This confirms the immobilization of the ZnO on the cellulose fibre surface with the formation of the covalent bonds.
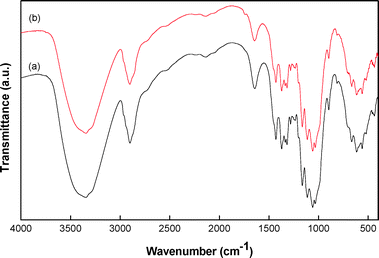
In order to further prove the immobilization of ZnO NWs on the cellulose fibers of paper matrices, the specimens were investigated by FTIR spectroscopy. The FTIR spectra of 0.0Z-P were compared with a representative specimen 7.0Z-P as shown in Fig. 5. Several bands in the region of 400–800 cm−1, attributed to Iβ and Iα phases of cellulose, were observed (Fig. 5).21,25,26 The bands in the range of 900–1300 and 1300–1500 cm−1 can be attributed to C–O and C–H vibrations of cellulose (Fig. 5).21,25,26 It is interesting to note that the intensity of the broad band observed around 3345 cm−1 corresponding to the –OH stretching vibration and the band at 1640 cm−1 due to the –OH bending vibration of the cellulose fibers decreased in the paper matrix 7.0Z-P compared to that in the 0.0Z-P. This corroborates the results obtained from the XPS that the hydroxyl groups of cellulose were interacting with the Zn2+. Such a decrease in intensity of the –OH band is common when there is a decrease in the –OH groups in the specimen and was observed earlier in the immobilization of the TiO2 nanoparticles.26
To support our proposed mechanism of the formation of the covalent bonds between the zinc cations and oxygen from the hydroxyl group of the cellulose, a leaching experiment was carried out. In a typical experiment, the 7.0Z-P paper matrix was sonicated in 25 mL of H2O for 5 min at an ultrasonic power of 300 W. The paper matrix was removed from the solvent and the leaching of ZnO NWs was investigated by ICP-OES. It was observed that ca. 10.0% of the ZnO was removed from the prepared paper matrix. Considering a high-power sonication that could be enough to clean the surface could only remove a part of the ZnO that was immobilized. Furthermore, the sonication could have disintegrated some of the fibers along with the ZnO NWs. That could have added to the above amount. Interestingly, when a similar leaching experiment was carried out manually by shaking, no ZnO could be detected in the ICP-OES. This confirms our claim of having a covalent bond formation between the Zn2+ and the cellulose fibers; otherwise, a major part of ZnO would have been leached out from the paper matrix.
The antibacterial activity of paper matrices was investigated by inhibiting the growth of E. coli in the presence of visible light for 3, 6 and 9 h. The results of antibacterial activity is shown in Fig. 6 and summarised in Table 2. Paper matrices without ZnO NW (0.0Z-P) did not show any substantial reduction in the bacterial growth. Only 2.0–5.0% reduction in the bacterial growth could be observed in 9 h. However, paper matrices 2.0Z-P, 7.0Z-P, 10.0Z-P and 18.0Z-P showed around 62.58, 66.34, 83.11 and 95.88% reduction in bacterial growth in 3 h. On further increasing the exposure time to 6 h, bacterial growth reduction of around 95.30, 98.72, 99.74 and 99.97% was observed for 2.0Z-P, 7.0Z-P, 10.0Z-P and 18.0Z-P, respectively. On 9 h exposure, the paper matrix 2.0Z-P showed 97.48% reduction in the bacterial growth; however, paper matrices 7.0Z-P, 10.0Z-P and 18.0Z-P showed almost complete reduction, i.e. 99.81, 99.98 and 99.99%, respectively, in the bacterial growth. The antibacterial activity of the ZnO has been studied earlier for various nanostructures including NWs.48–50 The mechanism of the antibacterial efficacy of ZnO has been attributed to the generation of highly reactive oxygen species (ROS) particularly HO˙ in the presence of moisture that leads to the formation of H2O2 which is harmful to cells.1,10,48–50 The formed peroxide penetrates the bacterial cell wall and causes fatal damage to the cell membranes, DNA, and cell proteins and thus leads to bacterial death.1,10,48–50
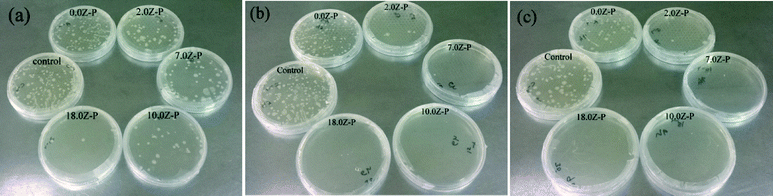 | ||
| Fig. 6 Images of antibacterial activity shown by the prepared paper matrices under visible light illumination for (a) 3, (b) 6 and (c) 9 h. | ||
| Sample ID | 3 h | 6 h | 9 h | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CFU mL−1 | LR | R (%) | CFU mL−1 | LR | R (%) | CFU mL−1 | LR | R (%) | |
| 0.0Z-P | 15.3 × 105 | 0.02 | 5.08 | 15.6 × 105 | 0.02 | 2.70 | 15.6 × 105 | 0.01 | 2.70 |
| 2.0Z-P | 6.0 × 105 | 0.42 | 62.58 | 7.5 × 104 | 1.34 | 95.30 | 4.0 × 104 | 1.61 | 97.48 |
| 7.0Z-P | 5.4 × 105 | 0.47 | 66.34 | 2.0 × 104 | 1.98 | 98.72 | 3.0 × 103 | 2.57 | 99.81 |
| 10.0Z-P | 2.7 × 105 | 0.77 | 83.11 | 4.0 × 103 | 2.62 | 99.74 | 0.2 × 103 | 3.97 | 99.98 |
| 18.0Z-P | 6.0 × 104 | 1.42 | 95.88 | 0.3 × 103 | 3.66 | 99.97 | 0.12 × 103 | 5.15 | 99.99 |
Conclusions
The ZnO NWs have been immobilized on the surface of the cellulose fibers of the paper matrices by a single-step hydrothermal method. The synthesis and immobilization of the NWs occur simultaneously, that helps in obtaining a high retention %; however, a low retention % could be achieved if the incorporation of NWs in the paper matrix will be done by an ex situ method as reported by our group.32 The ZnO NW-immobilized paper matrices showed promising antibacterial activity by completely inhibiting the growth of E. coli in the presence of visible light in 6 to 9 h depending on the ZnO NW contents. Thus, the presently used hydrothermal method is an effective method for the immobilization of metal oxide NWs on cellulose fibers of paper matrices and could be extended for the immobilization of other metal oxide nanostructures in paper products. The complete deactivation of E. coli by the ZnO NW-immobilized paper matrices demonstrates that these paper matrices have the potential to be used in household and industrial purposes such as antibacterial wipes, food packaging, etc.Acknowledgements
This work was financially supported by IIT Roorkee, India under grant no. FIG-400131-DPT/12-13.Notes and references
- N. C. T. Martins, C. S. R. Freire, C. P. Neto, A. J. D. Silvestre, J. Causio, G. Baldi, P. Sadocco and T. Trindade, Colloids Surf., A, 2013, 417, 111 CrossRef CAS PubMed.
- A. R. Gliga, S. Skoglund, I. O. Wallinder, B. Fadeel and H. L. Karlsso, Part. Fibre Toxicol., 2014, 11, 11, DOI:10.1186/1743-8977-11-11.
- A. Gerber, M. Bundschuh, D. Klingelhofer and D. A. Groneberg, J. Occup. Med. Toxicol., 2013, 8, 32 CrossRef PubMed.
- C. S. Yah, Biomed. Res., 2013, 24, 400 CAS.
- S. Prabhu and E. K. Poulose, Int. Nano Lett., 2012, 2, 32 CrossRef.
- C. Greulich, D. Braun, A. Peetsch, J. Diendorf, B. Siebers, M. Epple and M. Koller, RSC Adv., 2012, 2, 6981 RSC.
- Y.-N. Chang, M. Zhang, L. Xia, J. Zhang and G. Xing, Materials, 2012, 5, 2850 CrossRef CAS PubMed.
- K. Liu, X. Lin and J. Zhao, Int. J. Nanomed., 2013, 8, 2509 Search PubMed.
- A. Adamcakova-Dodd, L. V. Stebounova, J. S. Kim, S. U. Vorrink, A. P. Ault, P. T. O'Shaughnessy, V. H. Grassian and P. S. Thorne, Part. Fibre Toxicol., 2014, 11, 15, DOI:10.1186/1743-8977-11-15.
- V. Khatri, K. Halasz, L. V. Trandafilovic, S. Dimitrijevic-Brankovic, P. Mohanty, V. Djokovic and L. Csoka, Carbohydr. Polym., 2014, 109, 139 CrossRef CAS PubMed.
- C. Chawengkijwanich and Y. Hayata, Int. J. Food Microbiol., 2008, 123, 288 CrossRef CAS PubMed.
- S. Mangalassary, J. Food Process. Technol., 2012, 3, 1 Search PubMed.
- T. V. Duncan, J. Colloid Interface Sci., 2011, 363, 1 CrossRef CAS PubMed.
- M. J. Hajipour, K. M. Fromm, A. A. Ashkarran, D. J. Aberasturi, I. R. Larramendi, T. Rojo, V. Serpooshan, W. J. Parak and M. Mahmoudi, Trends Biotechnol., 2012, 30, 511 CrossRef PubMed.
- A. Toolabia, M. R. Zareb, A. Rahmani, E. Hoseinzadehd, M. Sarkhoshe and M. Zaref, J. Basic. Appl. Sci. Res., 2013, 3, 221 Search PubMed.
- E. V. Skorb, L. I. Antonouskaya, N. A. Belyasova, D. G. Shchukin, H. Mohwald and D. V. Sviridov, Appl. Catal., B, 2008, 84, 94 CrossRef CAS PubMed.
- K. Marsh and B. Bugusu, J. Food Sci., 2007, 72, 39 CrossRef PubMed.
- R. K. Padhan, A. A. Gupta, R. P. Badoni and A. K. Bhatnagar, Polym. Degrad. Stab., 2013, 98, 2592 CrossRef CAS PubMed.
- H. K. Webb, J. Arnott, R. J. Crawford and E. P. Ivanova, Polymer, 2013, 5, 1 Search PubMed.
- H. Li, S. Fu and L. Peng, Fibers Polym., 2013, 14, 1794 CrossRef CAS PubMed.
- K. Ghule, A. V. Ghule, B. J. Chen and Y. C. Ling, Green Chem., 2006, 8, 1034 RSC.
- H. Koga, T. Kitaoka and H. Wariishi, J. Mater. Chem., 2009, 19, 2135 RSC.
- M. Jaisai, S. Baruah and J. Dutta, Beilstein J. Nanotechnol., 2012, 3, 684 CrossRef PubMed.
- S. Baruah, M. Jaisai, R. Imani, M. M. Nazhad and J. Dutta, Sci. Technol. Adv. Mater., 2010, 11, 055002 CrossRef.
- I. Chauhan and P. Mohanty, RSC Adv., 2014, 4, 57885 RSC.
- I. Chauhan and P. Mohanty, Cellulose, 2015, 22, 507 CrossRef CAS PubMed.
- F. Cheng, J. W. Betts, S. M. Kelly, D. W. Wareham, A. Kornherr, F. Dumestre, J. Schallere and T. Heinze, J. Mater. Chem. B, 2014, 2, 3057 RSC.
- T. A. Dankovich, Environ. Sci.: Nano, 2014, 1, 367 RSC.
- T. A. Dankovich and J. A. Smith, Water Res., 2013, 63, 245 CrossRef PubMed.
- K. Manjunath, T. N. Ravishankar, D. Kumar, K. P. Priyanka, T. Varghese, H. R. Naika, H. Nagabhushana, S. C. Sharma, J. Dupont, T. Ramakrishnappa and G. Nagaraju, Mater. Res. Bull., 2014, 57, 325 CrossRef CAS PubMed.
- J. Maa, J. Liu, Y. Bao, Z. Zhu, X. Wang and J. Zhang, Ceram. Int., 2013, 39, 2803 CrossRef PubMed.
- I. Chauhan, S. Chattopadhya and P. Mohanty, Mater. Express, 2013, 3, 343 CrossRef CAS PubMed.
- S. Skoglund, T. A. Lowe, J. Hedberg, E. Blomberg, I. O. Wallinder, S. Wold and M. Lundin, Langmuir, 2013, 29, 8882 CrossRef CAS PubMed.
- S. K. Mehta, S. Kumar, S. Chaudhary and K. K. Bhasin, Nanoscale Res. Lett., 2009, 4, 1197 CrossRef CAS PubMed.
- H. Hu, X. Huang, C. Deng, X. Chen and Y. Qian, Mater. Chem. Phys., 2007, 106, 58 CrossRef CAS PubMed.
- Y. Iguchi, H. Ichiura, T. Kitaokas and H. Tanaka, Chemosphere, 2003, 53, 1193 CrossRef CAS.
- X. Chen, X. Qian and X. An, BioResources, 2011, 6, 2435 CAS.
- L. He, B. He and L. Zhao, BioResources, 2014, 9, 1361 Search PubMed.
- R. S. Niranjan, Y. K. Hwang, D.-K. Kim, S. H. Jhung, J.-S. Chang and I. S. Mulla, Mater. Chem. Phys., 2005, 92, 384 CrossRef CAS PubMed.
- K. J. Lethy, D. Beena, V. P. M. Pillai and V. Ganesan, J. Appl. Phys., 2008, 104, 033515 CrossRef PubMed.
- B. Mayer and Y. Xia, Adv. Mater., 2002, 14, 279 CrossRef.
- P. Mohanty, J. Park and B. Kim, J. Nanosci. Nanotechnol., 2006, 6, 3380 CrossRef CAS PubMed.
- P. Mohanty, T. Kang and B. Kim, J. Phys. Chem. B, 2006, 110, 791 CrossRef CAS PubMed.
- P. Mohanty, I. Yoon, T. Kang, K. Seo, K. S. K. Varadwaj, W. Choi, Q.-H. Park, J. P. Ahn, Y. D. Suh, H. Ihee and B. Kim, J. Am. Chem. Soc., 2007, 129, 9576 CrossRef CAS PubMed.
- Z. Guo, S. Wei, B. Shedd, R. Scaffaro, T. Pereira and H. T. Hahn, J. Mater. Chem., 2007, 17, 806 RSC.
- Z. Tan and D. H. C. Chua, J. Electrochem. Soc., 2011, 158, K112 CrossRef CAS PubMed.
- Y. Lv, L. Yu, H. Huang, Y. Feng, D. Chen and X. Xie, Nanotechnology, 2012, 23, 065402 CrossRef PubMed.
- X. Wang, F. Yang, W. Yang and X. Yang, Chem. Commun., 2007, 4419 RSC.
- R. K. Duttaa, B. P. Nenavathua, M. K. Gangishetty and A. V. R. Reddy, Colloids Surf., B, 2012, 94, 143 CrossRef PubMed.
- Y. Xie, Y. He, P. L. Irwin, T. Jin and X. Shi, Appl. Environ. Microbiol., 2011, 77, 2325 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |