Anti-P2 structured Na0.5NbO2 and its negative strain effect†
Abstract
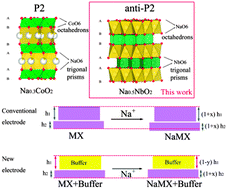
Layer-structured oxides are studied for their essential roles in various applications (e.g. high-energy batteries and superconductors) due to their distinctive physical structures and chemical properties. Most of the layered AxMO2 (A = alkali ions, M = transition metals) are composed of MO6 octahedra and various A coordination polyhedra such as octahedra (O), tetrahedra (T) or trigonal prisms (P). Herein, we report a new layered oxide material, anti-P2 Na0.5NbO2, which is composed of NbO6 trigonal prisms and NaO6 octahedra. Its lattice shrinks as sodium (Na) ions are intercalated in it and expands when the ions are deintercalated (a negative volume or strain effect). Analysis by X-ray absorption spectroscopy and density functional theory (DFT) calculations indicates that the negative volume effect is mainly a result of the enhanced interlayer (Na–O) interaction and the weakened Nb–Nb and Nb–O bonding in the O–Nb–O slab upon Na intercalation. Moreover, Na0.5NbO2 exhibits high structural stability, a long cycle life and prominent rate performance for Na-ion batteries. These distinctive features make Na0.5NbO2 an ideal “volume buffer” to compensate for positive-strain electrode materials. These findings will arouse great interest in anti-P2 layered oxides for materials science and applications, and enrich the understanding of novel negative-strain materials for energy storage either as excellent independent active electrode materials or as volume buffers for constructing long-life composite electrodes made of positive-strain materials.

 Please wait while we load your content...
Please wait while we load your content...