Open Access Article
Open Access ArticleStructure of the high voltage phase of layered P2-Na2/3−z[Mn1/2Fe1/2]O2 and the positive effect of Ni substitution on its stability†
Elahe
Talaie‡
a,
Victor
Duffort‡
a,
Hillary L.
Smith
b,
Brent
Fultz
b and
Linda F.
Nazar
*a
aDepartment of Chemistry, Waterloo Institute for Nanotechnology, University of Waterloo, 200 University Ave W, Waterloo, Ontario N2L 3G1, Canada. E-mail: lfnazar@uwaterloo.ca
bDepartment of Applied Physics and Materials Science, California Institute of Technology, 1200 E. California Blvd., Pasadena, CA, USA
First published on 3rd July 2015
Abstract
A combination of operando X-ray diffraction, pair distribution function (PDF) analysis coupled with electrochemical measurements and Mössbauer spectroscopy elucidates the nature of the phase transitions induced by insertion and extraction of sodium ions in P2-Na0.67[NiyMn0.5+yFe0.5−2y]O2 (y = 0, 0.10, 0.15). When phase transitions are avoided, the optimal cathode material – P2-Na0.67Fe0.2Mn0.65Ni0.15O2 – delivers 25% more energy than the unsubstituted material, sustaining high specific energy (350 Wh kg−1) at moderate rates and maintains 80% of the original energy density after 150 cycles – a significant improvement in performance vs. the unsubstituted analogue. The crystal structure of the high voltage phase is solved for the first time by X-ray PDF analysis of P2-Na0.67−zFe0.5Mn0.5O2 (where z ∼ 0.5), revealing that migration of the transition metals – particularly Fe3+ – into tetrahedral sites in the interlayer space occurs at high potential. This results in new short range order between two adjacent layers. Although the transition metal migration is reversible as proven by electrochemical performance, it induces a large disfavourable cell polarization. The deleterious high voltage transition is mitigated by substitution of Fe3+ by Mn4+/Ni2+, giving rise to better cycling performance. Moreover, as demonstrated by 57Fe Mössbauer spectroscopy, the much lower ratio of Fe4+O6 to Fe3+O6 observed systematically across the range of Ni content – compared to the values expected from a purely ionic model – suggests redox activity involves the O-2p orbitals owing to their overlap with the transition metal-3d orbitals.
Broader contextAs the critical need for an energy transition from fossil fuel to more sustainable sources becomes clearer, the need for large scale electrochemical energy storage arises. Li-ion batteries, which have revolutionized portable electronics and electrical vehicles in just over two decades, will likely fail to meet the cost requirements for such technology, directing new focus towards sodium batteries. Development of low cost electrode materials for sodium based systems is rapidly becoming a topic of high interest in the scientific community. These future generation systems based on oxide insertion hosts employing the Fe3+/Fe4+ redox couple offer high energy density approaching that of Li-ion materials, low environmental impact and low cost; making these materials highly desirable positive electrode candidates. However, commercial use is so far hindered by several challenges that include capacity fading. In the present work, we have employed P2-Nax[Fe0.5−2yMn0.5+yNiy]O2 layered oxides to study the phase transitions that impede the rate capability and capacity retention of these materials. Using in-depth structural and spectroscopic analysis, we unravel the nature of phenomena specific to the Fe3+/Fe4+ redox couple, highlighting the issues that will need to be addressed to improve the design of new iron containing positive electrodes for sodium batteries. This approach shows the way forward to diminish capacity fading by developing compositions with tailored substitution that minimize structural changes on cycling. |
Introduction
Sodium-ion batteries have generated considerable interest recently with respect to developing sustainable and low-cost electrical energy storage systems. The high abundance of sodium and competitive energy densities of Na insertion host materials make Na-ion batteries an especially promising alternative to Li-ion battery technology for large scale applications such as electrical grid storage. Layered transition metal oxides NaxMO2, 0 ≤ x ≤ 1 and M = Ti, V, Cr, Mn, Fe, Co or Ni and their various combinations,1–13 have been synthesized and their electrochemical properties investigated. Layered sodium transition metal oxides crystallize in several polymorphs dependent on their sodium content and synthesis conditions (temperature and partial pressure of oxygen). The transition metal ions reside within layers of edge sharing MO6 octahedra while the sodium ions are accommodated between the layers. The most common polymorphs for NaxMO2 are O3 and P2, according to a classification by Delmas et al.14 in which sodium ions occupy octahedral (O) or trigonal prismatic (P) sites, respectively; structural distortion is denoted by a prime (i.e., P′2). The number following the sodium coordination environment describes the number of MO2 layers in a unit cell. These polymorphs based on oxygen close packing differ in their oxygen arrays; namely ABCABC stacking for O3 and ABBA stacking for P2.Sodium layered oxides offer significant advantages over their lithium counterparts. The ionic radius difference between Na+ and the transition metal ions prevents cation mixing which is frequently observed in lithium metal oxides. Redox couples that are inactive vs. Li can be used in sodium cells, such as Cr3+/4+.4 Finally, the higher vacancy concentrations tolerated by sodium layered oxides offer larger capacities. However, the strong Na+/vacancy interactions result in multiple structural transitions upon cycling, considerably affecting the electrode ageing properties. For example, the sodium equivalent to the iconic LiCoO2 follows an O3, O′3, P′3, P3, P′3 path as the sodium content decreases.15 Two approaches can be used to limit the extent of this problem. Limiting the cutoff voltage to 4.1 V to avoid the P2–O2 transition in Nax[Mn2/3Ni1/3]O2 results in an increase in capacity retention from 64% to over 95% after 10 cycles.16 Chemical modification can also retard structural transitions, as observed in P2-Na0.80[Li0.12Ni0.22Mn0.66]O2 which maintains its structure up to 4.4 V and exhibits more than 95% of its initial capacity after 50 cycles.17
Following the initial report of the auspicious properties of P2-NaxFe0.5Mn0.5O218 that demonstrated the activity of the Fe3+/4+ redox couple in sodium cells, layered oxides Nax[Mn1−yFey]O2 based on low-cost and non-toxic Mn and Fe have received considerable interest.18–25 Their major drawbacks were identified as poor capacity retention due to structural transitions24 and air sensitivity leading to carbonate insertion that inhibits electrode performance.23 Nickel substitution seemed successful in addressing these points. P2-Nax[Fe0.5−yNiyMn0.5+y]O2 oxides show improved air stability, better capacity retention and increase in the average discharge potential.23,26,27 However, the structural transition at high voltage remains unexplained to date.
Here, we elucidate the nature of the structural evolution of P2-Na0.67[Mn0.5Fe0.5]O2 and P2-Na0.67[Mn0.65Ni0.15Fe0.2]O2 upon cycling, and directly correlate this to electrochemical performance. The structure of rigorously air-protected pristine compounds was solved using combined X-ray and neutron powder diffraction (XRPD and NPD). The phase diagram of the two compositions as a function of sodium content is determined using operando diffraction experiments, and the structure of the unknown high voltage phase is solved by X-ray pair distribution function (PDF) analysis and 57Fe Mössbauer spectroscopy for the first time. We demonstrate that the extended stability range of the P2 phase in the Ni substituted composition allows the storage of almost 25% more energy compared to the unsubstituted oxide when the cycling range is selected to prevent structural transitions.
Experimental
Synthesis
Na0.67[Mn0.5+yNiyFe0.5−2y]O2 phases with y = 0, 0.1, 0.15 were synthesized by a solid state method. Na2CO3 (EMD Millipore, ≥99.5%), Mn2O3 (Sigma-Aldrich, 99%), NiO (Sigma-Aldrich, 99.8%), and Fe2O3 (Sigma-Aldrich, ≥99%) powders were used as starting materials. For each composition, a mixture of a stoichiometric amount of precursors was pelletized and heated in air at 750 °C for 4 hours followed by a final step at 900 °C for 6 hours. Due to the sensitivity of these materials under ambient atmosphere, samples were then subjected to an additional heat treatment under vacuum at 600 °C and transferred directly to an argon filled glovebox (MBraun, O2 and H2O < 0.1 ppm) as described in our previous study.23Na0.14Fe0.5Mn0.5O2 and Na0.1Fe0.2Mn0.65Ni0.15O2 were obtained by chemical oxidation of the corresponding air-protected P2 pristine materials in a 2 fold excess of NO2BF4 in acetonitrile. In an argon filled glovebox, 400 mg of sample were dispersed in 12 mL of acetonitrile (HPLC grade, Sigma Aldrich), and 688 mg of NO2BF4 (98%, Sigma-Aldrich) were added to the suspension. After 24 h under magnetic stirring, the solution was filtered and washed with 2 × 20 mL of acetonitrile.
Materials characterization
The X-ray powder diffraction (XRPD) data were recorded using Cu-Kα radiation on a PANalytical Empyrean diffractometer equipped with a PIXcel bidimensional detector. The samples were loaded in glass capillaries (Ø = 0.3 mm) in an argon filled glovebox and sealed in order to protect them from air exposure. Rietveld quality patterns were collected in Debye–Scherrer geometry using a parabolic X-ray mirror in the incident beam. Neutron powder diffraction (NPD) experiments were recorded on the time of flight spectrometer POWGEN at the Spallation Neutron Source at Oak Ridge National Laboratory. The powders were loaded in 8 mm vanadium sample cans in an argon filled glovebox and sealed with a copper gasket. The crystal structures of air protected samples were refined using combined Rietveld refinement of the NPD and XRPD datasets within the FullProf28 software suite.Operando diffraction experiments were conducted in a homemade cell, mounted on a PANalytical Empyrean diffractometer. The diffraction patterns were collected in Bragg–Brentano geometry using Cu-Kα radiation and a PIXcel detector with a Ni Kβ filter. During the acquisition, the cells were cycled at a constant current rate of 13 mA g−1 (C/20) with a pattern collection time of 30 min, i.e. Δx ≈ 0.02 in NaxMO2. The evolution of the lattice parameters during the operando diffraction experiments was determined by means of Le Bail fitting.29
Reciprocal space data for pair distribution function (PDF) analysis were obtained using a PANalytical Empyrean outfitted with Ag-Kα radiation, a Rh Kβ filter and a NaI scintillation point detector. In order to maximize the diffracted intensity, a divergent beam was used in combination with a 1 mm glass capillary (filled in an argon filled glovebox) and the receiving slits were set to 2 mm. The total collection time was 48 h per sample. The data reduction was performed using PDFGetX3 software30 and real space data was fitted using PDFgui.31 The damping factor associated with this instrumental configuration was calibrated by refining the structure of a silicon sample.
Mössbauer spectrometry was performed with a conventional constant acceleration system with a radiation source of 57Co in a Rh matrix. Velocity and isomer shift calibrations were performed with reference to a room-temperature α-Fe spectrum. The spectrometer linewidth obtained from the calibration is 0.31 mm s−1. This was used as the linewidth for all spectral components resulting from fitting with Lorentzian doublets.
Energy dispersive X-ray spectroscopy (EDS) (EDAX) and inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Prodigy high dispersion ICP, Teledyne Leeman Labs) were used to confirm the molar ratio of transition metals in each composition. The sodium content was determined to be 65% of transition metals in all three compositions by ICP-AES.
Electrochemistry
The electrochemical performance of the positive electrode materials was measured in 2325 coin cells using Na metal as the counterelectrode. Electrode fabrication was performed inside a glovebox under argon (O2 and H2O < 0.1 ppm) to avoid air contamination. Each active material was mixed with 10 wt% carbon black and 10 wt% polyvinylidene fluoride (PVDF) (Aldrich average Mw ∼ 534![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) suspended in N-methyl-2-pyrrolidinone (NMP) (Sigma-Aldrich, 99.5%) and cast on aluminum foil with a typical loading of 7–9 mg cm−2. Electrodes were dried in a vacuum oven at 90 °C overnight. The electrolyte was 1 M NaClO4 (Alfa Aesar, ≥98%) in propylene carbonate (BASF, 99.98%) with 2 vol% of 4-fluoro-1,3-dioxolan-2-one (FEC) (Sigma-Aldrich, 99%). The electrodes were separated with glass fiber separators (Merck Millipore). Galvanostatic cycling was performed using a MPG-2 (bio-logic) cycler at 24 °C with a current density of 13 mA g−1 (C/20) or 26 mA g−1 (C/10 rate).
000) suspended in N-methyl-2-pyrrolidinone (NMP) (Sigma-Aldrich, 99.5%) and cast on aluminum foil with a typical loading of 7–9 mg cm−2. Electrodes were dried in a vacuum oven at 90 °C overnight. The electrolyte was 1 M NaClO4 (Alfa Aesar, ≥98%) in propylene carbonate (BASF, 99.98%) with 2 vol% of 4-fluoro-1,3-dioxolan-2-one (FEC) (Sigma-Aldrich, 99%). The electrodes were separated with glass fiber separators (Merck Millipore). Galvanostatic cycling was performed using a MPG-2 (bio-logic) cycler at 24 °C with a current density of 13 mA g−1 (C/20) or 26 mA g−1 (C/10 rate).
Results and discussion
Structural and electrochemical overview
The crystal structures of rigorously air protected Na0.67[Mn0.6Ni0.1Fe0.3]O2 and Na0.67[Mn0.65Ni0.15Fe0.2]O2 were examined by combined Rietveld refinement of X-ray and neutron time of flight diffraction data (Fig. 1 and Table S1, ESI†). Both compositions crystallize in an undistorted P2 structure (P63/mmc) (Fig. 2; Table S1, ESI†), similar to Na0.67[Mn0.5Fe0.5]O2.23 The substitution of Fe3+ by Mn4+/Ni2+ only impacts the cell parameters, which follow the evolution of the cation diameter, i.e. the cell parameters (a = 2.9429(1) Å, c = 11.1881(3) Å) for Na0.67[Mn0.5Fe0.5]O2 decrease as Fe3+ ions (radius 0.65 Å) are partially replaced by Mn4+/Ni2+ ions (radii of 0.53 Å, and 0.69 Å, respectively).32In the P2 structure, sodium ions are distributed over two prismatic sites – Nae and Naf – that share edges and faces, respectively, with the MO6 octahedra (Fig. 2). The occupancy of the Nae site is about twice that of Naf, showing that the former site is more stable than the latter due to longer Na–M distances. Configurational disorder in the sodium layer is frequently observed in the P2 structure and is thought to underlie the good mobility of sodium ions in the host structure.23,33,34 Similar to those studies, this disorder is modelled here by splitting the Nae(2d) and Naf(2b) sites into lower symmetry 6 h Wyckoff positions. The combined refinement of the neutron and X-ray datasets confirms that the Ni, Fe, and Mn cations are statistically distributed over the transition metal site. This prevents the long range ordering of the sodium ion vacancies which are very common in sodium layered materials. These rearrangements trigger structural transitions that give rise to potential step features on the charge/discharge profiles in P2-Na2/3VO2,35 P2-Na2/3CoO2,36 and P2-Na2/3[Mn2/3Ni2/3]O2,37 resulting in poor cyclability and limited rate capability. In contrast, smooth voltage profiles and perfectly superimposable electrochemical profiles on first and second charges of Na0.67[Mn0.5+yNiyFe0.5−2y]O2 (y = 0, 0.10, 0.15) imply facile and reversible sodium (de)intercalation from all three compositions (Fig. 3) in agreement with previously published results.23
 | ||
| Fig. 3 First two galvanostatic charge/discharge cycles and specific capacity of P2-Na0.67[Mn0.5+yNiyFe0.5−2y]O2 (y = 0, 0.1, 0.15) over 25 cycles at 13 mAh g−1 (C/20). | ||
The initial capacity for the three compositions is about 200 mAh g−1. They exhibit similar capacity fading, which is slightly exaggerated in the first five cycles. The electrochemical cycling curves of Na0.67[Mn0.5+yNiyFe0.5−2y]O2 (y = 0, 0.10, 0.15) can be divided in two regions based on the magnitude of the cell polarization. Very small polarization is observed in the low voltage region. Greater polarization is noted in the high voltage range that clearly diminishes as the nickel content increases. The specific energy marginally increases (∼3%) with the nickel content, from 545 Wh kg−1 for y = 0 to 561 Wh kg−1 for y = 0.15.
The structural modifications induced by nickel substitution are minor, and thus it seems appropriate to conclude that the improved performance of the Ni-substituted materials arise from the change in charge compensation mechanism from the Fe3+/4+ couple to Ni2+/4+. Since Fe4+ has a d4 electronic configuration, it is expected to show significant Jahn–Teller stabilization, possibly explaining the large polarization observed at high voltage. In order to determine whether high polarization regions originate intrinsically from the Fe3+/4+ couple or from structural features we carried out a comparative study on the structural evolution upon cycling of P2-Nax[Fe0.5Mn0.5]O2 and P2-Nax[Fe0.2Mn0.65Ni0.15]O2. The results below disprove Jahn–Teller effects, showing instead that a mechanism involving transition metal migration is at hand.
Operando XRD study of P2-NaxFe0.5Mn0.5O2 and P2-NaxFe0.2Mn0.65Ni0.15O2 upon cycling
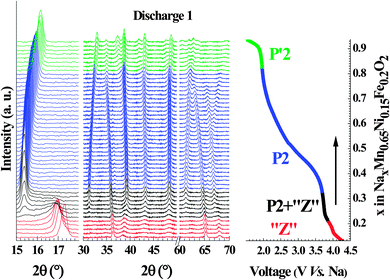
Operando monitoring of the structural evolution upon cycling shows that both title compositions undergo transitions to yield the same three phases. The evolution of the diffraction patterns is shown for the first discharge of Nax[Fe0.2Mn0.65Ni0.15]O2 (Fig. 4) and the phase diagram over a complete cycle for both compositions (x = 0, 0.15) is displayed in Fig. 5. In agreement with previously published work on P2-Na0.67[Fe0.5Mn0.5]O2,18,24 the initial P2 structure is preserved over a wide range of stoichiometry. It converts to a distorted P′2 phase at low voltage and to an uncharacterized high potential phase, labeled “Z”24 or OP4.18 It will be referred here as the “Z” phase since the OP4 structure does not match our structural data (see below). All the observed transitions are fully reversible; the high and low voltage phases convert back to the pristine P2 phase as shown by the perfect overlap of the evolution of the cell parameters as a function of the sodium content during the first and second charge (Fig. S1, ESI†). | ||
| Fig. 5 Phase evolution within: (a) Na0.67[Mn0.5Fe0.5]O2 and (b) Na0.67[Mn0.65Ni0.15Fe0.2]O2 as a function of the sodium content during first cycle. The sign * shows the starting point of cycling. | ||
![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) m2 space group, compatible with the OP4 structure and consisting of a mixture of O and P type of stacking. As we show in the next section, this phase is not an OP4 type.
m2 space group, compatible with the OP4 structure and consisting of a mixture of O and P type of stacking. As we show in the next section, this phase is not an OP4 type.
The sharp contraction of the interlayer spacing in moving from the P2 to the Z phase (Fig. 6b and d) can be related to a transition from a P to O stacking type, due to the fact that the oxygen atoms of two different transition metal layers are not aligned along the c-axis in O-type structures. However, the evolution of the interlayer distance is opposite to what is generally observed upon sodium de-intercalation in O and P structures.15,17,36,39–41 This means that, contrary to sodium extraction, the mechanism driving this transformation implies an increasingly strong inter-layer interaction. This transition occurs at different sodium content in the case of NaxFe0.5Mn0.5O2 (x = 0.35) and NaxFe0.2Mn0.65Ni0.15O2 (x = 0.29). If the structural reorganization was driven by Na+/vacancies ordering as usually observed in sodium layer oxides, the transition would be expected to occur at the same sodium concentration.
Study of the transition from the P2 to the high voltage phase Z phase and its structure
The stoichiometry of a sample oxidized by an excess of NO2BF4 in acetonitrile, Na0.14Fe0.5Mn0.5O2, was determined by ICP-AES. The material showed a very similar diffraction pattern to that observed for samples in the high voltage region of the operando experiment (Fig. S2, ESI†). The in-plane cell parameter and interlayer spacing, 2.89 Å and 5.22 Å respectively, correspond to those for the operando composition Na0.22Fe0.5Mn0.5O2. Even by using a large excess of oxidant and successive oxidation steps, it was not possible to extract more sodium despite the high potential of the NO2+/NO2 couple (about 4.7 V vs. Na). The larger peak width of the electrochemically oxidized sample, as well as the appearance of small additional signals shows – as expected – that chemical oxidation produces a less homogeneous sample than the slower galvanostatic oxidation.
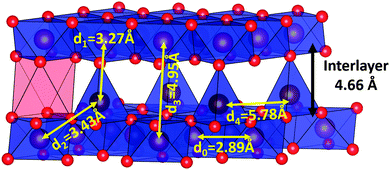
The intensity of the second peak (Fig. 7, yellow shading) is largely dominated by the first neighbor M–M correlations (in-plane MOh–MOh) which are at 2.94 Å in the pristine material. As expected, this distance decreases slightly to 2.89 Å when the sample is oxidized, following the evolution of the in-plane cell parameter. The decrease in intensity of this peak (associated with the growth of a new signal at 3.35 Å) is direct evidence of modification of the hexagonal lattice of the transition metal element on oxidation. Since in-plane distortion due to a cooperative Jahn–Teller effect (as occurs in the low voltage orthorhombic phase) can be ruled out, differentiation of the M–M distance is attributed to the atomic displacement of transition metal atoms perpendicular to the close packed hexagonal lattice, i.e. along c. These shifted species will interact with the adjacent layers. This explains why the interlayer distance shrinks considerably as sodium continues to be extracted (Fig. 6b, red markers), while the opposite trend is usually observed (i.e. where sodium extraction triggers expansion of the interlayer distance).15,17,36,39–41 The mechanism proposed here is different from the cation mixing observed in lithium layered oxides. Sodium layered oxides are generally thought to be resistant to migration of the transition metal into the alkali layer, owing to the different ionic radii of Na+ and the transition metal ions. However, when the concentration of sodium vacancies is high, the transition metal ion migrates to an essentially empty layer. Migration of Li+ from the transition metal layer to the sodium layer has been demonstrated by NMR measurements in P2-Nax[Li0.12Ni0.22Mn0.66]O2.17 Irreversible transition metal migration into the sodium layer was also proposed in O3-NaxCrO241 and O3-NaxFeO211 based on the decrease of the interlayer distance and evolution of the pre-peak in X-ray absorption measurements. We show here, to the best of our knowledge, the first structural evidence of this mechanism.
The next group of peaks between 4 Å and 5.5 Å, due to in-plane M–M and M–O interactions (Fig. 7, lavender shading), remain similar in both pristine and oxidized samples, showing the overall conservation of the MO2 layer. For distances larger than 6 Å, interlayer interactions start to be visible in the PDF profile of the pristine P2-Na0.67Fe0.5Mn0.5O2 sample. The disappearance of the interlayer M–M distances from the radial distribution curve of the chemically oxidized sample (6.32 Å, 8.14 Å, 9.57 Å; shown in green shading in Fig. 7) is particularly indicative of the destruction of the P2-type stacking. The fact that these very intense peaks cannot be found anywhere in the PDF curve of the oxidized sample is evidence that the long range ordering between the layers is completely destroyed. In order to model the PDF, supercells containing 20 metal transition layers were generated in the P1 space group and each MO2 layer was randomly shifted in the ab plane. Owing to the low concentration of sodium and its weak scattering factor, it was not taken into account. The number of M cations moving out of plane was probed by gradually increasing the size of the supercell in the ab plane. Initially, the layers were evenly spaced along the c direction based on the assumption that the first diffraction peak observed at ∼5.22 Å was indeed a (00l) peak. Partial organization based on the P2, O2, P3 and O3 stacking schemes were tested but failed to explain the recorded PDF curve.
The good agreement between measured and calculated PDF curves up to 30 Å shows that despite the poor diffraction pattern of the oxidized phase, the coherence length in the in-plane direction is large. The conservation of the primary structure of the layers clearly plays a major role in the good reversibility of the transition.
The smaller inter-bilayer distance observed for the nickel substituted compound indicates that the thickness of the bilayer is controlled by the nature of the tetrahedral species rather than their concentration. The MO4 site in “Z”-Na0.1Fe0.2Mn0.65Ni0.15O2 is smaller (M–O ∼ 1.8 Å) than in “Z”-Na0.14Fe0.5Mn0.5O2 (M–O ∼ 1.9 Å), suggesting that the average diameter of the tetrahedrally coordinated cation is smaller in the nickel substituted oxide (see below).
| Component | IS (mm s−1) | QS (mm s−1) | area (%) | ||
|---|---|---|---|---|---|
| Ni = 0.15 | Pristine | Fe3+O6 | 0.24 | 0.72 | 100.0 |
| Charged | Fe3+O6 | 0.25 | 0.71 | 84.2 | |
| Fe4+O6 | 0.00 | 0.70 | 15.8 | ||
| Ni = 0.10 | Pristine | Fe3+O6 | 0.24 | 0.73 | 100.0 |
| Charged | Fe3+O6 | 0.26 | 0.79 | 69.5 | |
| Fe3+O4 | 0.17 | 1.53 | 6.5 | ||
| Fe4+O6 | 0.00 | 0.69 | 24.0 | ||
| Ni = 0.00 | Pristine | Fe3+O6 | 0.25 | 0.75 | 100.0 |
| Charged | Fe3+O6 | 0.28 | 0.76 | 50.9 | |
| Fe3+O4 | 0.17 | 1.51 | 16.7 | ||
| Fe4+O6 | 0.00 | 0.69 | 32.5 | ||
The spectra of the charged materials (x ≈ 0.15, Fig. 10d–f) were fitted using three components (Table 1). The main component arises from Fe3+O6 in an octahedral environment and has a nearly identical isomer shift and quadrupolar splitting values compared to its pristine counterpart. The second component with an isomer shift of 0.17 mm s−1 and a large quadrupolar splitting of ∼1.5 mm s−1 is assigned to Fe3+O4 (tetrahedral coordination) owing to the non-centrosymmetry of its ligand environment. The third component with an isomer shift close to zero and a quadrupolar splitting of ∼0.7 mm s−1 is consistent with Fe4+O6 in sodium layered oxides.44–47
We note that the refined ratio of Fe4+O6 (Table 1) is systematically lower than what is expected for Na0.15[Mn0.5+yNiyFe0.5−2y]O2 according to a simple, purely ionic model; i.e. 70%, 50% and 25% respectively for y = 0, 0.1, 0.15. This large discrepancy shows that the high voltage charge compensation mechanism is more complex than the straightforward oxidation of Fe3+ to Fe4+. Indeed, X-ray absorption measurements (XAS) of P2-Nax[Mn0.5Fe0.5]O2 have showed that in contrast to the Mn K-edge, the energy of the Fe K-edge transition was essentially unaffected by sodium extraction,18 suggesting that the electronic configuration of iron remains similar throughout the redox process.
It was recently proposed that ligand-to-metal charge transfer (LMCT) plays an important role in the electrochemical properties of sodium layered oxides.44 It is particularly interesting to note that in perovskite structures containing Fe4+ such as CaFeO3 and SrFeO3, the LMCT energy was found to be null or negative.48–50 The Fe(IV) oxidation state can be stabilized by transferring electrons from the oxide framework to the metal centers. This explains why the perovskites do not exhibit Jahn–Teller distortion, and accounts for the very small shift observed in the XAS spectra of non-stoichiometric AFeO3−δ with A = Sr, Ca.50 We propose that in the case of Na0.15[Mn0.5+yNiyFe0.5−2y]O2 a similar mechanism occurs; however in contrast to the perovskites that exhibit metallic behavior, the electron hopping rate is sufficiently low in the case of the layered oxides to exhibit localized Fe3+ states by Mössbauer spectroscopy.
The charge transfer from the anionic framework to some of the iron atoms weakens the chemical bonding of the surrounding metal centers. The weakly bonded atoms are therefore more prone to the migration phenomena previously identified by PDF. Based on the intensity of the tetrahedral signal in Na0.15Fe0.5Mn0.5O2, we see that roughly 2/3 of the transition metal atoms in the tetrahedral sites are Fe3+, indicating preferential migration of iron. The fact that both iron and manganese ions migrate is further supported by previously reported XAS measurements18 which show the appearance of pre-peaks characteristic of non-centrosymmetric coordination polyhedra in both the Mn and Fe spectra, when the sample is charged from 3.8 V to 4.5 V vs. Na.
As the nickel content increases, the amount of tetrahedrally coordinated iron decreases from 16.6% in Na0.15Fe0.5Mn0.5O2 to 6.5% in Na0.15Fe0.3Mn0.6Ni0.1O2. The signal was too small to be accurately refined in Na0.15Fe0.2Mn0.65Ni0.15O2. This is in agreement with the PDF results obtained for this phase showing that the concentration of tetrahedral species is very low. Moreover, the smaller MO4 (M–O ∼1.8 Å) site suggests that a cation smaller than Fe3+ migrates into this site. Tetravalent manganese is a likely candidate since its migration is observed in the non-substituted sample and the expected bond length for tetrahedral Mn4+ is 1.77 Å. Fe4+ and Ni4+ are usually not encountered in tetrahedral coordination but cannot be completely ruled out based on the data presented here.
Upon substitution of Fe by Mn/Ni, the lower concentration of Fe4+ implies that fewer atoms are impacted by the LMCT that stabilizes this valence state. Therefore the number of transition metal ions susceptible to migrate to the interlayer space is smaller, delaying the P2 to “Z” phase transition. This is consistent with the lower overpotentials observed in the electrochemical profiles of the nickel substituted oxides (Fig. 3); the lower number of MO4 tetrahedra binding the bi-layers together implies a better reversibility of the transition. This has significant implications for the electrochemistry, as discussed below.
Electrochemistry: effect of structural transitions on cycling
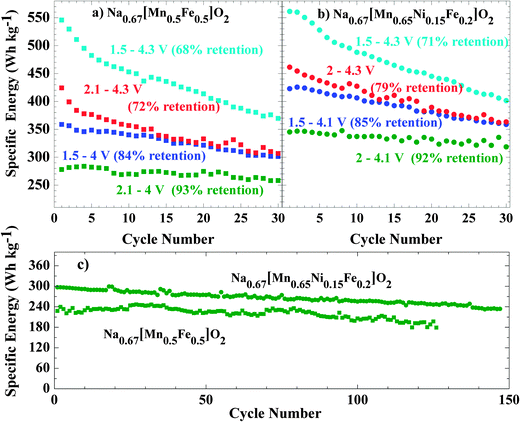
To investigate the effect of phase transitions on the electrochemical performance of Nax[Mn0.5Fe0.5]O2 and Nax[Mn0.65Ni0.15Fe0.2]O2, these materials were cycled in four different voltage ranges allowing either: (i) both structural transitions to occur, (ii) and (iii) only one of the two, (iv) none at all (Fig. 11). Based on the operando study (Fig. 5), we determined that P2-Nax[Mn0.5Fe0.5]O2 is stable within the 4–2.1 V window, whereas the Ni substituted composition exhibits a larger stability window of 4.1–2 V. Preventing the formation of the orthorhombic P′2 phase by changing the low cutoff voltage does not induce significant change in the cycling profile of either composition. The observed polarization can then directly be related to intrinsic properties of the P2 phase such as the sodium mobility.
 | ||
| Fig. 11 The galvanostatic charge/discharge profile of Nax[Mn0.5Fe0.5]O2 and Nax[Mn0.65Ni0.15Fe0.2]O2 cycled at C/20 in different voltage windows. | ||
In contrast, when the high cutoff voltage is set to prevent formation of the bilayer phase, the cell polarization at high voltage drastically decreases. This shows that the polarization at high potential is solely due to the energy required to convert the bilayer structure of the Z phase to the original P2 structure. A small residual polarization is observed around the Na0.5Fe0.5Mn0.5O2 stoichiometry, i.e. around the potential jump, and was previously attributed to the decrease in electronic conductivity due to the absence of transition metals with a mixed valence state.24 The absence of a similar jump in the NaxMn0.65Ni0.15Fe0.2O2 shows that this composition remains electronically conductive over the whole range of sodium concentration. The oxidation of nickel and iron overlap, explaining why no potential jump is observed.
Limiting the cycling range is an efficient way to greatly increase the life span of sodium batteries based on P2-Nax[Mn0.5+yNiyFe0.5−2y]O2 electrodes benefitted by Ni substitution. Cycling in the 4.3–1.5 V window results in only a 3% energy density gain for Nax[Mn0.65Ni0.15Fe0.2]O2 compared to NaxFe0.5Mn0.5O2, (561 Wh kg−1 on the first discharge compared to 545 Wh kg−1 for the latter). However reducing the voltage window in order to maintain the P2 structure increases the energy stored by the nickel containing sample by 25% (345 Wh kg−1vs. 278 Wh kg−1 for the non-substituted electrode).
Conclusions
We present here the phases of P2-NaxFe0.2Mn0.65Ni0.15O2 as a function of the sodium content. The sodium (de)insertion mechanism is similar to that of the parent oxide P2-NaxFe0.5Mn0.5O2. The pristine P2 phase converts at low voltage to a P′2 orthorhombic phase due to a cooperative Jahn–Teller effect that stabilizes the Mn3+ cations. At high voltage, the migration of the transition metal into a tetrahedral coordination environment perturbs the long range stacking order of the MO2 layers. The structure of this semi-crystalline phase, solved for the first time using X-ray PDF analysis, shows that the migration of the transition metal in the interlayer space induces the formation of short range order between two adjacent layers with the O2 stacking scheme. In contrast to other oxides for which this mechanism was proposed – O3-NaxCrO241 and O3-NaxFeO211 – the structural transformation in P2-Nax[Fe0.5−2yMn0.5+yNiy]O2 is fully reversible, despite the significant polarization that results. This difference between the O3 and P2 structures is most likely due to the fact that O2 stacking is not made accessible by translation of the MO2 layers in an O3 structure. In this respect, P2 based structures are better suited for the design of high voltage positive electrode for sodium ion batteries.The impact of both structural transitions on the cycling properties of the electrodes was investigated by adjusting the cutoff voltage. As expected, the high voltage structural transition has a much more significant impact due to the reorganization of the MO2 layer stacking scheme. If both transitions are avoided, 80% specific energy is maintained after 150 cycles with non-optimized electrodes, representing a significant improvement vs. ∼70% after 30 cycles in the full electrochemical window. In other words, cycling is stabilized at the expense of capacity. Substitution of Fe3+ by Mn4+/Ni2+ decreases the concentration of species at the origin of the high voltage transition, effectively delaying its occurrence. This additional capacity allows P2-NaxFe0.2Mn0.65Ni0.15O2 to deliver 25% more energy than the unsubstituted oxide when it is cycled in a voltage range that inhibits any structural transition.
The observations reported show that the Fe3+/4+ redox couple is well suited to the design of high voltage positive electrodes for sodium ion batteries, provided that transition metal migration is prevented. Further studies are needed to fully understand the role of LMCT in these oxides and how to employ this effect to prevent cation migration. Mixing iron with larger cations should help increase the size of the MO6 octahedra, hence inhibiting population of the tetrahedral site. Similarly, substituting Mn3+ by a higher voltage non-Jahn–Teller active cation should also greatly improve the material performance, bringing sodium ion batteries closer to market application.
Acknowledgements
We thank the Natural Sciences and Engineering Council of Canada for generous financial support of this work through their Discovery and Canada Research Chair programs, and BASF SE for ongoing support through the research network in Electrochemistry and Batteries. Mössbauer work, performed at Caltech, was supported as part of EFree, an Energy Frontier Research Center, under Award No. DE-SG0001057. Experiments performed at ORNL’s Spallation Neutron Source by UWaterloo were sponsored by the Scientific User Facilities Division, Office of Basic Energy Sciences, U.S. Department of Energy. We warmly thank A. Huq and M. Kirkham at the ORNL for their invaluable help during measurements on the POWGEN diffractometer, and Hiruy Haile for his assistance in the conception and realization of the operando cell. We also acknowledge M. Gateshki and M. Sommariva from PANalytical for their help with the PDF experimental setup.References
- J. Billaud, G. Singh, R. A. Armstrong, E. Gonzalo, V. Roddatis, M. Armand, T. Rojo and P. G. Bruce, Energy Environ. Sci., 2014, 7, 1387 CAS.
- A. Maazaz, C. Delmas and P. Hagenmuller, J. Inclusion Phenom., 1983, 1, 45–51 CrossRef CAS.
- D. Hamani, M. Ati, J.-M. Tarascon and P. Rozier, Electrochem. Commun., 2011, 13, 938–941 CrossRef CAS PubMed.
- S. Komaba, C. Takei, T. Nakayama, A. Ogata and N. Yabuuchi, Electrochem. Commun., 2010, 12, 355–358 CrossRef CAS PubMed.
- A. Mendiboure, C. Delmas and P. Hagenmuller, J. Solid State Chem., 1985, 57, 323–331 CrossRef CAS.
- S. Okada, Y. Takahashi, T. Kiyabu, T. Doi, J. Yamaki and T. Nishida, ESC Meet. Abstr., 2006, 201 Search PubMed.
- C. Delmas, J.-J. Braconnier, C. Fouassier and P. Hagenmuller, Solid State Ionics, 1981, 3, 165–169 CrossRef.
- S. Miyazaki, S. Kikkawa and M. Koizumi, Synth. Met., 1983, 6, 211–217 CrossRef CAS.
- M. H. Han, E. Gonzalo, G. Singh and T. Rojo, Energy Environ. Sci., 2015, 8, 81–102 CAS.
- J. Billaud, R. J. Clément, A. R. Armstrong, J. Canales-Vázquez, P. Rozier, C. P. Grey and P. G. Bruce, J. Am. Chem. Soc., 2014, 136, 17243–17248 CrossRef CAS PubMed.
- N. Yabuuchi and S. Komaba, Sci. Technol. Adv. Mater., 2014, 15, 043501 CrossRef.
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Chem. Rev., 2014, 114, 11636–11682 CrossRef CAS PubMed.
- D. Kundu, E. Talaie, V. Duffort and L. F. Nazar, Angew. Chem., 2015, 54, 3431–3448 CrossRef CAS PubMed.
- C. Delmas, C. Fouassier and P. Hagenmuller, Physica B+C, 1980, 99, 81–85 CrossRef CAS.
- Y. Lei, X. Li, L. Liu and G. Ceder, Chem. Mater., 2014, 26, 5288–5296 CrossRef CAS.
- D. H. Lee, J. Xu and Y. S. Meng, Phys. Chem. Chem. Phys., 2013, 15, 3304–3312 RSC.
- J. Xu, D. H. Lee, R. J. Clément, X. Yu, M. Leskes, A. J. Pell, G. Pintacuda, X.-Q. Yang, C. P. Grey and Y. S. Meng, Chem. Mater., 2014, 26, 1260–1269 CrossRef CAS.
- N. Yabuuchi, M. Kajiyama, J. Iwatate, H. Nishikawa, S. Hitomi, R. Okuyama, R. Usui, Y. Yamada and S. Komaba, Nat. Mater., 2012, 11, 512–517 CrossRef CAS PubMed.
- B. Mortemard de Boisse, D. Carlier, M. Guignard and C. Delmas, J. Electrochem. Soc., 2013, 160, A569–A574 CrossRef CAS PubMed.
- J. S. Thorne, R. A. Dunlap and M. N. Obrovac, J. Electrochem. Soc., 2013, 160, A361–A367 CrossRef CAS PubMed.
- J. Xu, S. L. Chou, J. L. Wang, H. K. Liu and S. X. Dou, ChemElectroChem, 2014, 1, 371–374 CrossRef CAS PubMed.
- S. Kalluri, K. Hau Seng, W. Kong Pang, Z. Guo, Z. Chen, H.-K. Liu and S. X. Dou, ACS Appl. Mater. Interfaces, 2014, 6, 8953–8958 CAS.
- V. Duffort, E. Talaie, R. Black and L. F. Nazar, Chem. Mater., 2015, 27, 2515–2524 CrossRef CAS.
- B. Mortemard de Boisse, D. Carlier, M. Guignard, L. Bourgeois and C. Delmas, Inorg. Chem., 2014, 53, 11197–11205 CrossRef CAS PubMed.
- E. Gonzalo, M. Han, J. L. del Amo, B. Acebedo, M. Casas-Cabanas and T. Rojo, J. Mater. Chem. A, 2014, 2, 18523–18530 CAS.
- D. Yuan, X. Hu, J. Qian, F. Pei, F. Wu, R. Mao, X. Ai, H. Yang and Y. Cao, Electrochim. Acta, 2014, 116, 300–305 CrossRef CAS PubMed.
- I. Hasa, D. Buchholz, S. Passerini, B. Scrosati and J. Hassoun, Adv. Energy Mater., 2014, 4, 1400083 Search PubMed.
- J. Rodríguez-Carvajal, Phys. B, 1993, 192, 55–69 CrossRef.
- A. Le Bail, Powder Diffr., 2005, 20, 316–326 CrossRef CAS.
- P. Juhas, T. Davis, C. L. Farrow and S. J. Billinge, J. Appl. Crystallogr., 2013, 46, 560–566 CrossRef CAS.
- C. Farrow, P. Juhas, J. Liu, D. Bryndin, E. Božin, J. Bloch, T. Proffen and S. Billinge, J. Phys.: Condens. Matter, 2007, 19, 335219 CrossRef CAS PubMed.
- R. D. Shannon, Acta Crystallogr., 1976, 32, 751–767 CrossRef.
- Y. Ono, N. Kato, Y. Ishii, Y. Miyazaki and T. Kajitani, T., J. Jpn. Soc. Powder Powder Metall., 2003, 50, 469–474 CrossRef CAS.
- N. Yabuuchi, R. Hara, M. Kajiyama, K. Kubota, T. Ishigaki, A. Hoshikawa and S. Komaba, Adv. Energy Mater., 2014, 4, 1301453 Search PubMed.
- M. Guignard, C. Didier, J. Darriet, P. Bordet, E. Elkaïm and C. Delmas, Nat. Mater., 2013, 12, 74–80 CrossRef CAS PubMed.
- R. Berthelot, D. Carlier and C. Delmas, Nat. Mater., 2011, 10, 74–80 CrossRef CAS PubMed.
- Z. Lu and J. R. Dahn, J. Electrochem. Soc., 2001, 148, A1225–A1229 CrossRef CAS PubMed.
- J.-P. Parant, R. Olazcuaga, M. Devalette, C. Fouassier and P. Hagenmuller, Solid State Chem., 1971, 3, 1–11 CrossRef CAS.
- M. Sathiya, K. Hemalatha, K. Ramesha, J. M. Tarascon and A. S. Prakash, Chem. Mater., 2012, 24, 1846–1853 CrossRef CAS.
- Y.-N. Zhou, J.-J. Ding, K.-W. Nam, X. Yu, S.-M. Bak, E. Hu, J. Liu, J. Bai, H. Li and Z.-W. Fu, J. Mater. Chem. A, 2013, 1, 11130–11134 CAS.
- K. Kubota, I. Ikeuchi, T. Nakayama, C. Takei, N. Yabuuchi, H. Shiiba, M. Nakayama and S. Komaba, J. Phys. Chem. C, 2015, 119, 166–175 CAS.
- Y.-Y. Hu, Z. Liu, K.-W. Nam, O. J. Borkiewicz, J. Cheng, X. Hua, M. T. Dunstan, X. Yu, K. M. Wiaderek, L.-S. Du, K. W. Chapman, P. J. Chupas, X.-Q. Yang and C. P. Grey, Nat. Mater., 2013, 12, 1130–1136 CrossRef CAS PubMed.
- D. Zeng, J. Cabana, J. Bréger, W.-S. Yoon and C. P. Grey, Chem. Mater., 2007, 19, 6277–6289 CrossRef CAS.
- X. Wang, G. Liu, T. Iwao, M. Okubo and A. Yamada, J. Phys. Chem. C, 2014, 118, 2970–2976 CAS.
- J. S. Thorne, S. Chowdhury, R. A. Dunlap and M. N. Obrovac, J. Electrochem. Soc., 2014, 161, A1801–A1805 CrossRef PubMed.
- J. Zhao, L. Zhao, N. Dimov, S. Okada and T. Nishida, J. Electrochem. Soc., 2013, 160, A3077–A3081 CrossRef CAS PubMed.
- Y. Takeda, K. Nakahara, M. Nishijima, N. Imanishi, O. Yamamoto, M. Takano and R. Kanno, Mater. Res. Bull., 1994, 29, 659–666 CrossRef CAS.
- A. E. Bocquet, T. Mizokawa, T. Saitoh, H. Namatame and A. Fujimori, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 3771–3784 CrossRef CAS.
- M. Takano, N. Nakanishi, Y. Takeda, S. Naka and T. Takada, Mater. Res. Bull., 1977, 12, 923–928 CrossRef CAS.
- A. E. Bocquet, A. Fujimori, T. Mizokawa, T. Saitoh, H. Namatame, S. Suga, N. Kimizuka, Y. Takeda and M. Takano, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 1561–1570 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ee01365h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |