Open Access Article
Open Access ArticleA zinc–iron redox-flow battery under $100 per kW h of system capital cost†
Ke
Gong
a,
Xiaoya
Ma
a,
Kameron M.
Conforti
b,
Kevin J.
Kuttler
a,
Jonathan B.
Grunewald
a,
Kelsey L.
Yeager
a,
Martin Z.
Bazant
bc,
Shuang
Gu
*a and
Yushan
Yan
*a
aDepartment of Chemical and Biomolecular Engineering, University of Delaware, Newark, Delaware 19716, USA. E-mail: shuang.gu@wichita.edu; yanys@udel.edu
bDepartment of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
cDepartment of Mathematics, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
First published on 9th September 2015
Abstract
Redox flow batteries (RFBs) are one of the most promising scalable electricity-storage systems to address the intermittency issues of renewable energy sources such as wind and solar. The prerequisite for RFBs to be economically viable and widely employed is their low cost. Here we present a new zinc–iron (Zn–Fe) RFB based on double-membrane triple-electrolyte design that is estimated to have under $100 per kW h system capital cost. Such a low cost is achieved by a combination of inexpensive redox materials (i.e., zinc and iron) and high cell performance (e.g., 676 mW cm−2 power density). Engineering of the cell structure is found to be critical to enable the high power density. Our cost model shows that a Zn–Fe RFB demonstrates the lowest cost among some notable RFBs and could reach the 2023 cost target set by the U.S. Department of Energy ($150 per kW h).
Broader contextThe widespread deployment of renewable energies such as wind and solar calls for energy-storage systems to smooth out their intermittent electricity generation. Redox-flow batteries (RFBs) are scalable energy-storage devices with great design flexibility due to their decoupled energy and power functions. The most important prerequisite for RFBs to be economically viable is low capital cost. In this work, we present a zinc–iron (Zn–Fe) RFB that uses inexpensive redox materials yet offers high cell performance, and thus achieves a very low system capital cost under $100 per kW h. |
The widespread deployment of renewable energies such as wind and solar calls for energy-storage systems to smooth out their intermittent electricity generation. Various technologies have been introduced, including physical methods such as pumped hydro and compressed air, and electrochemical methods such as regenerative fuel cells and rechargeable batteries.1–7 Redox flow batteries (RFBs) are one of the most promising systems due to their excellent scalability. Freeing the redox pairs from the solid electrodes into flowing electrolytes allows independent engineering for energy (through electrolytes) and power (through electrodes), leading to flexibility in system design and deployment.8–12 One of the prerequisites for RFBs to be widely employed in energy storage is their low capital cost. To make electricity-storage systems economically viable, the US Department of Energy has set a system capital cost target of $150 per kW h by 2023,13 and an even lower target of $100 per kW h is needed to match the existing grid-level storage technologies of pumped hydro and compressed air. However, these targets have not been met so far due to the high cost of redox pairs and/or the low power density of cells. For example, the most developed all-vanadium (denoted as all-V) RFBs currently have a system capital cost around ∼$300–$800 per kW h.14 Here we design a new RFB that uses low-cost redox pairs (i.e., zinc and iron, denoted as a Zn–Fe RFB) and demonstrates high power density (e.g., 676 mW cm−2); the Zn–Fe RFB therefore offers a potential system capital cost of less than $100 per kW h.
The RFB system cost has two major contributions: electrolyte cost and stack cost:
| Csys ≈ Ce + Cs = Ue/Veff + Us/(t·I·Veff) | (1) |
From eqn (1), a low system cost can be achieved by minimizing Ue and/or Us, or maximizing Veff. Low Ue and Us can be obtained by using low cost redox pairs and stack materials, while high Veff requires high reversible cell voltage, and small overpotential and internal resistance, as shown in eqn (2).
| Veff = Vrev − η − I·R | (2) |
Current RFBs cannot satisfy simultaneously low Ue and high Veff at high current densities. As a reference, the challenges facing two notable RFBs are described here. All-V RFBs recently achieved a dramatic improvement in their Veff at high current density by reduction of internal resistance, but their Ue is too high.15 The vanadium material alone would cost $64 per kW h (Table S1, ESI†). On the other hand, Cr–Fe RFBs have low Ue, but their Veff is low due to the intrinsically low reversible cell voltage and sluggish kinetics of the Cr redox pair.16 It is therefore imperative to find two redox pairs that have low elemental cost, fast redox kinetics, and high reversible voltage.
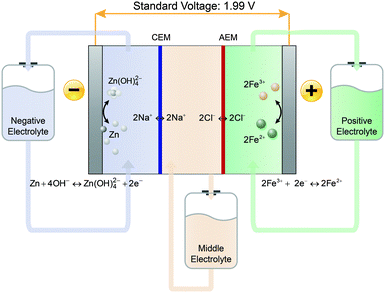
Zn and Fe are two elements with the potential to satisfy these low cost requirements. Specifically, the use of Zn in a basic environment and Fe in an acidic environment has been seen in many RFBs due to their low elemental cost, facile redox kinetics, and desirable standard potential (ϕZn(II)/Zn = −1.22 V vs. SHE in base, pH = 14; and ϕFe(III)/Fe(II) = 0.77 V vs. SHE in acid, pH = 0).17,18 In addition, the long-standing concern of Zn dendrite formation is precluded by the flowing electrolyte in RFBs.19 Each of the two redox pairs has been separately used in the construction of many promising RFBs;17,18,20 however, the combination of the two redox pairs in one cell cannot be realized by the conventional single-membrane cell configuration. In this work, the Zn–Fe RFB is fabricated using a double-membrane design that enables the use of redox pairs of different ion charges and supporting electrolytes of different pHs.21 The working principles of the Zn–Fe RFB based on the basic Zn redox pair and the acidic Fe redox pair are illustrated in Fig. 1, and the negative and positive electrode reactions are shown in eqn (3) and (4), respectively.
| Negative electrode: Zn + 4OH− ↔ Zn(OH)42− + 2e− | (3) |
| Positive electrode: 2Fe3+ + 2e− ↔ 2Fe2+ | (4) |
A sufficiently low cell resistance is then the remaining barrier to achieving the low cost. Unlike traditional single-membrane RFBs, the double-membrane design used by the Zn–Fe RFB requires an additional membrane and electrolyte, which adds challenges for managing the cell resistance. The study shows that the total cell resistance consists of two components: ohmic resistance (Ro) mostly from the two membranes and three electrolytes; and concentration-polarization resistance (Rcp) caused by insufficient ion diffusion in the middle electrolyte.23 Specifically, the concentration-polarization resistance originates from the accumulation or depletion of Na+ and Cl− ions in the vicinity of either membrane when a current flows through the cell (Fig. S2a, ESI†). Compared with OH− anions (in negative electrolyte) and H+ cations (in positive electrolyte), the Na+ cations and Cl− anions have much smaller ion diffusion coefficients, and as such the middle electrolyte, to a great extent, controls Rcp.
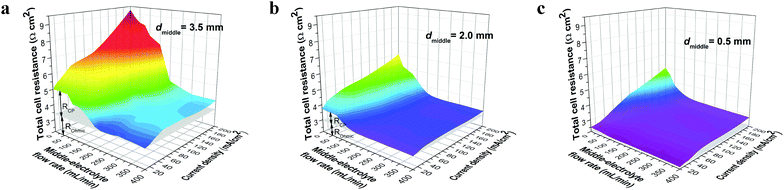
R o can be managed by optimizing the electrolyte concentration and reducing the thickness of the middle electrolyte (Fig. S3a and b, ESI†). With an optimal electrolyte concentration of 2.5 mol L−1 and a small electrolyte thickness of 0.5 mm, an Ro as low as 2.3 Ω cm2 was obtained, which is sufficiently low for the Zn–Fe RFB, considering its close-to-2 V standard voltage. Rcp, on the other hand, depends on various structural and operational parameters24 (Fig. S2b, ESI†). Rcp can be quantitatively measured by subtracting the constant Ro from total cell resistance obtained at different flow rates and/or different current densities.
Fig. 2 shows the impact of flow rate and current density on Rcp with different thicknesses of the middle electrolyte (0.5, 2. 0, and 3.5 mm). We found that the operational and structural parameters can be correlated to a dimensionless number X characterizing Rcp: X = (Q·d−1·w−1)/(I·F−1·C−1), where, Q is the flow rate; d and w are thickness and width of the middle electrolyte, respectively; I is current density; F is the Faraday's constant; C is the salt concentration of the middle electrolyte. The quantity X represents the ratio of vertical velocity of convection (direction of electrolyte flow) to horizontal velocity of ion diffusion (direction of current flow). From regression analysis, increasing X can effectively depress Rcp to a negligible level (Fig. S4a, ESI†). This relationship illustrates the general resistance behavior with respect to cell structural and operational parameters. The theoretically calculated Rcp also has a similar trend with respect to the dimensionless number X (Fig. S4b and c, ESI†), as compared with measured Rcp. Both results show that Rcp can be limited to around 0.1 Ω cm2 if X is larger than 105, suggesting that the manipulation of cell structural and operational parameters is effective to minimize Rcp.
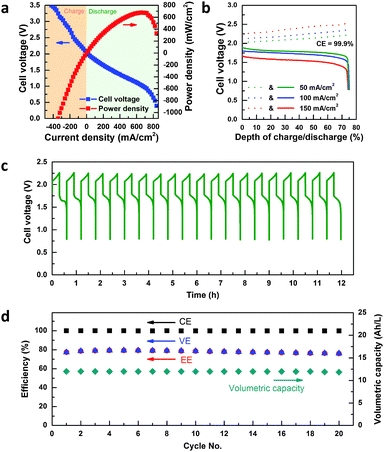
With an engineered middle electrolyte, the Zn–Fe RFB achieves high performance (Fig. 3). A peak power density of 676 mW cm−2 was delivered during discharge at a current density of 660 mA cm−2 at 70% of state of charge (Fig. 3a, state of charge, SOC, is calculated as a percentage via dividing available capacity for discharge by total capacity of the battery). This peak power density is the highest among all RFBs based on zinc (∼200 mW cm−2 for the Zn–Br RFB to our best estimation25) or iron (257 mW cm−2 for the H–Fe RFB20) and among the highest among advanced RFBs including quinone-Br RFBs (600 mW cm−2),26 all-V RFBs (1300 mW cm−2),27 and H–Br RFBs (1450 mW cm−2).28 In addition, even at I = 600 mA cm−2, Veff remains at 1.1 V, which is comparable to Vrev of some of the most advanced RFBs. The charge polarization also demonstrated high power density, comparable to discharge power density. Zn–Fe RFBs also showed a very high coulombic efficiency of 99.9% regardless of current density (Fig. 3b), indicating excellent isolation of two redox pairs, owing to the double-membrane cell configuration. In addition, the Zn–Fe RFB showed no decrease in coulombic efficiency (99.9%), voltage efficiency (76%) and capacity after 20 cycles at 80 mA cm−2 current density with 75% state of charge (SOC) swing (Fig. 3c and d).
Low element cost and high performance make the Zn–Fe RFB very attractive in terms of the total capital cost. To quantify the capital cost of the double-membrane RFB system, we adapted the cost model for all-V and Fe-V RFBs developed by the Pacific Northwest National Lab (PNNL).29 Based on the excellent PNNL model that (1) considered the geometry of cell, the configuration of stack, and auxiliary RFB components, (2) adopted the reliable pricing information, and (3) minimized the total capital cost, in this work we have expanded the model: (1) from a single-membrane configuration to a double-membrane configuration, and (2) from all-vanadium chemistry to various other redox chemistries. Note that the cost analysis was based on the small-scale experimental results. Although the model has already considered the performance losses during scaling up, the practical performance of the Zn–Fe RFB in a large scale may vary.
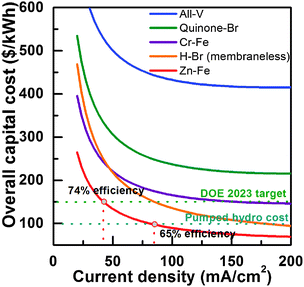
Fig. 4 shows the results of the total capital cost of a 1 MW/8 MW h system for Zn–Fe RFBs and a few most notable RFBs, including all-V,29 quinone-bromide,26 hydrogen-bromide (membraneless)30 and chromium-iron.18 Cost contributions and round-trip efficiency are also mapped over the range of current densities (Fig. S5, ESI†). At a current density of 40 mA cm−2, a total capital cost of $150 per kW h is projected (with 74% of overall energy efficiency at the system level), which meets the 2023 DOE's cost target ($150 per kW h). Increasing current density will result in an even lower overall capital cost but decreased system efficiency, for example, a current density of 80 mA cm−2 (65% system efficiency) will lower the total capital cost to $100 per kW h. It should be noted that the designed discharge duration is also an important factor determining the total capital cost of RFB systems. The system capital cost decreases with increasing discharge duration, and it is below $150 per kW h when the discharge duration is greater than 5 hours (Fig. S6, ESI†). In this work, we focus on engineering the middle electrolyte, leaving out the optimization of membranes and electrodes common to most RFB efforts. The system efficiency is expected to be improved by further reducing the cell resistance via adopting highly-conductive and selective membranes and engineering electrodes.
Our results demonstrate that the Zn–Fe RFB can deliver high power density with inexpensive materials, making it the one of the most cost-effective RFB systems. The Zn–Fe RFB is able to meet the $100 per kW h of the capital cost (with 65% system efficiency), although a significant amount of work is still needed such as long-term durability testing and scale-up to large cells and stacks before industrial implementation.
Acknowledgements
This work was funded by the US Department of Energy through ARPA-E Award (DE-AR0000346). We thank Vish Viswanathan of the PNNL for help and sharing details of the PNNL RFB's open-source cost model.Notes and references
- Z. G. Yang, J. L. Zhang, M. C. W. Kintner-Meyer, X. C. Lu, D. W. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577–3613 CrossRef CAS PubMed.
- J. Liu, J. G. Zhang, Z. G. Yang, J. P. Lemmon, C. Imhoff, G. L. Graff, L. Y. Li, J. Z. Hu, C. M. Wang, J. Xiao, G. Xia, V. V. Viswanathan, S. Baskaran, V. Sprenkle, X. L. Li, Y. Y. Shao and B. Schwenzer, Adv. Funct. Mater., 2013, 23, 929–946 CrossRef CAS PubMed.
- J. Rugolo and M. J. Aziz, Energy Environ. Sci., 2012, 5, 7151–7160 Search PubMed.
- U. Eberle, B. Muller and R. von Helmolt, Energy Environ. Sci., 2012, 5, 8780–8798 Search PubMed.
- H. Ibrahim, A. Ilinca and J. Perron, Renewable Sustainable Energy Rev., 2008, 12, 1221–1250 CrossRef CAS PubMed.
- U. Eberle, M. Felderhoff and F. Schuth, Angew. Chem., Int. Ed., 2009, 48, 6608–6630 CrossRef CAS PubMed.
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- P. Leung, X. H. Li, C. P. de Leon, L. Berlouis, C. T. J. Low and F. C. Walsh, RSC Adv., 2012, 2, 10125–10156 RSC.
- W. Wang, Q. T. Luo, B. Li, X. L. Wei, L. Y. Li and Z. G. Yang, Adv. Funct. Mater., 2013, 23, 970–986 CrossRef CAS PubMed.
- M. Skyllas-Kazacos, M. H. Chakrabarti, S. A. Hajimolana, F. S. Mjalli and M. Saleem, J. Electrochem. Soc., 2011, 158, R55–R79 CrossRef CAS PubMed.
- A. Z. Weber, M. M. Mench, J. P. Meyers, P. N. Ross, J. T. Gostick and Q. H. Liu, J. Appl. Electrochem., 2011, 41, 1137–1164 CrossRef CAS.
- C. P. de Leon, A. Frias-Ferrer, J. Gonzalez-Garcia, D. A. Szanto and F. C. Walsh, J. Power Sources, 2006, 160, 716–732 CrossRef PubMed.
- Grid Energy Storage, U.S. Department of Energy, 2013.
- R. F. Service, Science, 2014, 344, 352–354 CrossRef CAS PubMed.
- D. S. Aaron, Q. Liu, Z. Tang, G. M. Grim, A. B. Papandrew, A. Turhan, T. A. Zawodzinski and M. M. Mench, J. Power Sources, 2012, 206, 450–453 CrossRef CAS PubMed.
- N. H. Hagedorn, NASA redox storage system development project final report, National Aeronautics and Space Administration, Lewis Research Center, 1984 Search PubMed.
- J. Cheng, L. Zhang, Y. S. Yang, Y. H. Wen, G. P. Cao and X. D. Wang, Electrochem. Commun., 2007, 9, 2639–2642 CrossRef CAS PubMed.
- L. H. Thaller, 9th Intersociety Energy Conversion Engineering Conference Proceedings, 1974, 924–928.
- L. Zhang, J. Cheng, Y. S. Yang, Y. H. Wen, X. D. Wang and G. P. Cao, J. Power Sources, 2008, 179, 381–387 CrossRef CAS PubMed.
- M. C. Tucker, K. T. Cho and A. Z. Weber, J. Power Sources, 2014, 245, 691–697 CrossRef CAS PubMed.
- S. Gu, K. Gong, E. Z. Yan and Y. S. Yan, Energy Environ. Sci., 2014, 7, 2986–2998 CAS.
- R. Holze, Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology, Electrochemical Thermodynamics and Kinetics, Springer, 2007 Search PubMed.
- R. D. Patel, K.-C. Lang and I. F. Miller, Ind. Eng. Chem. Fundam., 1977, 16, 340–348 CAS.
- Y. Tanaka, J. Membr. Sci., 2003, 216, 149–164 CrossRef CAS.
- L. Zhang, H. Zhang, Q. Lai, X. Li and Y. Cheng, J. Power Sources, 2013, 227, 41–47 CrossRef CAS PubMed.
- B. Huskinson, M. P. Marshak, C. Suh, S. Er, M. R. Gerhardt, C. J. Galvin, X. Chen, A. Aspuru-Guzik, R. G. Gordon and M. J. Aziz, Nature, 2014, 505, 195–198 CrossRef CAS PubMed.
- M. L. Perry, R. M. Darling and R. Zaffou, Stationary and Large Scale Electrical Energy Storage 2, 2013, 53, 7–16 Search PubMed.
- K. T. Cho, P. Albertus, V. Battaglia, A. Kojic, V. Srinivasan and A. Z. Weber, Energy Sci. Technol., 2013, 1, 596–608 CrossRef PubMed.
- V. Viswanathan, A. Crawford, D. Stephenson, S. Kim, W. Wang, B. Li, G. Coffey, E. Thomsen, G. Graff, P. Balducci, M. Kintner-Meyer and V. Sprenkle, J. Power Sources, 2014, 247, 1040–1051 CrossRef CAS PubMed.
- W. A. Braff, M. Z. Bazant and C. R. Buie, Nat. Commun., 2013, 4 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ee02315g |
| This journal is © The Royal Society of Chemistry 2015 |