Probabilistic modelling of engineered nanomaterial emissions to the environment: a spatio-temporal approach†
Tian Yin
Sun‡
ab,
Gulliver
Conroy‡
cd,
Erica
Donner
c,
Konrad
Hungerbühler
b,
Enzo
Lombi
c and
Bernd
Nowack
*a
aEmpa – Swiss Federal Laboratories for Materials Science and Technology, Technology and Society Laboratory, Lerchenfeldstrasse 5, CH-9014 St. Gallen, Switzerland. E-mail: nowack@empa.ch
bInstitute for Chemical and Bioengineering, ETH Zürich, CH-8093 Zürich, Switzerland
cCERAR, University of South Australia, Building X, University Boulevard, Mawson Lakes, South Australia 5095, Australia
dCRC CARE, PO Box 486, Salisbury, South Australia 5106, Australia
First published on 10th April 2015
Abstract
For the environmental risk assessment of engineered nanomaterials (ENM) knowledge about environmental concentrations is crucial. Soils and sediments are considered sinks for ENM and thus a better understanding of the spatial and temporal variability of concentrations is needed. In this work we use South Australia as a case study for a region with significant biosolids and treated wastewater application on soils, representing a system with almost “closed loops”. The probabilistic material flow modelling approach was extended to include a temporal modelling of ENM production and biosolids handling and transfer onto soils, focusing on nano-TiO2, nano-ZnO, nano-Ag, Carbon Nanotubes(CNT) and fullerenes. The results thus not only incorporate the uncertainty on ENM flows but also the spatial and temporal variability of ENM concentrations between 2005 and 2012. The ENM concentrations in different waste amended soils vary by more than 2 orders of magnitude due to different biosolids and wastewater application rates. Because of the almost complete transformation of nano-ZnO and nano-Ag during wastewater treatment, we also modelled the total flows of Zn and Ag derived from the nanoparticles and compared their modelled concentrations to measured total Ag and Zn concentration in biosolids and soils in South Australia. The modelled Ag concentration derived from nano-Ag is 50-times smaller than measured Ag in soils and 10-times in biosolids. For Zn the respective values are 250 and 7. If in the future the accumulation continues with the same rate as in 2012 it would take about 170 years until a regulatory threshold value of 500 ug Ag per kg of soil would be reached. For Zn, it will take 930 years. The results from this modelling highlight that regional and site-specific conditions need to be considered when assessing the environmental risks of nanomaterials.
Nano impactThis study provides predicted environmental ENM concentrations at a regional resolution with temporal ENM accumulation in soils taken into account. This is particularly relevant as metal concentrations in soil are already used as a critical indicator for assessing risk in soils. In this study we not only modelled nano-Ag and nano-ZnO flows and concentrations but also the total “nano-Ag derived Ag” and the total “nano-ZnO derived Zn”. Additionally the information of the annual Zn and Ag load to soils is compared to the background concentration of Zn and Ag in soils and local environmental guidelines to provide a context for discussion of the management of waste streams post WWTP. |
Introduction
To date, quantification of engineered nanomaterials (ENM) at the trace concentrations relevant in environmental samples is, in most cases, not feasible.1,2 A modelling approach is thus required in order to fill the existing data gaps and gain information about current environmental emissions and concentrations of ENM, and subsequent environmental exposures.3 Mueller and Nowack4 used a material flow model and took all known commercial products containing ENM into account to predict their environmental release. In their study, uncertainty and variability of data were addressed by including a realistic emissions scenario, as well as a worst case (i.e. high emissions) scenario. Gottschalk et al. went further and developed a stochastic material flow model by applying a Monte Carlo approach.5,6 This stochastic method enabled a full range of possible values to be inserted as model parameters and generated results in the form of probability distributions.6 Both studies were necessarily based on the limited information available at that time about the behaviour of ENM in technical and natural systems. Taking advantage of the considerably greater amount of published information now available on production, fate and behaviour of ENM, Sun et al. recently carried out a comprehensive and up-to-date prediction of ENM flows to the environment by extending the stochastic method.7 At almost the same time, Keller et al.8 for the first time revealed a mass-flow analysis of different ENM at a global level.All of the studies mentioned above were limited in their assessment of ENM accumulation in environmental compartments, e.g. sediments and soils, because they were restricted to an incremental yearly input of ENMs into the system. Only Gottschalk et al.6 made a simple projection of the increasing concentrations of ENMs in sediments and biosolids-treated soils, forecasting the period from 2001 to 2012 for the USA, based on some ENM market projections. These projections indicated that a range of responses are to be expected for different ENMs with different sources and properties. The possible accumulation of ENM in soils and sediments is now recognised as a very important issue, as the development and application of nanotechnology has grown exponentially in recent years9,10 and previous modelling studies have indicated that these compartments can act as ENM sinks.
Aside from the limited temporal aspects, these studies4,6–8,11,12 also generally assumed well-mixed and homogenous environmental compartments for a given country or region (e.g. the European Union). Some local information was reported by Gottschalk et al.13 who modelled spatially resolved ENM concentrations in 543 river sections at base flow, and in 21 locations based on 20-year flow data. The ENM input was allocated to the river sections by population-adjusted inflows from wastewater treatment plants (WWTP). Markus et al.11 combined the potential release of nano-forms of zinc (Zn), titanium (Ti), and silver (Ag) from nano-enhanced products per person and then predicted, by using this bottom-up approach, the contribution of these nanoparticles to the annual load in the Dutch reaches of the Rhine and Meuse rivers. A very similar principle was used by Dumont et al.14 By combining the information of household connectivity to sewerage, sewage treatment efficiency, the spatial distribution of sewage treatment plants, and their associated populations they calculated the monthly nano-Ag and nano-ZnO load from households to individual river sections. They used these loadings to model temporally varying nano-particle concentrations in rivers, lakes and wetlands by considering dilution, downstream transport, water evaporation, water abstraction, and nano-particle sedimentation. Keller et al.8 provided a global assessment of ENM emissions to the environment for ten ENMs by combining ENM market information and material flow modelling. In a follow-up study, Keller et al.12 down-scaled the global values to the regional and local level according to the population in each region and their developmental status based on the United Nations inequality-adjusted human development index (IHDI) for the major countries in each region. However, the production and use input data of the whole study relied solely on one single market report, and it is thus not transparent as to how the information was compiled. As a result, it is difficult to access the amount of uncertainty in the dataset itself, which therefore leads to an unknown uncertainty in all the results. An accurate prediction of localized environmental concentrations, especially ENM concentrations in soils, has therefore not yet been achieved.
As noted above, it is clear that, in addition to landfills as technical compartments, soils and sediments are the main sinks for ENMs following the use and release of products containing them. For soils, the main transfer occurs due to the application of biosolids onto certain agricultural soils and, in this situation, time-resolved modelling is the key to an accurate prediction of current concentrations. As only a small fraction of soils receive biosolids, and often in varying amounts, the inclusion of spatial aspects is also required to support the realistic prediction of soil concentrations. Recently Yang et al.15 showed the presence of nano-TiO2 particles in biosolids-treated soils, thereby adding experimental evidence to the predicted transfer and accumulation of ENMs in soils.
The goal of this study was to develop a mass flow model combining spatial and temporal aspects. The model is able to calculate the ENM emissions and concentrations for five materials (nano-TiO2, nano-ZnO, nano-Ag, CNT, and fullerenes) in a highly regionalized context, and is able to account for the accumulation of ENM in environmental compartments that represents sinks, e.g. soils and sediments. South Australia has been selected as a suitable case study for the elaboration of this model as it is a region where treated waste products such as biosolids and wastewater are very actively recycled, and for which detailed spatial and temporal data documenting the application of biosolids and treated wastewater to regional farms, gardens and pastures is available.
Methods
General system boundary
The mass-flow modelling approach used in this study follows the life-cycle principle. Initially, it tracks ENM distribution from their production to allocation in consumer products, and from the ‘use’ phase of products containing ENM to their ‘end of life’ disposal/recycling stage. Then, the ENM distribution within and between disposal processes (technical compartments) and from disposal to the environment is considered. In this study, nano-TiO2, nano-ZnO, nano-Ag, CNT and fullerenes were modelled. These ENM were selected on the basis of their significant production and application quantities, or due to the relatively abundant information available with respect to their production, applications and environmental fate and behaviour.The geographical focus of this study was the state of South Australia (SA). The life-cycle tracking of ENM in SA started from the point of consumption (i.e. use phase), rather than production. This is a reasonable starting point for SA based on the assumption that in a small region (in terms of population size) like SA, there is minimal ENM production and manufacturing of ENM enabled products. During and after the use phase of ENM enabled products, ENMs will however be released into technical and environmental compartments, and these transfers and subsequent accumulations were the focus of the model. The technical compartments included in this study were landfill, wastewater treatment plants (WWTP), community wastewater management systems (CWMS, which handle the primary treated effluent derived from septic tanks), recycling, and export (i.e. outside of SA). The environmental compartments considered were the atmosphere, the two ocean gulfs within South Australia (St. Vincent and Spencer gulfs), and the biosolids and sewage effluent treated soils in the region. ENM flows to air and atmospheric deposition were calculated but a comprehensive calculation of ENM concentration in air was excluded from this modelling study. A preliminary calculation of ENM concentration in air suggested very low values, which are negligible to the purpose of this study.7 ENM concentrations in ocean water were not calculated because complete sedimentation was assumed; only ENM flows (emissions) to ocean were calculated. The soil compartment in this study is a combination of biosolids treated soils (well-defined regional agricultural soils in this context), sewage effluent treated soils, and other soils (i.e. soils not receiving biosolids or sewage effluent and receiving ENM only from atmospheric deposition and direct release during the use of products containing ENM). Inland surface water was excluded from our study because unlike in Europe or the U.S.A, treated wastewater in SA is either recycled for soil irrigation or discharged into the ocean, as over 90% of the population live in coastal urban centres.16 Although ENM leaching from landfill, soils and resuspension from sediments is possible, in our study due to inadequately available knowledge they are assumed as final sinks, no output flows are modelled from these compartments.
Chemical transformations of nano-ZnO and nano-Ag during the wastewater treatment processes were also taken into account.17,18 Unlike treating all the transformed nano-ZnO and nano-Ag as eliminated as done in our previous study,7 here we also tracked the flows of nano-ZnO and nano-Ag that are transformed into non-nano forms. We call them “nano-ZnO derived Zn” and “nano-Ag derived Ag” respectively for the transformed forms of nano-ZnO and nano-Ag. It is noteworthy to point out that the “nano-Ag derived Ag” includes both the transformed forms of nano-Ag and the remaining nano-Ag.
Probabilistic material flow modeling
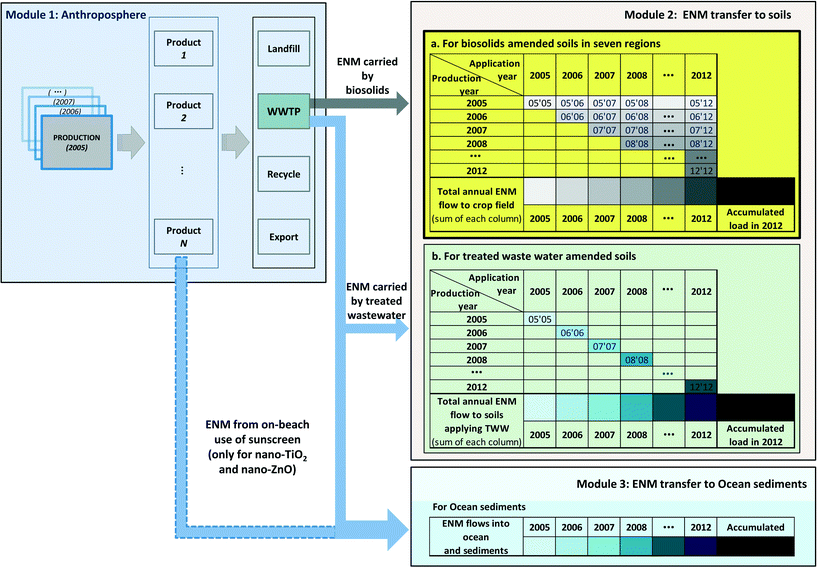
The model consists of three modules: module 1: anthroposphere; module 2: ENM transfer to soils; and module 3: ENM transfer to oceans. Module 1 calculates the yearly ENM flows in treated wastewater and to biosolids for the period considered (2005–2012). This module was based on the previous mass flow models4–7 but with adaptions to SA conditions. The first part of module 2 calculates the annual distribution and the accumulated load of ENM transferred with biosolids into different government-defined regional areas (see ESI†) where soils were amended with biosolids between 2005 and 2012. The second part of module 2 calculates the annual distribution of ENM and accumulated ENM load in soils irrigated with treated sewage effluent over this period. Module 3 calculates the annual and accumulated ENM load into ocean gulf waters and ocean sediments.Only a portion of the biosolids produced in SA was transported to soils for application in the year produced; the rest was stockpiled and gradually transported and applied onto soils over the following years, together with some proportion of biosolids produced in previous years. The annual mass of ENM flowing into the soil in each regional area is thus the sum of each year's ENM input from biosolids that are sourced from different production years. One aim of this study was to calculate the total accumulated ENM load into the regional soils over the period from 2005 to 2012. This was done by summing up the yearly ENM loads from 2005 to 2012, as Fig. 1 shows. The ENM concentrations in these regional soils were then calculated by dividing the accumulated ENM load by the corresponding receiving soil volume. This approach is well defined by REACH,19 but also adapted according to the Australian guidelines by using a different soil depth proposed.20 0.10 m21 and 0.03 m22 of depth for agricultural soils and sediments are used respectively for the calculation of compartment volumes.
The approach used to calculate ENM loads to soils treated by sewage effluent differs from that used for biosolids treated soils. Unlike the temporal variation in biosolids application onto soils, all the treated wastewater (sewage effluent) produced in one year is used in the same year. Hence, in this case, the ENM load to soils is directly related to the ENM concentration of that year's sewage effluent. As no data was available to define the area (therefore the volume) of these soils treated by sewage effluent, and only application rates of recycled water onto these soils were available,23,24 we calculated the ENM load to one unit area of these soils by combining the ENM concentration in treated wastewater given by module 1 and the wastewater application rates to these soils. The accumulated ENM loads carried by recycled water in these soils are then the sum of all the considered years' loads.
Module 3 calculates the ENM load to the two ocean gulfs and their sediments. These values are actually provided directly by the output of module 1. The portion of the treated wastewater that is not used for irrigation is discharged into the two ocean gulfs. For nano-TiO2 and nano-ZnO there is also a direct release of these ENMs into the ocean due to the on-beach use of sunscreen, in which nano-TiO2 and nano-ZnO are important active ingredients. As shown in Fig. 1, this presents an additional flow to the ocean besides the wastewater discharge. Each gulf has three major WWTP effluent discharge points, so in order to obtain more accurate estimates of ENM concentrations in sediments, the spatial distribution of these different discharge points is taken into account and the accumulated ENM loads are divided by the mass volume of the defined area that is affected by each discharge point (see ESI†). The accumulated ENM loads to individual discharge points are the sum of the products of the annual WWTP effluent discharge and the corresponding ENM concentrations in that effluent. The yearly discharge of WWTP effluent at each discharge point is given in the summary of Table S3 (ESI†); and the definition of discharge point areas is documented in the “Gulf Sedimentation Area Estimates & WWTP Discharges for 2005 – 2012” section of the ESI.† It is important to note that strong agglomeration and sedimentation is expected to occur in seawater.25 Therefore the calculation of concentrations in sediments is based on the assumption of complete sedimentation from seawater.
The definition of the areas of the biosolids treated soils in the seven regions is according to the areas of crop land in these regions. This information is given in the local government report.26 A summary of all compartment volumes used in the model is given in Table S1 (ESI†), with detailed information on the parameters and data used for the calculations.
ENM parameters, system parameters and assumptions
Given that no specific data for ENM use in South Australia is available, we estimated these values by downscaling the modelled European Union (EU) ENM use to that of SA according to the proportional difference in their Gross Domestic Product (GDP).7 This is based on the assumption that regional GDP is proportional to purchase power.12 It is an imperfect scaling factor but is stated to be more reasonable than using other factors such as population which disregards the difference in purchasing power of people living in countries with different development status.12 The base year data used was 2012. The extrapolation of annual ENM consumption between 2005 and 2011 was made by scaling the 2012 base year data according to the estimated development of world ENM production. The estimation was modelled on the basis of several market and patent studies.10,27,28 Each market and patent study provides a projection of how the technology or market can develop over time; this supports the use of quantitative ratios between the base year 2012 and other years over the period from 2005 to 2012. Projections of this ratio for the same year based on different studies were used as raw input data for probability modelling. The results can be found in Fig. S1 (ESI†).The evolution of all ENM flows over time in module 1 is based on the total ENM consumption in SA. It assumes all the transfer parameters in module 1 remain unchanged compared to 2012 and the ENM flows are proportional to the initial yearly total consumption. Our assumption is that the allocation of ENM to different consumer products and applications is region-independent, therefore the data used for Europe by Sun et al.7 can also be applied in the case of SA. ENM releases to the environment and waste streams are generally dependent on the product-type, irrespective of where they are used; however, when it comes to the end of life treatment of products different regional regulations may apply. In SA, 10% of plastics are recycled and 6% exported; 30% of consumer electronics are recycled and 19% exported; 66% of glass is recycled and 3% is exported;29 23% of metals are recycled and 67% are exported.30 Centralized wastewater treatment plants serve 92% of the population in SA, and the 8% of people living in rural areas dispose of their wastewater via septic tanks.31 No SA specific WWTP removal efficiencies are available for the five studied ENMs, therefore these data were drawn mostly from the summary by Sun et al.7 and updated by adding newly available WWTP removal studies.32–34 There is only one waste incineration plant in SA, and that is used to burn the small volume of medical, quarantine and pharmaceutical waste produced.35 Farmers in SA have used biosolids since the late 1960s and the use of biosolids is regulated by the SA Environmental Protection Authority.20,36 The biosolids applied to SA soil between 2005 and 2012 also include some stockpiled biosolids produced prior to this period, but due to their very low ENM concentrations compared to the biosolids produced in the last few years, we excluded this portion of ENM from our calculation. According to a relevant report,37 100% of biosolids are applied in agriculture in SA (92% directly used for agriculture, 8% composted and then applied in soils). The official data for stockpiled and transported biosolids produced between 2005 and 2012 is given in Table S2 (ESI†). About 30% of treated wastewater produced in SA is reused for irrigation, while the vast majority of the remaining 70% is discharged into the two ocean gulfs.38 On the basis of the available information for treated wastewater application rates in different soil types, we were able to calculate the ENM load per unit area of soil. For the category of grass, gardens and pastures 4.48 million litres (ML) of treated wastewater per hectare (ML ha−1 yr−1) are applied on average each year. The value for viticulture is 2.10 ML ha−1 yr−1 and for horticulture it is between 3 and 5 ML ha−1 yr−1.23 A summary of these data is made in Table S4 in ESI.†
Like most of the populated regions in Australia, SA is a state with a long coastline. A considerable amount of sunscreen application takes place near the beach, where ENM contained in sunscreen are released directly into ocean water instead of going through wastewater treatment plants. About 13% of the total sunscreen used in SA is estimated to be used during beach visits.39
A summary of the transfer coefficients (TC) of ENM from production to technical and environmental compartments can be found in Table S8 (ESI†).
Results
ENM mass flows in South Australia
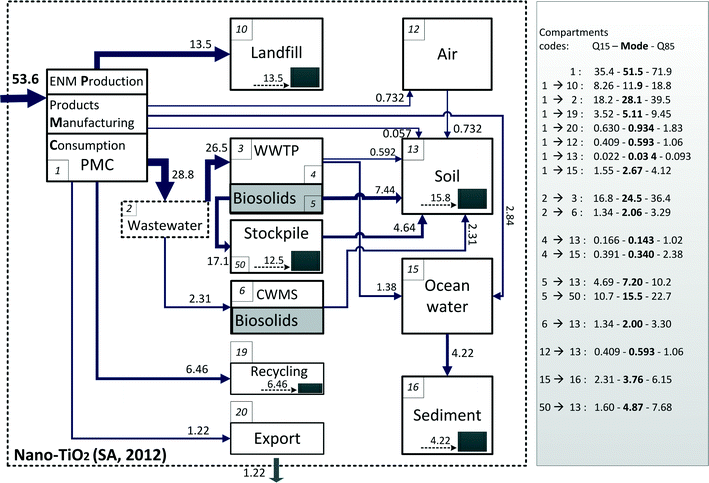
The flows of the five ENM were modelled using the volumes of ENM used in SA, ENM allocation to products, and SA-specific ENM transfer factors from products to technical and environmental compartments. Fig. 2 shows the mass flow for nano-TiO2 in 2012. Mass-flow charts for nano-ZnO, nano-Ag, CNT and fullerenes can be found in Fig. S4 (ESI†). The technical compartments (e.g. PMC, WWTP, WIP, landfill, recycling) and environmental compartments (air, soil, water and sediments) are expressed as boxes; flows between these processes and compartments are expressed as arrows, and the thickness of the arrows indicates the magnitude of the flows. The flows are determined by transfer coefficients (TC) that describe the exchange of ENM between and within these boxes. Flows leaving the system describe ENM flows out of the considered system boundary, e.g. export. ENM can also accumulate in landfills, soils and sediments, as indicated by the black squares in Fig. 2 with the accompanying yearly rate. In Fig. 2 the soil box is a combination of all regional soils receiving biosolids and/or treated wastewater, as well as the soils not receiving ENM via biosolids and sewage effluent but via ENM deposition from air and direct release during the use of ENM enabled products. The ocean and sediment compartment values shown in Fig. 2 represent the sum for the two gulfs and gulf sediments. The mass flow values shown on the flowchart are the mean values from the probability distributions, and mode values (values having the highest probability) and the quantile 15 and 85 are also presented in the figures.The total nano-TiO2 consumption in SA in 2012 was estimated to be 54 tonnes, and this value was used as the total input into our modelling system. The most prominent flow for nano-TiO2 in SA was from production, manufacturing, and consumption (PMC) to waste water (and further to WWTP). This is due to the fact that, like anywhere else, the major use of nano-TiO2 in SA is in cosmetics.7 During wastewater treatment more than 90% of nano-TiO2 is captured in biosolids. All of the nano-TiO2 contained in biosolids ends up in soils, either directly or in the form of compost. The nano-TiO2 flow from PMC to landfill constitutes another significant flow, which is a result of dumping all municipal solid wastes in landfills. In total about 5% of nano-TiO2 may be directly released into the ocean due to sunscreen use on beaches.
In our modelling we track the mass of the primary particles. Agglomeration plays a role in transferring particles from one compartment to another (e.g. from water to sediment), but does not affect the total mass of the primary particles. It should be noted, however, that ENM can be transformed or degraded before they reach environmental compartments. Nano-Ag and nano-ZnO can be transformed into other forms during product use and especially in wastewater transfer and in the anaerobic phase in WWTPs.17,18,33,40 According to Lombi et al.17 and Ma et al.33 all nano-ZnO is transformed to other Zn compounds during WWTP processes, which means that all of the Zn contained in WWTP biosolids and effluent is no longer nano-ZnO. These transformed flows are called “nano-ZnO derived Zn”. For nano-Ag, the above mentioned authors indicated in their studies that a small fraction can survive the WWTP processes. So the Ag flows from WWTPs and CWMSs are mainly transformed species of Ag together with a very small fraction of original nano-Ag. In the mass flow charts these forms are presented together (see Fig. S4, ESI†), but separate mass flow charts differentiating the nano and transformed flows of Ag are given in Fig. S5 (ESI†).
The modelled total consumptions of nano-ZnO, nano-Ag, CNT and fullerenes in SA are 10 tonnes, 0.18 tonnes, 2.1 tonnes and 0.12 tonnes respectively. Very similar to the findings for nano-TiO2 the prominent flows of nano-ZnO are from consumption to waste water due to a considerable fraction of nano-ZnO being used in personal care products. After WWTP processes, nano-ZnO is transformed into different chemical forms such as ZnS, Zn-ferrihydrite and Zn3(PO4)17,33 and then transported to soils. For nano-Ag the most prominent flow is to landfill; this is because one of its major applications is in plastics and electrical appliances. This is followed by lesser flows to waste water and downstream compartments. As mentioned above, after passing through wastewater transfer and treatment, most of the nano-Ag is transformed to Ag sulfides. Most of the CNT and fullerenes end up either in landfill or recycling, and this can be explained by the fact that most of these materials are applied in polymer composites.
Environmental concentrations of ENM
In Fig. 3 the development of the accumulated nano-TiO2 concentrations between 2005 and 2012 is shown for biosolids treated soils, effluent treated soils and the gulf sediments. The nano-TiO2 concentrations increased over time due to the yearly input into these compartments that function as nano-TiO2 sinks. The yearly input by biosolids varied, resulting in a non-linear increase. The accumulation curves for nano-TiO2 in soils and sediments provide references for the other four ENM, which follow similar trends. A summary table of accumulated concentrations of all five ENMs in 2012 is given in Table S9 (ESI†).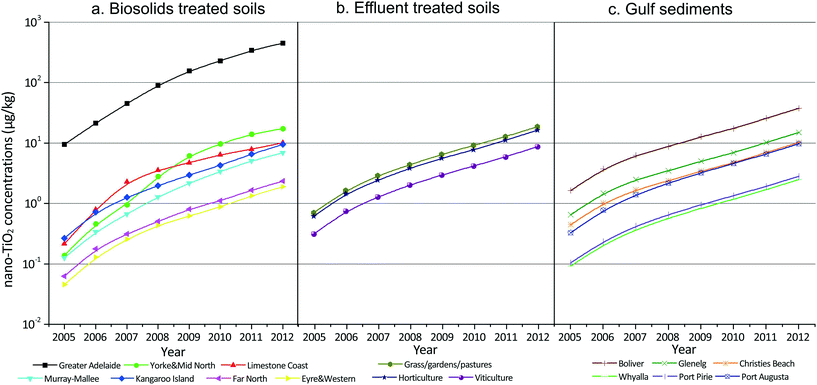 | ||
| Fig. 3 Accumulated nano-TiO2 concentrations (unit: μg kg−1) in biosolids amended soils, sewage effluent irrigated soils, and gulf sediments over the period from 2005 to 2012. Fig. 3a shows nano-TiO2 concentrations in the agricultural soils of seven different regions within the modelled area; Fig. 3b shows nano-TiO2 concentrations in three categories of soils irrigated with sewage effluent; and Fig. 3c shows nano-TiO2 concentrations in six treated wastewater discharge points within the two ocean gulfs. | ||
Comparing the concentrations in the different compartments, nano-TiO2 concentrations in soils amended with biosolids cover the broadest range, varying from 0.05 μg kg−1 for farms on Eyre and Western in 2005 to 450 μg kg−1 for farms in the Greater Adelaide area in 2012 (i.e. 4 orders of magnitude difference). These concentrations can effectively be divided into two clusters: the concentration for Greater Adelaide and the concentrations for the other six regions. The concentrations of nano-TiO2 in WWTP effluent treated soils and gulf sediments are in a very similar range as the concentrations in biosolids treated soils of the six regions except Greater Adelaide, which vary from 0.05 to 17 μg kg−1. In summary, the model indicates that the concentrations of nano-TiO2 in biosolids and WWTP effluent treated soils and in ocean sediments have increased 30–40 times over the 7 year period. The nano-TiO2 concentrations in the soils of the Greater Adelaide region are projected to be the highest among all environmental compartments considered within the model boundaries. This is due to the fact that, compared with the other regions, in the Greater Adelaide region a similar or even larger yearly biosolids load is applied to a much smaller area (i.e. smaller receiving soil volume). The soil volumes in some other regions are 1 to 2 orders of magnitude higher than that of the Greater Adelaide region. In contrast to Greater Adelaide, soils of Kangaroo Island receive the lowest loads and have the lowest concentrations due to the relatively low biosolids production and use in this area.
Concentrations in WWTP effluent treated soils including horticulture; viticulture; and grassland, gardens, and pastures are less variable than concentrations in biosolids treated soils and in gulf sediments. Among the three land-use categories irrigated with WWTP effluents, nano-TiO2 concentrations are almost the same, increasing from less than 1 μg kg−1 in 2005 to more than 10 μg kg−1 in 2012. Concentrations in viticultural soils are only half that of the other two land-uses due to the lower application rate of sewage effluent in viticulture.
With respect to the concentrations in sediments, overall the predicted concentrations increase 30-fold over the seven years between 2005 and 2012. The lowest concentration predicted by the model is for the sediment at Whyalla. This is estimated to increase from 0.09 μg kg−1 in 2005 to 2.5 μg kg−1 in 2012. The highest concentration is linked to the sediment affected by Bolivar (the largest WWTP in SA), with the concentration in the affected area predicted to be more than 10 times higher than the concentration in the least affected sediment.
A direct comparison between the accumulations of the five ENM has been made for the Greater Adelaide soils, the region where the highest ENM concentrations were found. We can see from Fig. 4 that the order of ENM concentrations is well correlated with the order of the total input volumes of the ENM. Nano-TiO2 is predicted to be present in the highest concentrations, accumulating from about 9.5 μg kg−1 in 2005 to 450 μg kg−1 in 2012, followed by nano-ZnO (in this case the “nano-ZnO derived Zn”), with the “nano-ZnO derived Zn” concentrations predicted to be 5 times lower than those for nano-TiO2. As mentioned in the previous section, after wastewater treatment processes, all nano-ZnO is expected to be transformed into other chemical forms; therefore none of the original nano-ZnO released from products is predicted to be transported onto soils with biosolids. The majority of the nano-Ag in biosolids is also known to be transformed into non-nano forms e.g. Ag2S.18 We therefore calculated the concentrations of the “nano-Ag derived Ag” (here both the transformed forms of nano-Ag and the remaining nano-Ag) ending up in soil as well as the concentration of only remaining nano-Ag. The concentration of “nano-Ag derived Ag” is about 2 orders of magnitude lower than that of “nano-ZnO derived Zn”. The concentration of CNT and fullerenes are a further order of magnitude lower. Nano-Ag has the lowest concentration in soil, ranging from 0.0002 μg kg−1 in 2005 to 0.02 μg kg−1 in 2012.
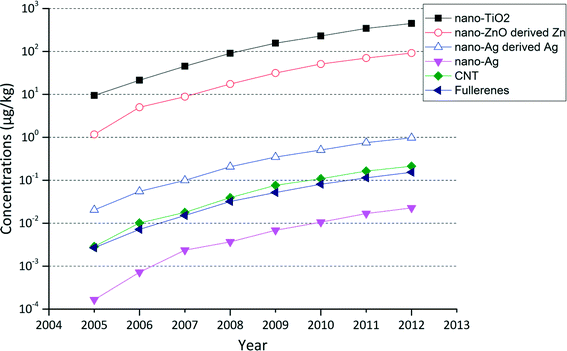 | ||
| Fig. 4 Evolution of accumulated concentrations of ENM and metals derived from their nano form in soil of the Greater Adelaide area receiving biosolids between 2005 and 2012. | ||
Discussion
Compared to previous ENM mass flow studies,4,6,7 the present study increased the temporal and spatial resolution of the predicted environmental concentrations of ENM. It includes temporal aspects by considering the accumulation of ENM in environmental sinks over a seven-year period instead of only one single year's input and accumulation of ENM in soils, therefore provides actual concentrations instead of only the predicted yearly increase in concentration. The increase of ENM concentrations in biosolids amended soils is caused by a combination of increasing production of ENM – and therefore increasing concentrations in biosolids – and the yearly addition of biosolids to the soils. We have used the conservative assumption that soils are the final sink for the ENM transported along this pathway. Leaching and runoff are minimal in SA as due to the small amount of precipitation the large majority of the watercourses are dry for the majority of the year and potential evapotranspiration exceeds water supply, although it should be noted that wind erosion may occur. The model was also conservative in that it did not consider the possible transformations of ENM in soil/sediments. These compartments were considered in our model as final sinks, although of course dissolution/phase transformation or leaching or sediment transport may affect the concentrations. However, to include these reactions in the model a coupling with process-based environmental fate models would be needed.41When compared to the studies by Sun et al.7 and Gottschalk et al.,6 the results for biosolids treated soils in the EU are 3–4 times higher than the ENM concentrations predicted for soils in the Greater Adelaide area. We note that the nano-ZnO concentrations cannot be directly compared because results in Sun et al.7 indicate the concentration of untransformed nano-ZnO reaching the soils while the results presented here only report the concentrations of total “nano-ZnO derived Zn” because no nano-ZnO survives in biosolids, the only source modelled in our study. The differences between the results for the EU and for this study are caused by the smaller biosolids production and relatively larger volumes of soils in Greater Adelaide. The soils of Greater Adelaide with the highest ENM load received accumulatively 292 tons km−2 of biosolids between 2005 and 2012; in contrast, European soils received 2000 tons km−2 in a single year in 2012. Even within SA the accumulated concentrations of ENM in biosolids treated soils vary by two orders of magnitude, ranging from 1.9 μg kg−1 of nano-TiO2 in Eyre and Western soils to 450 μg kg−1 of nano-TiO2 in Greater Adelaide soils. These results indicate that ENM soil concentrations will be very strongly influenced by local practices. For SA, one important practice is the transportation of biosolids produced in metropolitan Adelaide (SA state capital) to different regions across the state for use in soil amendment by farmers. This practice helps explain the soil concentrations for the two groups of regions in Fig. 3a. Greater Adelaide is likely to receive most transported biosolids per unit area because it is the closest to metropolitan Adelaide. Since farmers pay the cost of transportation, the cluster of regions beyond Greater Adelaide (Murray-Mallee, Limestone Coast, Yorke and Mid North, Eyre and Western, Kangaroo Island) are likely to receive less transported biosolids per unit area because transport is relatively expensive.
SA is also special in that a significant part of the treated wastewater is used for irrigation of some crops and trees. This results in an almost closed loop for ENM as on the one hand the ENM captured by the WWTP (this is the major part of the ENM released to wastewater) are transferred to some soils whereas the ENM not captured are transferred directly other soils. In such a situation an almost complete transfer of all ENM to soils can be expected which is completely different to a region that does not allow biosolids transfer onto soils (instead landfilling or incineration may be used). In such a region landfills and sediments will be final sinks for ENM whereas in SA these final sinks are mainly soils.
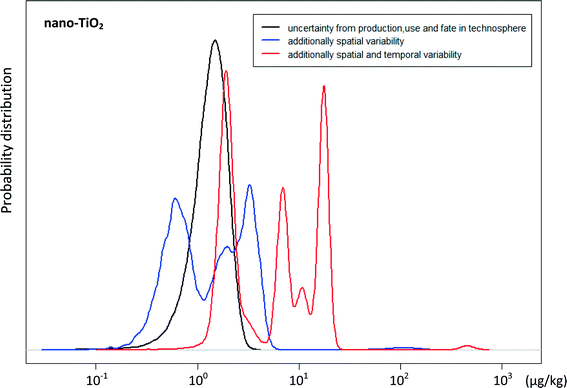
The availability of extensive data detailing the annual distribution of biosolids to individual regional farms enabled a more precise positioning on target soils receiving biosolids and therefore a higher resolution of the predicted environmental concentrations of ENM. In order to visualize the importance of spatial and temporal variability, in Fig. 5 the probability distributions of nano-TiO2 concentrations from different stages of the modelling are plotted. The black curve shows the uncertainty of the model output from production/use/fate in technosphere and represents the average nano-TiO2 concentration in biosolids treated soils in SA. This curve was calculated by dividing the total annual flow of nano-TiO2 onto soils in 2012 by the total volume of these soils. Here only the uncertainty of nano-TiO2 production and transfer along its life cycle before ending up in soils is reflected. The blue curve represents the additional spatial variability and is the probability density distribution of the 2012 incremental nano-TiO2 concentrations comprising all regional soils of SA. It involves the uncertainty of the model output from production, use and fate in technosphere as well as the regional variability of nano-TiO2 concentrations. Compared to the average curve, both higher and lower concentrations are observed. The red curve is the probability density distribution of the accumulated nano-TiO2 concentrations over the period between 2005 and 2012 comprising the seven regional soils. Compared to the blue curve apart from uncertainty in production, use, fate in technosphere and local variability it also takes the temporal variability of biosolids application onto soils into account. We can clearly see that the concentration variability derived from spatial and temporal biosolids application practice is much larger than the uncertainty caused by production data and transfer parameters.
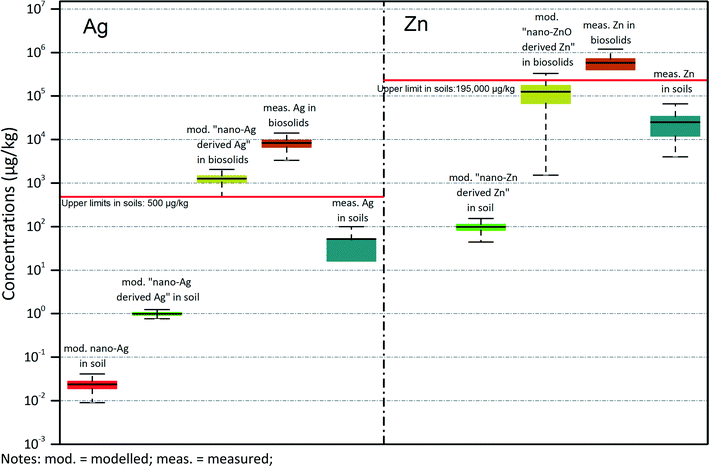
As this study provides accumulated concentrations in soils, it can be directly used for environmental risk assessment. This is particularly relevant as metal concentrations in soil are already used as a critical indicator for assessing risk in soils. In this study we not only modelled nano-Ag and nano-ZnO flows and concentrations but also the total “nano-Ag derived Ag” and the total “nano-ZnO derived Zn”. This was done by applying the transformation rate suggested by studies about the fate of metal nanomaterials during the wastewater processes.17,18,33,40 The inclusion of total “nano-ZnO derived Zn” and “nano-Ag derived Ag” modelling helps to see how much contribution nanomaterials make to the total metal concentrations in soils. We therefore compared modelled and measured concentrations of Ag and Zn in both biosolids and soils in SA (total measured concentrations of Ag and Zn are summarised in Table S10 of ESI†). Fig. 6 shows these comparisons with boxplots. Here the modelled concentrations of biosolids treated soils in Greater Adelaide were taken to represent the highest soil metal concentration induced by biosolids. Modelled nano-Ag concentrations and the concentration of nano-Ag derived Ag are separated by almost 2 orders of magnitude. The modelled concentration of nano-Ag derived Ag in biosolids is about 1000 μg kg−1, the measured concentration of total Ag in biosolids is about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg kg−1. About 10% of the total Ag contained in biosolids could be contributed by and derived from nano-Ag. The concentration of measured background Ag in soil is around 50 μg kg−1, which is between the modelled Ag concentrations in soils and the Ag concentrations in biosolids. Zinc is quite similar to Ag, but with all the corresponding concentrations increased by about 2 orders of magnitude. There is no modelled nano-Zn concentration in soils but only “nano-ZnO derived Zn”, because no nano-Zn survives the WWTP processes. The red line in the figure represents for the upper limits of maximally permitted Zn concentrations in soils in South Australia, which is 195 mg kg−1 for soils with a moderate pH as given by the SA biosolids guideline for the safe handling and reuse of biosolids.20 No corresponding value is available for Ag in Australia, so the upper limit for Ag in Fig. 6 is based on existing regulations in other countries, here the lowest value of 500 μg kg−1 was shown.42–44
000 μg kg−1. About 10% of the total Ag contained in biosolids could be contributed by and derived from nano-Ag. The concentration of measured background Ag in soil is around 50 μg kg−1, which is between the modelled Ag concentrations in soils and the Ag concentrations in biosolids. Zinc is quite similar to Ag, but with all the corresponding concentrations increased by about 2 orders of magnitude. There is no modelled nano-Zn concentration in soils but only “nano-ZnO derived Zn”, because no nano-Zn survives the WWTP processes. The red line in the figure represents for the upper limits of maximally permitted Zn concentrations in soils in South Australia, which is 195 mg kg−1 for soils with a moderate pH as given by the SA biosolids guideline for the safe handling and reuse of biosolids.20 No corresponding value is available for Ag in Australia, so the upper limit for Ag in Fig. 6 is based on existing regulations in other countries, here the lowest value of 500 μg kg−1 was shown.42–44
The modelled Ag and Zn concentrations are 50 and 250 times respectively smaller compared to the measured background soil concentrations of Ag and Zn. The difference between the current background soil concentration of Ag and the lowest upper limit concentration is 450 μg kg−1, as it is shown in Fig. 6. If assuming the current load of Ag onto soil remains constant – the biosolids induced Ag yearly increase in soil concentration is 2.6 μg kg−1 y−1 it will take 170 years to reach the lowest threshold in SA. The difference between the currently measured Zn background concentration in soils and the upper limit in SA is about 170 mg kg−1. The current yearly increase in concentrations of Zn in soils in Greater Adelaide is 182 μg kg−1 y−1 According to this rate, it will take 930 years to reach the limit.
Two issues in relation to soil concentrations need to be highlighted: we assumed soils to be final sinks for ENM, so no downward leaching or surface erosion was considered, also further dissolution/degradation reactions were not included. The modelled soil concentrations therefore represent maximal values and more process-based models will be needed to quantify the importance of these processes for the long-term fate of ENM in soils.
Our work also provides the first concentration prediction for marine sediments in two different ocean gulfs. The concentrations are in the μg kg−1 range for nano-TiO2 and are thus much lower than predicted for river sediments.7 These concentrations can serve as baseline concentrations for marine ecotoxicologists when studying sediment-dwelling organisms. These concentrations were obtained by assuming complete sedimentation in the area affected by ENM, an assumption that it is reasonable given the fast sedimentation of ENM in seawater.45 However, we have to consider that highest sedimentation will occur close to the discharge point and thus a gradient in sedimentation with increasing distance from the coast is expected.
Acknowledgements
The authors would like to thank SA Water, and in particular Jon Mikuzis and Nirmala Dinesh, for providing the Biosolids Activity Annual Reports for SA and for their comments on the manuscript. The authors would also like to acknowledge the financial support from the Australian Research Council through grant FT100100337, FT130101003 and DP120101115. The authors would also like to thank the Swiss National Science Foundation within the National Research Programme “Opportunities and Risks of Nanomaterials” (NRP 64) for the financial support to TS. CRC CARE is also acknowledged for providing a top-up scholarship to GC.References
- B. Kim, M. Murayama, B. P. Colman and M. F. Hochella, J. Environ. Monit., 2012, 14, 1128–1136 CAS.
- F. von der Kammer, P. L. Ferguson, P. A. Holden, A. Masion, K. R. Rogers, S. J. Klaine, A. A. Koelmans, N. Horne and J. M. Unrine, Environ. Toxicol. Chem., 2012, 31, 32–49 CrossRef CAS PubMed.
- F. Gottschalk, T. Sun and B. Nowack, Environ. Pollut., 2013, 181, 287–300 CrossRef CAS PubMed.
- N. C. Mueller and B. Nowack, Environ. Sci. Technol., 2008, 42, 4447–4453 CrossRef CAS.
- F. Gottschalk, R. W. Scholz and B. Nowack, Environ. Model. Softw., 2010, 25, 320–332 CrossRef PubMed.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS PubMed.
- T. Y. Sun, F. Gottschalk, K. Hungerbühler and B. Nowack, Environ. Pollut., 2014, 185, 69–76 CrossRef CAS PubMed.
- A. Keller, S. McFerran, A. Lazareva and S. Suh, J. Nanopart. Res., 2013, 15, 1–17 CrossRef.
- M. C. Roco, Nanotechnology Research Directions for Societal Needs in 2020:Retrospective and Outlook, 2011 Search PubMed.
- LUX RESEARCH INC, Statement of Findings: Sizing Nanotechnology's Value Chain, 2004 Search PubMed.
- A. A. Markus, J. R. Parsons, E. W. M. Roex, G. C. M. Kenter and R. W. P. M. Laane, Sci. Total Environ., 2013, 456–457, 154–160 CrossRef CAS PubMed.
- A. A. Keller and A. Lazareva, Environ. Sci. Technol. Lett., 2013, 1, 65–70 CrossRef.
- F. Gottschalk, C. Ort, R. W. Scholz and B. Nowack, Environ. Pollut., 2011, 159, 3439–3445 CrossRef CAS PubMed.
- E. Dumont, A. C. Johnson, V. D. J. Keller and R. J. Williams, Environ. Pollut., 2015, 196, 341–349 CrossRef CAS PubMed.
- Y. Yang, Y. Wang, P. Westerhoff, K. Hristovski, V. L. Jin, M.-V. V. Johnson and J. G. Arnold, Sci. Total Environ., 2014, 485–486, 441–449 CrossRef CAS PubMed.
- Australian Bureau of Statistics, Regional Population Growth, Australia and New Zealand,2001-02, Report cat. no. 3218.0.
- E. Lombi, E. Donner, E. Tavakkoli, T. W. Turney, R. Naidu, B. W. Miller and K. G. Scheckel, Environ. Sci. Technol., 2012, 46, 9089–9096 CrossRef CAS PubMed.
- R. Kaegi, A. Voegelin, B. Sinnet, S. Zuleeg, H. Hagendorfer, M. Burkhardt and H. Siegrist, Environ. Sci. Technol., 2011, 45, 3902–3908 CrossRef CAS PubMed.
- REACH, Registration, Evaluation, Authorisation and Restriction of Chemical substances, European Commission, 2007.
- SA EPA, South Australian biosolid guideline for the safe handling and reuse of biosolids (draft), 2009.
- CSIRO, The Adelaide Coastal Waters Study, final report, CSIRO, 2007.
- ECB, Technical Guidance Document on Risk Assessment, European Chemicals Bureau; Institute for Health and Consumer Protection, Dublin, 2003.
- S. Laurenson, A. Kunhikrishnan, N. Bolan, R. Naidu, J. McKay and G. Keremane, Int. J. Environ. Sci. Dev., 2010, 1, 176–180 CrossRef.
- South Australian Wine Industry Incorporated, fact sheet: ‘Leading Environmental Practice – Keeping Our Members Informed’, http://www.winesa.asn.au/_r1720/media/system/attrib/file/420/LEP004%20-%20Bulletin%20-20Water%20v1-0%20June%202013.pdf Accessed 03/11, 2013.
- A. A. Keller, H. Wang, D. Zhou, H. S. Lenihan, G. Cherr, B. J. Cardinale, R. Miller and Z. Ji, Environ. Sci. Technol., 2010, 44, 1962–1967 CrossRef CAS PubMed.
- PIRSA, Crop and Pasture Report South Australia, Primary Industries and Regions SA (PIRSA), Adelaide, 2013.
- European Commission, The economic development of nanotechnology - An indicators based analysis, 2006.
- F. Piccinno, F. Gottschalk, S. Seeger and B. Nowack, J. Nanopart. Res., 2012, 14 Search PubMed.
- C. Brulliard, R. Cain, D. Do, T. Dornom, K. Evans, B. Lim, E. Olesson and S. Young, The Australian recycling sector, E. Department of Sustainability, Water, Population and Communities (DSEWPaC), 2012 Search PubMed.
- C. Colby, M. Rawson and K. Heinrich, Zero Waste SA: Recycling Activity in South Australia, 2010–11. Report prepared by Rawtec for Government of South Australia, 2012.
- Local Government Association of South Australia, Existing CWMS, http://www.lga.sa.gov.au/page.aspx?u=1117, Accessed 08/10, 2014.
- L. Li, G. Hartmann, M. Döblinger and M. Schuster, Environ. Sci. Technol., 2013, 47, 7317–7323 CAS.
- R. Ma, C. Levard, J. D. Judy, J. M. Unrine, M. Durenkamp, B. Martin, B. Jefferson and G. V. Lowry, Environ. Sci. Technol., 2013, 48, 104–112 CrossRef PubMed.
- J. Hedberg, C. Baresel and I. Odnevall Wallinder, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2014, 49, 1416–1424 CrossRef CAS PubMed.
- SA EPA, Solid Waste Incineration, http://www.epa.sa.gov.au/environmental_info/waste/solid_waste/incineration, Accessed 08/11, 2014.
- UN-HABIATAT, Global Atlas of Excreta, Wastewater Sludge, and Biosolids Management: Moving Forward The Sustainable and Welcome Uses of A Global Resource, 2008.
- P. Darvodelsky, Biosolid snapshot, E. Department of Sustainability, Water, Population and Communities (DSEWPaC), 2012 Search PubMed.
- SA Water, Recycled Water Overview, http://www.sawater.com.au/SAWater/Education/OurWastewaterSystems/Recycled+Water+Overview.htm, Accessed 06/11, 2014.
- G. Conroy, T. Y. Sun, E. Donner, K. Hungerbuehler, B. Nowack and E. Lombi, Estimation of sunscreen use in South Australia Search PubMed, unpublished work.
- E. Lombi, E. Donner, S. Taheri, E. Tavakkoli, Å. K. Jämting, S. McClure, R. Naidu, B. W. Miller, K. G. Scheckel and K. Vasilev, Environ. Pollut., 2013, 176, 193–197 CrossRef CAS PubMed.
- A. Praetorius, R. Arvidsson, S. Molander and M. Scheringer, Environ. Sci.: Processes Impacts, 2013, 15, 161–168 CAS.
- Ministry of the Environment. Soil, ground water and sediment standards for use under Part XV.1 of the Environmental Protection Act., https://dr6j45jk9xcmk.cloudfront.net/documents/998/3-6-3-sediment-standards-en.pdf.
- L. S. Sonon and J. Gaskin, Metal concentration standards for land application of biosolids and other by-products in Georgia, Available at: http://athenaeum.libs.uga.edu/bitstream/handle/10724/12112/B1353.pdf?sequence=1, 2009 Search PubMed.
- ESdat, in Circular on target values and intervention values for soil remediation, 2000.
- K. Garner and A. Keller, J. Nanopart. Res., 2014, 16, 1–28 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5en00004a |
| ‡ Joint first authorship. |
| This journal is © The Royal Society of Chemistry 2015 |