Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbon dioxide utilisation for production of transport fuels: process and economic analysis
Ioanna
Dimitriou
*a,
Pelayo
García-Gutiérrez
a,
Rachael H.
Elder
a,
Rosa M.
Cuéllar-Franca
b,
Adisa
Azapagic
b and
Ray W. K.
Allen
a
aUK Centre for Carbon Dioxide Utilization, Department of Chemical and Biological Engineering, University of Sheffield, Mappin Street, Sheffield, S1 3JD, UK. E-mail: i.dimitriou@sheffield.ac.uk; Fax: +44 (0)114 2227501; Tel: +44 (0)114 2227594
bSchool of Chemical Engineering and Analytical Science, The University of Manchester, The Mill, Room C16, Sackville Street, Manchester M13 9PL, UK
First published on 12th May 2015
Abstract
Utilising CO2 as a feedstock for chemicals and fuels could help mitigate climate change and reduce dependence on fossil fuels. For this reason, there is an increasing world-wide interest in carbon capture and utilisation (CCU). As part of a broader project to identify key technical advances required for sustainable CCU, this work considers different process designs, each at a high level of technology readiness and suitable for large-scale conversion of CO2 into liquid hydrocarbon fuels, using biogas from sewage sludge as a source of CO2. The main objective of the paper is to estimate fuel production yields and costs of different CCU process configurations in order to establish whether the production of hydrocarbon fuels from commercially proven technologies is economically viable. Four process concepts are examined, developed and modelled using the process simulation software Aspen Plus® to determine raw materials, energy and utility requirements. Three design cases are based on typical biogas applications: (1) biogas upgrading using a monoethanolamine (MEA) unit to remove CO2, (2) combustion of raw biogas in a combined heat and power (CHP) plant and (3) combustion of upgraded biogas in a CHP plant which represents a combination of the first two options. The fourth case examines a post-combustion CO2 capture and utilisation system where the CO2 removal unit is placed right after the CHP plant to remove the excess air with the aim of improving the energy efficiency of the plant. All four concepts include conversion of CO2 to CO via a reverse water-gas-shift reaction process and subsequent conversion to diesel and gasoline via Fischer–Tropsch synthesis. The studied CCU options are compared in terms of liquid fuel yields, energy requirements, energy efficiencies, capital investment and production costs. The overall plant energy efficiency and production costs range from 12–17% and £15.8–29.6 per litre of liquid fuels, respectively. A sensitivity analysis is also carried out to examine the effect of different economic and technical parameters on the production costs of liquid fuels. The results indicate that the production of liquid hydrocarbon fuels using the existing CCU technology is not economically feasible mainly because of the low CO2 separation and conversion efficiencies as well as the high energy requirements. Therefore, future research in this area should aim at developing novel CCU technologies which should primarily focus on optimising the CO2 conversion rate and minimising the energy consumption of the plant.
Broader contextCarbon capture and utilisation (CCU) has recently become the focus of large scale international attention not only because it has the potential to reduce anthropogenic CO2 emissions which contribute to climate change but also because it could generate value from waste CO2 through the synthesis of fuels and chemicals. Globally, consumption of fuels is two orders of magnitude higher than that of chemicals; therefore, CO2 utilisation technologies should focus primarily on fuel synthesis to create significant economic value and to make a substantial contribution to the reduction of CO2 emissions. Successful market-entry of CO2-to-fuels technologies strongly depends on their economic competitiveness. In this article, a techno-economic assessment of the manufacture of transport hydrocarbon fuels from waste CO2 is performed through process simulation, cost modelling and sensitivity analysis. Unlike other studies, the present techno-economic assessment only employs the best currently available and proven CCU technologies. The aim is to support policy makers and businesses in their decision-making by establishing whether the production of liquid transport fuels from CO2 using current technology is economically feasible and identifying the modifications required to improve the economic competitiveness of CCU processes. |
1. Introduction
Carbon capture and storage (CCS) has attracted significant attention in recent years as a possible technology to help mitigate climate change by reducing CO2 emissions into the atmosphere.1 However, CCS requires high investment costs and poses risks associated with the need for long-term storage and potential leakage of CO2.2,3 Carbon capture and utilisation (CCU) is being proposed as a complementary technology to CCS with the aim of both reducing CO2 emissions and consumption of fossil resources by utilising CO2 as a feedstock for the production of chemicals and fuels.4–8Global CO2 emissions from fossil fuel combustion were approximately 31 Gt in 20119 and are likely to rise to 57 Gt in 2050.10 Currently, CO2 is only used for the production of chemicals, such as urea, salicylic acid and polycarbonates. However, in order to make a significant contribution to reducing CO2 emissions, its utilisation should focus primarily on the conversion to fuels since the market for chemicals is two orders of magnitude lower than that for fuels.5 Oxygenates and hydrocarbons can be produced via hydrogenation of CO2 and could offer feasible alternatives for the transportation sector, reducing its dependency on fossil fuels. CO2 hydrogenation for oxygenate production is at present the most intensively investigated area of CO2 utilisation with methanol synthesis from CO2 and H2 already being demonstrated at bench- and pilot-scale plants in Asia11,12 and Europe.13
Conversely, the production of hydrocarbon fuels from syngas (H2 and CO) produced from CO2 and H2 (e.g. via reverse water-gas-shift reaction) is yet to be demonstrated. This is mainly due to the fact that the production of hydrocarbons from CO2 and H2 requires a higher amount of hydrogen and energy than oxygenates.5 However, there is a noticeable lack of published techno-economic feasibility studies in this area that could potentially support this argument. It should also be noted that hydrocarbons produced from syngas via Fischer–Tropsch synthesis (the established industrial process for converting syngas to liquid fuels) are specifically attractive because of their unlimited compatibility with conventional fuels in any proportion and thus, unlike alcohols and ethers, can readily be incorporated and integrated with conventional markets and supply chains.
As highlighted above, the high hydrogen and energy requirements associated with CO2-to-fuels pathways is one of the main issues for the application of these technologies. In order to make such a process economically and environmentally sustainable, hydrogen should be made from a non-fossil resource or produced within the process itself. The latter would also decrease the overall operating costs, especially since fossil-derived hydrogen is still significantly cheaper than that produced from renewable technologies.14 One non-fossil source of hydrogen could be biogas produced by anaerobic digestion of wet waste, such as sewage sludge. Such biogas contains mainly CO2 and methane, the latter of which can be utilised to produce hydrogen (e.g. via steam reforming),15 thus avoiding the depletion of natural gas, currently used for hydrogen production. The CO2 from the biogas can be separated and utilised for the production of fuels. Within the EU alone, around 10 million tonnes (dry basis) of sewage sludge are generated per year and the digestion of each tonne could produce approximately 590 m3 of methane.16 Other wastes could result in even higher methane yields; for example, food waste can generate up to 3.5 times more methane per tonne than sewage sludge.17 Therefore, anaerobic digestion of waste could be an important source of hydrogen for a CO2 hydrogenation-to-fuels process. It could also be a suitable target process for CO2 utilisation technologies since it requires moderate capital investment.
This is the topic of this paper which examines different CO2 capture and utilisation process concepts for the conversion of sewage sludge to liquid hydrocarbon fuels. The aim of the study is to identify the most promising process configurations in terms of conversion efficiencies and costs. For these purposes, a comprehensive techno-economic assessment has been carried out to examine the technical and economic performance of four conceptual designs, considering only the best available and proven technologies: amine CO2 capture, steam reforming, reverse water-gas-shift (RWGS) process and Fischer–Tropsch synthesis.
An overview of the CCU process concepts developed in this work is presented in the next section. Section 3 outlines the methodology for process modelling and the economic assessment followed by the results in Section 4. A sensitivity analysis is carried out in Section 5 which examines the effect of key economic and technical parameters on the production costs of liquid fuels, including capital and energy costs as well as the CO2 conversion rate in the RWGS reactor.
2. Process description
2.1 Process overview
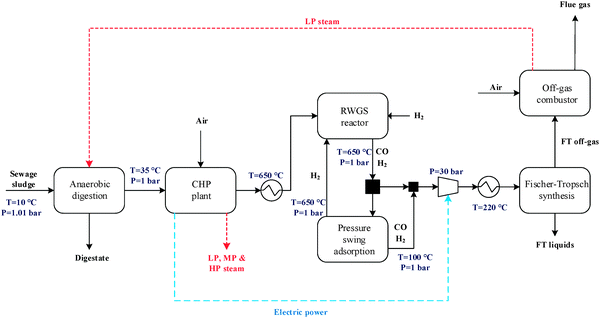
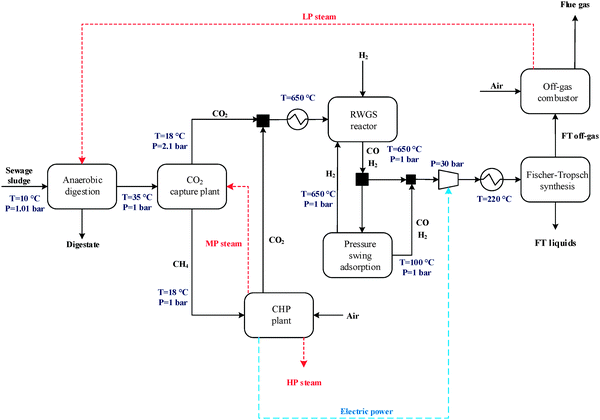
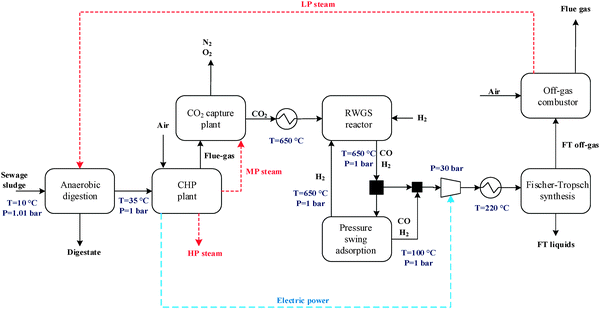
The general CO2 utilisation system considered in this study allows the production of liquid hydrocarbon fuels from sewage sludge, as shown in Fig. 1. It consists of six sections: anaerobic digestion of sewage sludge, CO2 capture, heat and power generation, syngas production, conversion of CO2 to CO and fuel synthesis. This general concept is realised in four different design configurations which are based on typical biogas utilisation applications. These are summarised in Table 1 along with the main process steps. The different process sections and designs are depicted in more detail in Fig. 2–5. The first process design (PD-MEA in Table 1 and Fig. 2) incorporates a monoethanolamine (MEA) gas treatment unit which is often used to upgrade biogas to the same standards as natural gas by removing CO2 and other contaminants.18 The second case (PD-CHP1, Table 1 and Fig. 3) is based on another biogas application: combustion of untreated biogas in a combined heat and power (CHP) unit to produce electricity and heat. The third design (PD-CHP2, Fig. 3) comprises an MEA CO2 capture system placed before the CHP plant which in this case is fed with the upgraded biogas (i.e. more concentrated in CH4) rather than untreated biogas as in the second case. Thus, this is a pre-combustion CO2 capture system. The final configuration (PD-CHP3, Fig. 5) is similar to PD-CHP2 but the MEA unit is now placed after the CHP plant so that this process design is based on post-combustion CO2 capture.7 In this case, the MEA unit also allows the removal of the excess air used in the CHP plant which acts as an inert diluent, decreasing the efficiency of the downstream processes and necessitating higher power consumption for the subsequent syngas compression. The feedstock for all the process concepts is sewage sludge, a by-product of wastewater treatment. In all four cases, the plant produces liquid hydrocarbon fuels (gasoline and diesel). The different process sections and designs are described in more detail below.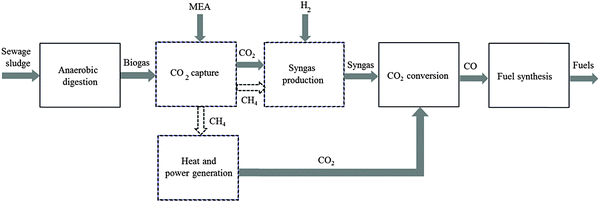 | ||
| Fig. 1 An overview of the CO2 utilisation system for production of synthetic fuels [MEA: monoethanolamine. Dashed lines represent steps which are not present in all design options; see Table 1 for details. In some design cases the heat and electricity generation plant is placed before CO2 capture]. | ||
| Process sections | PD-MEA | PD-CHP1 | PD-CHP2 | PD-CHP3 |
|---|---|---|---|---|
| a Monoethanolamine (MEA) CO2 capture plant. b Combined heat and power (CHP). c Methane steam reforming. d Reverse water-gas-shift reaction. e Fischer–Tropsch synthesis. | ||||
| Anaerobic digestion | ✓ | ✓ | ✓ | ✓ |
| CO2 capturea | ✓ | ✓ | ✓ | |
| Heat and power generationb | ✓ | ✓ | ✓ | |
| Syngas productionc | ✓ | |||
| CO2 conversion | ||||
| RWGSd | ✓ | ✓ | ✓ | ✓ |
| Hydrogen recovery | ✓ | ✓ | ✓ | ✓ |
| Fuel synthesise | ✓ | ✓ | ✓ | ✓ |
 | ||
| Fig. 2 Process flow diagram for PD-MEA [the flue gas from the off-gas combustor in all design configurations contains N2 (68–88 vol%), CO2 (8–20 vol%), argon and oxygen]. | ||
2.2 Anaerobic digestion
Anaerobic digestion (AD) is a process in which biodegradable organic material is broken down by microorganisms in the absence of air. The produced biogas, consisting of around 65 vol% CH4, 35 vol% CO2 and trace gases such as H2S, H2, N2 and NH3, is commonly utilised in heat and electricity co-generation plants.16 In this study, the digestion conditions are mesophilic (35 °C, 1 atm) which are associated with lower equipment costs and energy requirements compared to thermophilic systems (>50 °C, 1 atm).19 A standard size AD plant typically used in the wastewater industry in the UK is considered with twin digesters of 2000 m3 each with a residence time of 15 days (B.C. Franklin and L. Styles, AECOM, personal communication, May 2013). This corresponds to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111 kg h−1 of sludge fed into the plant.
111 kg h−1 of sludge fed into the plant.
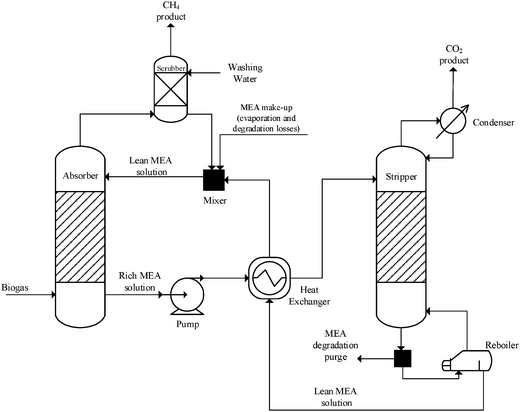
2.3 CO2 capture
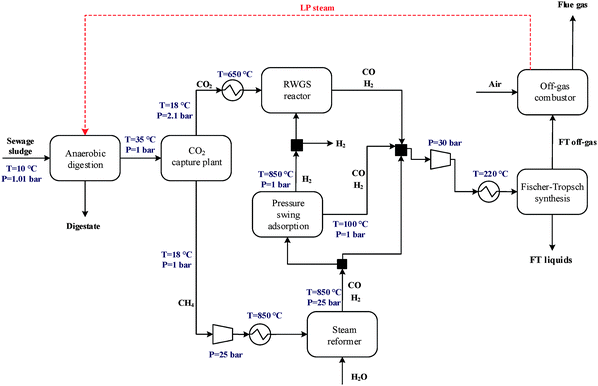
The flow diagram of the CO2 capture unit is shown in Fig. 6. To convert CO2 into a liquid fuel, a concentrated stream of CO2 needs to be generated by isolating it from biogas. Among the available technologies to capture CO2 from a gas stream, amine-based regenerative systems have been identified as the most suitable technology that has achieved commercial success.7In the packed absorption column, the biogas is fed counter-currently with an MEA aqueous solution (usually 15–35 wt%) which reacts with and absorbs CO2 in the biogas to form an MEA carbamate soluble salt. The gas stream lean in CO2 is released from the top of the absorber while the MEA solution rich in CO2 is pumped to a heat exchanger in which the solution is heated to about 120 °C and then fed into the stripping column. MEA is regenerated in the stripper and recycled to the absorber for re-use (lean MEA solution, Fig. 6). The regeneration conditions are maintained by the reboiler which uses low-pressure steam. Steam which acts as stripping gas in the column, is recovered in the condenser and fed back to the stripper, while the concentrated CO2 stream is released from the top of the stripper for downstream processing. Process conditions in the capture plant are summarised in Table 2.
| Parameters | Value |
|---|---|
| CO2 removal efficiency (%) | 70 |
| MEA solution (wt%) | 30 |
| Absorber pressure (bar) | 1.013 |
| Stripper pressure (bar) | 2.1 |
2.4 Heat and power generation
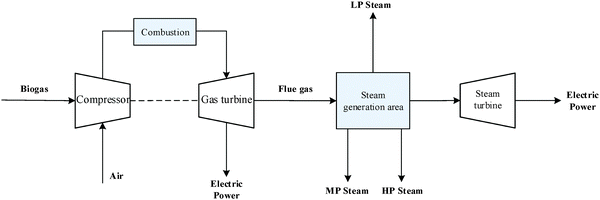
An outline of the combined heat and power (CHP) plant is shown in Fig. 7. Firstly, biogas is compressed from 1 bar to 8 bar. It is then mixed with steam (8 bar) and compressed air and then burned in the combustor to produce hot gas at 583 °C. Steam is used to lower the combustion temperature (below 750 °C) to minimise NOX formation. The hot gas is first passed through a gas turbine for electricity generation and then to the steam generation area to recover heat. In the steam generation area, the gas passes through five heat exchangers and is cooled down by water or steam. Consequently, three different grade steams are generated: low-pressure (LP) steam at 1.013 bar, medium-pressure (MP) steam at 5 bar and high-pressure (HP) steam at 24 bar.2.5 Syngas production
The main process for producing syngas currently used in Fischer–Tropsch synthesis is steam reforming of methane which is a well-understood and proven technology.22 In this study, the upgraded biogas from the CO2 removal section is utilised either in a methane steam reformer (PD-MEA concept, Fig. 2) or the co-generation unit (PD-CHP2 concept, Fig. 4). The CH4-rich gas stream leaving the MEA absorption column is mixed with steam (2.6 MPa) and the resulting mixture is preheated to 500 °C and introduced to the catalytic reforming reactor.15 The steam/methane mixture is passed through a set of externally heated reformer tubes filled with nickel catalyst, where it is converted to CO and H2 at 900 °C and 25 bar according to the following reaction:| CH4 + H2O ⇄ CO + 3H2 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, an excess of steam (H2O
1, an excess of steam (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH4 = 1.2
CH4 = 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is used to prevent deactivation of the catalyst from carbon deposition.15
1) is used to prevent deactivation of the catalyst from carbon deposition.15
2.6 CO2 conversion
| CO2 + H2 ⇄ CO + H2O | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 = 3
CO2 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is used to prevent carbon (coke) deposition on the catalyst surface. The feed CO2-rich gas is produced by the MEA plant in the process concepts PD-MEA, PD-CHP2 and PD-CHP3, while in PD-CHP1, CO2 is generated in the CHP plant.
1) is used to prevent carbon (coke) deposition on the catalyst surface. The feed CO2-rich gas is produced by the MEA plant in the process concepts PD-MEA, PD-CHP2 and PD-CHP3, while in PD-CHP1, CO2 is generated in the CHP plant.
2.7 Fuel synthesis
Fischer–Tropsch (FT) converts a mixture of CO and H2 (syngas) in the presence of a catalyst to a variety of organic compounds, mainly hydrocarbon products of variable chain length. The FT reactions are highly exothermic and can be represented by the following basic reaction equation:| nCO + (2n + 1)H2O ⇄ CnH2n+2 + nH2O | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO molar ratios of around 2 in order to achieve a high FT liquid production.30
CO molar ratios of around 2 in order to achieve a high FT liquid production.30
In this study, the FT reactor is operated at 30 bar and 220 °C and is assumed to be similar to the Sasol Slurry phase distillate reactor which is designed for LTFT synthesis.26,28 The reactor's gas effluent is passed to a three phase separator to remove water and heavy hydrocarbons from the residual vapour. The FT off-gas which mainly consists of light hydrocarbons (C1–C4) and unconverted syngas is combusted to generate low pressure steam for the anaerobic digesters, whereas the liquid fuels are sent to a central refinery plant for further upgrading.
As mentioned earlier, a wide range of products are obtained from the FT synthesis, therefore a quantitative approximation of product distribution is necessary. The most widely used approach to tackle this problem is the Anderson–Schulz–Flory (ASF) product distribution.31 According to this method, the adsorbed carbon chain can either undergo further addition of a –CH2– group or the chain can terminate.31 The ASF-product distribution model is represented by the following equation:
| Cn = αn−1(1 − α) | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO ratio in the feed gas, reactor type and operating conditions.
CO ratio in the feed gas, reactor type and operating conditions.
FT synthesis results in the production of various products, thus it is not a highly selective process. However, it offers the possibility to cover the entire range of petrochemical products so that gasoline, jet fuel and diesel can be produced with adequate process control. FT products are high quality and ultra clean fuels, free of sulphur and aromatic compounds and, unlike other fuels such as dimethyl ether and alcohols, they can be easily assimilated in the existing transport infrastructure, concerning both vehicle engines and distribution channels.
3. Modelling methodology
The process flowsheets of the four evaluated CCU cases were developed using the process simulation software Aspen Plus32 to estimate material balances, energy and utility requirements as the inputs for the techno-economic analysis.Various thermodynamic methods have been used to model the different unit operations considered in this study. The Aspen Physical Property System guide33 was used to ensure that the property methods are tailored to the different classes of compounds and operating conditions. The property method used for most unit operations is the Peng–Robinson with Boston–Mathias modifications (PR–BM) which is recommended for gas processing and refinery applications and provides accurate results for hydrocarbon mixtures and light gases, such as H2 and CO2.33 The non-random-two-liquid (NRTL) method with the Redlich–Kwong (RK) equation of state is used to simulate the anaerobic digestion process. The MEA gas treating unit is modelled using the electrolyte-NRTL based property method ENRTL-RK which is suitable for mixed electrolyte systems up to medium pressures. This method uses the RK equation of state for estimating the vapour phase properties.
The anaerobic digester is modelled using the Aspen Plus yield reactor block (RYield). The mass yields of CH4, CO2 and digestate were calculated separately considering a biogas production of 0.6 m3 per kg of volatile solids loading.19,34 It was assumed that neither NH3 nor H2S are present in biogas since the former is not produced when sewage sludge is used as feedstock and the latter is present in very low concentrations.35Table 3 shows the component mass yields calculated for the anaerobic digestion process.
| Component | Mass yield (kg kg−1 sludge) |
|---|---|
| Digestate | 0.9792 |
| CO2 | 0.0124 |
| CH4 | 0.0084 |
In the CO2 capture plant, the absorber and stripper columns are simulated using the RadFrac block which is suitable for modelling all types of multistage vapour–liquid fractionation operations. As mentioned previously, the thermodynamic and physical properties are estimated using the ENRTL-RK method coupled with an electrolyte calculation option which models the electrolyte solution chemistry and consists of five equilibrium reactions.36 Design specifications are used to obtain the desired molar split fractions in both the absorber and the stripper. In the absorber, a design specification measured the CO2 flow rate in the stack stream and adjusted the lean MEA flow rate to ensure that a target recovery of 70% is achieved. In the stripper, a design specification measured the CO2 molar concentration in the CO2 product stream and adjusted the reflux ratio to achieve a 98 vol% purity target.21 The number of minimum equilibrium stages was 5 for the absorber and 10 for the stripper.
In the CHP unit, compressed biogas is mixed with steam and air (10% excess) and fed into the combustor. The combustor is simulated using a Gibbs reactor block (RGibbs) which models single-phase chemical equilibrium by minimizing the Gibbs free energy, subject to atom balance constraints. Steam is generated inside a network of heat exchangers while electricity is produced in steam and gas turbines by assuming common isentropic and mechanical efficiencies.37 The CHP plant simulation was based on a natural gas CHP model previously developed by AspenTech.38 The main modification made to this model was to replace the natural gas feed stream with the biogas outlet stream of the anaerobic digestion plant.
Both the steam reformer and the RWGS reactor are modelled using a stoichiometric reactor block (RStoic). Reaction stoichiometry was specified for each of them as well as the fractional conversion of relevant components (80% for CH4 and 65% for CO2, respectively).12,39
FT synthesis is modelled using a yield reactor block (RYield). The mass yields of the produced hydrocarbons were calculated in a separate spreadsheet using the ASF distribution model described in Section 2.6 with a chain growth probability of 0.85 which favours the production of middle distillates. The single-pass CO conversion was set to 80%.40,41 Even though such a CO conversion value is relatively high, it can be achieved in slurry phase reactors employing cobalt catalysts.26,42 The ASF hydrocarbon distribution was taken up to a carbon number of 100. Production of aromatics, oxygenates and olefins is assumed to be negligible in this study since the presence of these compounds is typically small for LTFT synthesis.25,43
4. Cost estimation methodology
The capital and operating costs were estimated for each CCU process concept using the software Aspen Process Economic Analyzer (APEA) which, like Aspen Plus, is licenced by Aspen Technology. APEA was linked to Aspen Plus to estimate costs by utilising the output results of the Aspen Plus simulations. The UK was set as the default country which defines several economic parameters in APEA, such as currency, salary rates, equipment costs and construction materials.44Table 4 summarises the assumptions as well as the prices of raw materials and utilities specified in APEA. These prices were converted to GBP (£) and updated to 2013 where necessary. The same economic assumptions were used for all four process designs to allow fair comparisons between them. As discussed previously, the feedstock for all the evaluated CCU cases is sewage sludge. The CCU facility is considered to be part of a large wastewater treatment part and thus the sludge costs are zero since sludge is a by-product of wastewater treatment.| a APEA default values (country base: UK). b Average sale price.47 c The quoted price is for hydrogen produced from water electrolysis which is a low-carbon technology.14 d Assumed. e Average sale price for molecular sieve 13X.48 f Electricity price for small UK industrial consumer.49 g Natural gas price for small UK industrial consumer.49 | |
|---|---|
| General economic parameters | |
| Base year | 2013 |
| Plant life | 20 years |
| Plant annual operating hours | 8000 |
| Loan interest rate | 10% |
| Tax | 40%a |
| Contingency | 18%a |
| Working capital | 5% of TCIa |
| Raw material prices | |
| MEA | £1 per kgb |
| Hydrogen | £4 per kgc |
| Fischer–Tropsch catalyst | £22 per kg45 |
| Reformer catalyst | £22 per kg45 |
| RWGS catalyst | £22 per kgd |
| PSA packing | £0.85 per kge |
| Utility prices | |
| Electricity | £0.1127 per kW hf |
| Natural gas | £0.0319 per kW hg |
| Cooling water | £0.21 per m3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
The capital investment is comprised of installed equipment costs, indirect costs (e.g. contingency), tax and working capital.44 The capital investment required to establish the project is considered to be borrowed and repaid over the lifetime of the project (20 years) at a loan interest rate of 10% per annum. To estimate the fuel production costs of the CCU system, the annual amount required to pay back the loan on capital needs to be determined first:
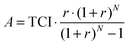 | (5) |
The total annual costs consist of capital annuities as well as operating costs: raw material, utilities, labour and maintenance costs. The fuel production costs are calculated by dividing the total annual costs by the amount of FT fuels (gasoline and diesel) produced in a year. The price inflation of equipment and raw materials is not considered for the ease of comparison between the evaluated CCU concepts. For the same reason, government subsidies, CO2 credits and by-product revenues are excluded from the economic analysis.
5. Results
5.1 Mass and energy balances
Table 5 presents the mass and energy balances for all four CCU process concepts evaluated in this study. For all considered cases, the sewage sludge input is the same in terms of mass flow and energy content so that results are directly comparable. The energy balances in Table 5 show that sewage sludge is the major source of energy input in the system. The PD-MEA concept, which includes separation of CO2 from the biogas stream and subsequent steam methane reforming, produces 43 kg h−1 of hydrocarbon fuels which corresponds to 513 kW (LHV) of fuel energy oput. The second CCU concept, PD-CHP1, which involves biogas combustion in a CHP plant without upstream CO2 scrubbing, results in a lower fuel production of 33.9 kg h−1 (405 kW). Table 5 also shows that the PD-CHP2 concept produces almost the same fuel output (33.7 kg h−1 or 403 kW) as PD-CHP1. The final CCU design produces 28.8 kg h−1 or 344.5 kW of hydrocarbon fuels which is lower than the other three cases since only 70% of the CO2 in the CHP product gas is captured in the MEA unit (see also Section 2.3), while the rest exits the top of the absorber and is discarded through a stack. However, it should be noted that PD-CHP3 is the only design that produces electricity as a by-product, whereas the amount of electricity produced in the other three designs is insufficient to cover their electricity requirements (for these concepts, the cost of purchasing the extra electricity has been taken into account in the calculations).| PD-MEA | PD-CHP1 | PD-CHP2 | PD-CHP3 | |
|---|---|---|---|---|
| a Values are given on a dry ash-free basis. b Total plant electricity requirements. c Electricity generation within the plant. | ||||
| Plant inputs | ||||
| Sewage sludge (kg h−1)a | 333.3 | 333.3 | 333.3 | 333.3 |
| Sewage sludge (kW) (LHV)a | 1944.4 | 1944.4 | 1944.4 | 1944.4 |
| Hydrogen (kg h−1) | — | 32.8 | 32.6 | 22.9 |
| Hydrogen (kW) (LHV) | — | 1093 | 1086.3 | 763.9 |
| Natural gas (kg h−1) | 50.7 | 33.7 | 45.3 | 75.7 |
| Natural gas (kW) (LHV) | 688.1 | 456.6 | 572.5 | 655.1 |
| Electricity (kW)b | 207 | 577 | 578 | 133 |
| Plant outputs | ||||
| Hydrogen (kg h−1) | 0.82 | — | — | — |
| Hydrogen (kW) (LHV) | 27.3 | — | — | — |
| Gasoline (kg h−1) | 12 | 4.6 | 4.5 | 7.9 |
| Gasoline (kW) (LHV) | 143.8 | 54.3 | 53.9 | 95.4 |
| Diesel (kg h−1) | 31 | 29.3 | 29.2 | 20.9 |
| Diesel (kW) (LHV) | 370 | 350.6 | 349 | 249.1 |
| Electricity (kW)c | 0 | 175 | 202 | 175 |
| Efficienciesa | ||||
| Fuel energy efficiency (%) | 26.4 | 20.8 | 20.7 | 17.7 |
| Plant energy efficiency (%) | 17.1 | 11.7 | 11.9 | 14 |
The PD-MEA concept produces more fuels than the other three cases because of the higher amount of syngas processed in FT synthesis as a result of its upstream methane steam reforming unit which converts methane to syngas instead of simply burning it in a CHP plant. This also significantly affects the hydrogen requirements of the individual concepts. In the case of PD-MEA, the amount of hydrogen produced from the methane steam reformer is higher than the hydrogen requirements of the RWGS reactor; therefore, some hydrogen is produced as by-product (see also Fig. 2). This is not the case for the other three concepts where additional hydrogen is required from an external source. The impact of the hydrogen price on the fuel production costs of PD-CHP1 and PD-CHP2 is examined in Section 5.4.
5.2 Energy efficiencies
Table 5 also shows the energy efficiencies of the four studied CCU designs. The fuel energy efficiency measures the fraction of the energy originally in the sewage sludge that ends up in the hydrocarbon fuel product. It is calculated by dividing the energy in the fuel output by the energy content of sewage sludge. Increased hydrocarbon production leads to higher energy efficiencies and thus the greatest efficiency is achieved by PD-MEA (26.4%). The next best options are PD-CHP1 and PD-CHP2 (∼20.8%) while PD-CHP3 is the least fuel-efficient (17.7%).The plant energy efficiency ηplant takes into account the total energy input (sludge, hydrogen, natural gas and electricity) and total energy output (fuels, hydrogen and electricity) and is calculated according to:
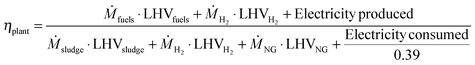 | (6) |
As for the fuel efficiency, PD-MEA also has the highest plant energy efficiency, estimated at 17.1%; this is due to the higher fuel output as well as the excess hydrogen production from which the other process designs do not benefit. PD-CHP3 shows the highest efficiency (14%) of all three CHP-based cases despite the fact that the fuel production is approximately 18% lower than that of the other concepts. The primary reason for this is that PD-CHP3 produces more than enough electricity to cover all the power requirements of the plant so there is no need to provide electricity externally. PD-CHP1 and PD-CHP2 achieve similar efficiencies (11.7% and 11.9%, respectively) which suggests that combustion of upgraded biogas in a CHP plant does not significantly benefit the overall CCU plant performance.
5.3 Energy requirements
The energy consumption calculated through the Aspen Plus simulations is used to assess the heat and power consumption in the different parts of the plant; these are given in Table 6. As mentioned in Section 2, steam produced from the combustion of FT off-gas and the LP steam from the CHP plant are used to supply heat to the anaerobic digesters which require approximately 640 kW of LP steam. The amount of LP steam produced by the CCU plant is sufficient to cover the energy requirements of the anaerobic digesters for PD-CHP1 and PD-CHP2, whereas this is not the case for PD-MEA and PD-CHP3. The most energy-intensive processing steps in terms of heat requirements are the RWGS CO2 conversion and steam reforming because they are both endothermic processes and thus require a significant amount of heat to operate. It should be noted that the CHP plant is fully integrated and does not require any external energy inputs (heat and electricity).38| PD-MEA | PD-CHP1 | PD-CHP2 | PD-CHP3 | |
|---|---|---|---|---|
| Heat (kW) | ||||
| Anaerobic digestion | 84 | — | — | 110 |
| MEA CO2 capture | 85 | — | 78 | 252 |
| Steam reforming | 329 | — | — | — |
| RWGS | 20 | 456 | 458 | 138 |
| Total | 518 | 456 | 536 | 500 |
| Power (kW) | ||||
| MEA CO2 capture | 0.3 | — | 0.3 | — |
| Steam reforming | 77 | — | — | — |
| RWGS | 69 | — | — | — |
| PSA | 57 | 29 | 29 | — |
| FT synthesis | 4 | 373 | 347 | −42 |
| Total | 207.3 | 402 | 376.3 | −42 |
Note that the FT synthesis is an exothermic process and thus requires cooling (water) rather than heating to maintain the operating temperature in the reactor. However, it is the most power consuming section for the PD-CHP1 and PD-CHP2 designs due to the compression of a large volume of processed syngas which contains excess air from the CHP plant. This is not an issue for PD-MEA which does not include a CHP unit. Similar is true for PD-CHP3 which employs an MEA unit right after the co-generation plant. Generally, it can be seen that none of the four CCU designs is energy self-sufficient with the exception of PD-CHP3 which produces surplus electricity but still requires additional heat. In this study, natural gas is used to cover the additional heating requirements of the plant, while electricity is bought from the grid, if needed.
5.4 Economic analysis
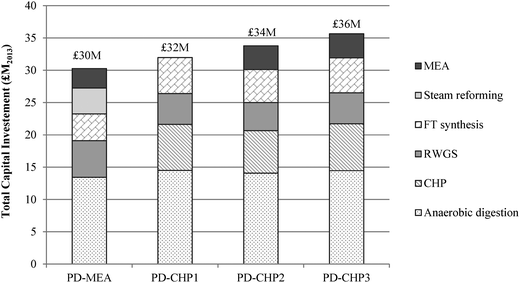
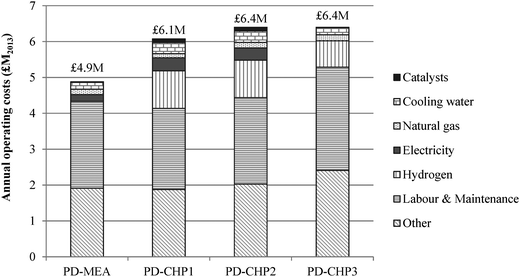
Fig. 8 shows the breakdown of capital costs for different parts of the CCU plant and the resulting total capital investment (TCI) for the four CCU process designs. The calculated TCI is expressed in 2013 million British Pounds and ranges from £30 (PD-MEA) to £36 million (PD-CHP3). The CHP-based cases are associated with higher capital costs than PD-MEA because of the CHP unit which increases the equipment costs and thus the TCI. As indicated in Fig. 8, anaerobic digestion represents 41–45% of the overall costs of the CCU plant. This is due to the two digestion reactors which handle a large volume of sewage sludge, as discussed in Section 2.2. The CHP unit is the second most expensive process section, contributing 19–22% to the TCI. The steam reforming, RWGS and FT synthesis sections necessitate similar capital investment ranging from 13–19% of the overall plant costs. Note that the capital costs of the PSA unit and the FT off-gas combustion are also included in the estimated capital investment of the RWGS and FT synthesis section, respectively. Finally, the capital costs of the MEA unit constitute the smallest fraction, requiring about 10% of the total capital investment.The annual operating and maintenance (O&M) costs are shown in Fig. 9. The operating costs include expenditure for materials (e.g., catalysts, hydrogen), utilities (e.g., electricity, natural gas), labour, maintenance and other costs (e.g., overheads, insurance). The O&M costs range from £4.9–6.4 million with PD-MEA resulting in the lowest expenditure owing to the lower equipment and utilities requirements associated with this process configuration. Labour and maintenance costs are the largest contributor to O&M costs and represent 37–45% of the total O&M expenditure. Other costs represent 31–39%, including the expense for MEA and PSA packing. Hydrogen contributes 12–17% to the total operating costs of the CHP-based designs. Catalyst and electricity costs are higher for PD-CHP1 and PD-CHP2 because of the higher volume of processed gas compared to the other two cases. Finally, cooling and heating utilities represent a small fraction of the total operating costs (2–5%).
The production costs per litre of gasoline and diesel are presented in Table 7 for the four CCU configurations, along with the contribution of capital costs (as capital annuity) and O&M expenditure. The calculated production costs do not include tax, duties, producer and retailer profits, marketing expenditure and distribution costs. As can be seen in Table 7, O&M costs are a more important contributor to the production costs than the capital investment as they represent 58–62% of the total production costs. PD-MEA has the lowest production costs at £15.8 per litre because of its lower capital and operating costs as well as higher fuel production compared to the other three cases. The next best option is PD-CHP at £23.2 per litre which has the lowest production costs among the three CHP-based designs. PD-CHP3 is associated with the highest fuel production costs at £29.6 per litre which is approximately 87% higher than for PD-MEA. The main reason for this is that this concept produces a significantly lower amount of liquid fuels than PD-MEA, as discussed in Section 5.1. From these it is clear that the amount of fuel produced (and thus conversion efficiencies) is a very important element of the production costs; thus, its effect is investigated in the sensitivity analysis later in the paper (Section 5.5).
| PD-MEA | PD-CHP1 | PD-CHP2 | PD-CHP3 | |
|---|---|---|---|---|
| Capital (£ per l) | 6.7 | 8.9 | 9.4 | 11.7 |
| O&M (£ per l) | 9.1 | 14.3 | 15.2 | 17.9 |
| Total (£ per l) | 15.8 | 23.2 | 24.6 | 29.6 |
Only the PD-MEA concept is considered as this process design has the lowest production costs. Twelve plant capacities are evaluated, ranging from 1 tonne (base case) to 1670 tonnes of liquid fuels produced per day. The latter capacity corresponds to the Bintulu gas-to-liquids (GTL) plant in Malaysia, one of the largest FT plants in the world, owned by Shell.51 The six-tenths factor rule52 was applied to estimate the investment costs of the scaled-up CCU plants as follows:
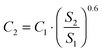 | (7) |
Using the above approach, the capital investment for the PD-MEA plant of the largest capacity considered here (1670 tonnes per day) is estimated at £2.6 billion. This is three times higher than the capital investment of the Bintulu plant, which cost $1.3 billion51 or £831 million (2013 exchange rate: £1 = $1.5653). Therefore, it is highly unlikely that the industry would invest in a CCU plant when they could instead build a conventional fuel production plant of the same capacity at a much lower cost, while also reducing financial and other risks by relying on a commercially proven, rather than a new technology.
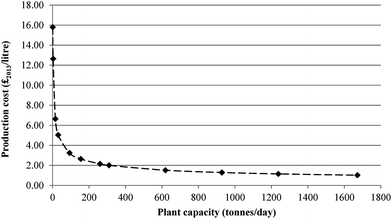
Fig. 10 shows the effect of scale on the costs of CCU fuels. For the largest plant capacity, the fuel production costs are almost 16 times lower than for the PD-MEA base case (£15.80 vs. £1.00 per litre). However, the effect of economy of scale levels off for capacities above 620 tonnes per day, with much smaller cost reductions thereafter. By comparison, the cost of producing conventional diesel in 2013 was £0.51 per litre and £0.47 per litre for gasoline54 (gate costs, excluding tax, duty, profits, marketing and distribution costs). This is around two times lower than the CCU fuel costs. Therefore, unless significant improvements are achieved in the conversion efficiencies of CCU technologies, along with introduction of government subsidies and incentives, it is highly unlikely that CCU fuels will be able to compete against conventional transport fuels, which despite their contribution to climate change, are still relatively cheap to produce.
The effect of economies of scale on the fuel production costs of the evaluated CCU process designs was also investigated using Aspen Plus and APEA. This also allows for comparisons with the costs estimated with the six-tenths rule above. Two scaled-up models of the PD-MEA concept were considered: a medium scale and a large scale plant at 850 and 1670 tonnes of fuel per day, respectively. Table 8 shows the capital investment and production costs of the two scaled-up designs calculated by APEA. For the medium plant capacity, the production cost drops to £2 per litre which is approximately eight times lower than that of the PD-MEA base case. As expected, the large scale plant with a capacity equal to the Bintulu plant has an even lower production cost at £1.2 per litre which is about 20% higher than the cost estimated with the six-tenths rule. The capital investment of the large scale process design estimated by APEA is £1.34 billion which is almost half of the equivalent six-tenths rule cost (£2.6 billion); however this is still 61% higher than the capital investment of the GTL Bintulu plant. Therefore, using either cost estimating method, it is clear that CCU fuels are currently significantly more expensive than conventional transport fuels.
| Process design | Plant capacity (t d−1) | Capital cost (£ billion) | Production cost (£ per l) |
|---|---|---|---|
| Base case | 1 | 0.03 | 15.8 |
| Medium capacity | 850 | 1.28 | 2.0 |
| Large capacity | 1670 | 1.34 | 1.2 |
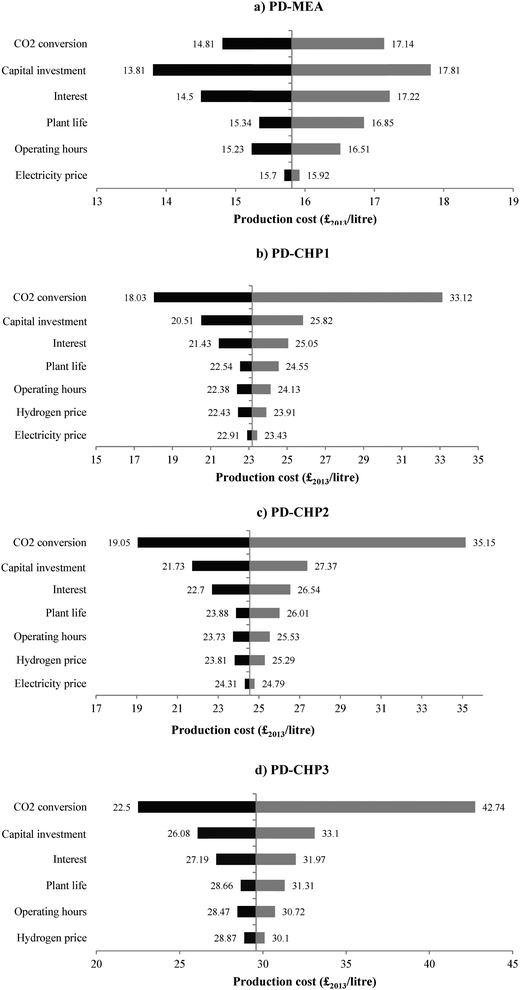
5.5 Sensitivity analysis
This section considers the effect of several key parameters on the overall fuel production costs: CO2 removal efficiency of the MEA CO2 capture plant, percentage of CO2 conversion in the RWGS reactor, capital costs, loan interest rate, plant life, operating hours, electricity and hydrogen prices. Fig. 11 shows the dependence of fuel production costs on the CO2 separation efficiency of the MEA CO2 unit for PD-MEA, PD-CHP2 and PD-CHP3. For 90% CO2 removal efficiency, production costs decrease to £13.8 per litre and £24.1 per litre for PD-MEA and PD-CHP3, respectively since more CO2 is converted to CO in the RWGS reactor which results in an increased fuel production (and thus lower production costs). This is not the case for PD-CHP2 where production costs are 32–33% higher than the base case cost with an increase in the CO2 removal efficiency. Generally, as the CO2 removal efficiency increases, the volume of gas which is passed to the stripper in the CO2 capture unit also increases and this results in higher energy requirements as well as higher equipment and operating costs. For PD-MEA and PD-CHP3, this increase in costs is outweighed by the increase in fuel production. However, in PD-CHP2, the same amount of CO2 in biogas will pass to the RWGS reactor regardless of the absorption efficiency of the MEA unit since the flue gas from the CHP plant and the concentrated CO2 gas stream are mixed just before the RWGS section (see Fig. 4). Therefore, in case of PD-CHP2 production costs will rise as there is no increase in production which could potentially outbalance the higher capital and operating costs of the MEA CO2 capture unit as the CO2 removal efficiency increases.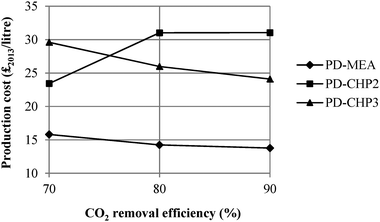 | ||
| Fig. 11 Sensitivity of fuel production cost to the CO2 removal efficiency of the MEA CO2 capture plant. | ||
For the other technical and economic parameters, the sensitivity analysis was carried out by changing each parameter in turn by ±30% of its base-case value (see Table 4) with the exception of the plant operating hours which were changed by ±10% since they cannot exceed the maximum hours per year. The results for the four design concepts are shown in Fig. 12. The bars show deviations from the original values of the model parameters with longer bars indicating a higher degree of sensitivity to a particular parameter.
In the case of PD-MEA, production costs are most sensitive to the capital investment costs. If the total capital is decreased by 30%, the fuel production cost drops to £13.81 per litre or 14.6% below the base case cost. However, errors of ±30% for capital investment estimates are typical40,50 and increased accuracy can only be achieved through very detailed and expensive analysis of a real case. Another important factor that increases the inherent uncertainties in projecting CCU capital costs is the different level of development of some of the technologies considered in this study. For example, the RWGS process has only been proven on a small scale and therefore is still under development as opposed to steam reforming which is a mature and well understood technology. The high sensitivity to variations in capital costs also emphasises the importance of economies of scale, which the studied CCU options do not benefit from yet as opposed to conventional fuel production plants.
For all CHP based models, production costs are most sensitive to changes in the CO2 conversion rate in the RWGS reactor. This suggests that the lower the fuel output, the more sensitive production costs are to variations of the CO2 conversion efficiency. The production costs of the CHP based models can be reduced by 24–29% for a CO2 conversion rate of 84.5% (30% higher than the base case). Therefore, improving the performance of the CO2 hydrogenation technology should be an early priority.
The loan interest rate is the second most sensitive parameter for PD-MEA and third for all CHP cases. Production costs can be decreased by 8–9% when the interest rate is reduced from 10% to 7%. Interest rates influence the capital annuities and can be controlled by agreeing fixed rates with the lender throughout the life of the project, significantly reducing the uncertainty associated with this economic parameter. Finally, production costs are less sensitive to the project's lifetime, electricity and hydrogen prices as well as operating hours.
6. Conclusions
This work has examined the technical and economic feasibility of four CCU process configurations for the production of liquid transport fuels. The process designs considered here incorporate existing CCU technologies for the conversion of a carbon source, in this case sewage sludge, to fuels. The first process configuration examined comprises separation of CO2 from biogas by MEA and steam reforming for conversion of methane to syngas, while the other three cases incorporate a CHP plant for co-generation of heat and power from biogas and the conversion of methane to CO2. All four cases include a RWGS reactor for converting CO2 to syngas and its subsequent conversion to fuels via Fischer–Tropsch synthesis. Detailed designs were developed in Aspen Plus to determine the technical and economic potential of the selected process configurations and identify the concept that has the lowest overall costs.The overall plant energy efficiency and production costs of the evaluated designs range from 12–17% LHV and £15.8–29.6 per litre of produced fuels, respectively. The process configurations which incorporate a CHP plant result in significantly lower efficiencies and higher costs than the process design with MEA CO2 capture and steam reforming. The primary reasons for this are the higher syngas production in the steam reforming process and the high capital and operating costs of CHP. The sensitivity analysis reveals that the fuel production costs are mainly influenced by variations in capital costs, the CO2 removal efficiency of the CO2 capture plant and the rate of CO2 conversion. This emphasises the importance of optimising current CCU technology, as well as the significance of economies of scale which greatly benefit commercial plants. For example, for the best design case, the costs of fuel production for a larger capacity plant (1670 tonnes per day), are £1–1.2 per litre, down from £16 per litre for a plant producing 1 tonne per day. However, this is still twice as high as the cost of conventional transport fuels. Therefore, fuel production with current CCU technologies is not yet economically viable primarily due to the: (i) low CO2 conversion in the RWGS process, (ii) low selectivity of the Fischer–Tropsch synthesis and (iii) relatively low CO2 separation efficiency in the MEA absorber. This highlights the need for new CCU technologies, some of which are currently being developed (e.g. ionic liquids for CO2 capture, co-electrolysis of CO2 and water, dry methane reforming).
Further research will be carried out to complement the analysis presented here, including the assessment of other less developed technologies (e.g. dry methane reforming) and products (e.g. methanol, formic acid), life cycle assessment to examine the environmental impacts of the studied CCU designs and the possibility of additional revenue from sales of by-products and avoided greenhouse gas emissions.
Acknowledgements
This work was carried out as part of the “4CU” programme grant, aimed at sustainable conversion of carbon dioxide into fuels, led by the University of Sheffield and carried out in collaboration with the University of Manchester, Queens University Belfast and University College London. The authors acknowledge gratefully the Engineering and Physical Sciences Research Council (EPSRC) for supporting this work financially (Grant No EP/K001329/1).References
- P. Markewitz, W. Kuckshinrichs, W. Leitner, J. Linssen, P. Zapp, R. Bongartz, A. Schreiber and T. E. Muller, Energy Environ. Sci., 2012, 5, 7281–7305 CAS.
- M. Aresta, Carbon Dioxide as Chemical Feedstock, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2010 Search PubMed.
- N. MacDowell, N. Florin, A. Buchard, J. Hallett, A. Galindo, G. Jackson, C. S. Adjiman, C. K. Williams, N. Shah and P. Fennell, Energy Environ. Sci., 2010, 3, 1645–1669 CAS.
- E. A. Quadrelli, G. Centi, J.-L. Duplan and S. Perathoner, ChemSusChem, 2011, 4, 1194–1215 CrossRef CAS PubMed.
- G. Centi and S. Perathoner, Catal. Today, 2009, 148, 191–205 CrossRef CAS PubMed.
- C. Song, Catal. Today, 2006, 115, 2–32 CrossRef CAS PubMed.
- A. J. Hunt, E. H. K. Sin, R. Marriott and J. H. Clark, ChemSusChem, 2010, 3, 306–322 CrossRef CAS PubMed.
- N. von der Assen, J. Jung and A. Bardow, Energy Environ. Sci., 2013, 6, 2721 CAS.
- International Energy Agency, CO2 Emissions from fuel combustion – Highlights, Paris, 2013 Search PubMed.
- International Energy Agency, Energy Technology Perspectives – Scenarios Strategies to 2050, Paris, 2010 Search PubMed.
- Mitsui Chemicals, http://http//www.mitsuichem.com/, 2014.
- O. Joo, K. Jung and Y. Jung, in Stud. Surf. Sci. Catal., ed. S.-E. Park, J.-S. Chang and K.-W. Lee, Elsevier, 2004 Search PubMed.
- Carbon Recycling International, http://www.carbonrecycling.is/, 2014.
- International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE), Renewable Hydrogen Report, http://www.iphe.net/renewablereport.html, 2014.
- R. B. Gupta, Hydrogen Fuel: Production, Transport and Storage, CRC Press, Boca Raton, Florida, USA, 2009 Search PubMed.
- L. Appels, J. Lauwers, J. Degrève, L. Helsen, B. Lievens, K. Willems, J. Van Impe and R. Dewil, Renewable Sustainable Energy Rev., 2011, 15, 4295–4301 CrossRef CAS PubMed.
- D. Gray, P. Suto and C. Peck, Anaerobic Digestion of Food Waste, Report EPA-R9-WST-06-004, US Environmental Protection Agency, Oakland, 2008 Search PubMed.
- E. Ryckebosch, M. Drouillon and H. Vervaeren, Biomass Bioenergy, 2011, 35, 1633–1645 CrossRef CAS PubMed.
- Metcalf and Eddy Inc., Wastewater Engineering – Treatment and Reuse, McGraw-Hill, 4th edn, 2003 Search PubMed.
- L. Zhu, G. W. Schade and C. J. Nielsen, Environ. Sci. Technol., 2013, 47, 14306–14314 CrossRef CAS PubMed.
- T. L. Sonderby, K. B. Carlsen, P. L. Fosbol, L. G. Kiorboe and N. von Solms, Int. J. Greenhouse Gas Control, 2013, 12, 181–192 CrossRef PubMed.
- S. Wang, G. Q. M. Lu and G. J. Millar, Energy Fuels, 1996, 10, 896–904 CrossRef CAS.
- The Linde Group, http://www.linde-engineering.com/, 2014.
- F. Lau, Techno-Economic Analysis of Hydrogen Production by Gasification of Biomass, Report DE-FC36-01GO11089, 2002 Search PubMed.
- T. Kaneko, F. Derbyshire, E. Makino, D. Gray, M. Tamura and K. Li, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000 Search PubMed.
- R. L. Espinoza, A. P. Steynberg, B. Jager and A. C. Vosloo, Appl. Catal., A, 1999, 186, 13–26 CrossRef CAS.
- P. L. Spath and D. C. Dayton, Preliminary Screening – Technical and Economic Assessment of Synthesis Gas to Fuels and Chemicals with Emphasis on the Potential for Biomass-Derived Syngas, Report NREL/TP-510-34929, National Renewable Energy Laboratory, 2003 Search PubMed.
- M. E. Dry, J. Chem. Technol. Biotechnol., 2001, 77, 43–50 CrossRef PubMed.
- M. J. A. Tijmensen, A. P. C. Faaij, C. N. Hamelinck and M. R. M. Hardeveld, Biomass Bioenergy, 2002, 23, 129–152 CrossRef CAS.
- A. De Klerk, Y.-W. Li and R. Zennaro, in Greener Fischer–Tropsch Processes for Fuels and Feedstocks, ed. P. M. Maitlis and A. De Klerk, John Wiley & Sons, Inc., 2013 Search PubMed.
- H. Schulz, Appl. Catal., A, 1999, 186, 3–12 CrossRef CAS.
- Aspen Technology, Aspen Plus Version 8.4, Burlington, MA, USA, 2013 Search PubMed.
- Aspen Technology, Aspen Physical Property System – Physical Property Methods, Burlington, USA, 2013 Search PubMed.
- L. Appels, J. Baeyens, J. Degrève and R. Dewil, Prog. Energy Combust. Sci., 2008, 34, 755–781 CrossRef CAS PubMed.
- S. Rasi, a. Veijanen and J. Rintala, Energy, 2007, 32, 1375–1380 CrossRef CAS PubMed.
- M. R. M. Abu-Zahra, L. H. J. Schneiders, J. P. M. Niederer, P. H. M. Feron and G. F. Versteeg, Int. J. Greenhouse Gas Control, 2007, 1, 37–46 CrossRef CAS.
- P. C. Hanlon, Compressor Handbook, McGraw-Hill, New York, 2001 Search PubMed.
- Aspen Technology, Aspen Plus Cogeneration Model, Burlington, USA, 2011 Search PubMed.
- K. Liu, C. Song and V. Subramani, Hydrogen and Syngas production and purification, Wiley-VCH, New Jersey, 2010 Search PubMed.
- E. D. Larson, H. Jin and F. E. Celik, Biofuels, Bioprod. Biorefin., 2009, 3, 174–194 CrossRef CAS PubMed.
- F. Trippe, M. Fröhling, F. Schultmann, R. Stahl, E. Henrich and A. Dalai, Fuel Process. Technol., 2013, 106, 577–586 CrossRef CAS PubMed.
- I. Bechtel Group, Slurry reactor design studies; Slurry vs. fixed-bed reactors for Fischer–Tropsch and methanol: final report, US Department of Energy Project No. DE-AC22-89PC89867, Pittsburgh Energy Technology Center, Pittsburgh, PA, 1990 Search PubMed.
- A. Sauciuc, Z. Abosteif, G. Weber, A. Potetz, R. Rauch, H. Hofbauer, G. Schaub and I. Dumitrescu, ICPS11 International Conference on Polygeneration Strategies, 30 August – 1 September 2011, ed. H. Hofbauer and M. Fuchs, Vienna, 2011 Search PubMed.
- Aspen Technology, Aspen Icarus Reference Guide – Icarus Evaluation Engine (IEE), Burlington, USA, 2013 Search PubMed.
- R. M. Swanson, A. Platon, J. A. Satrio and R. C. Brown, Fuel, 2010, 89, S11–S19 CrossRef CAS PubMed.
- R. K. Sinnott, Coulson and Richardson's Chemical Engineering Volume 6 – Chemical Engineering Design, Elsevier, 4th edn, 2005 Search PubMed.
- Alibaba Group, http://http//www.alibaba.com/showroom/monoethanolamine-price.html, 2014.
- Alibaba Group, http://http//www.alibaba.com/showroom/molecular-sieve-13x.html, 2014.
- Department of Energy and Climate Change, http://https://www.gov.uk/government/statistical-data-sets/international-domestic-energy-prices, 2014.
- P. Haro, P. Ollero, A. L. Villanueva Perales and A. Gómez-Barea, Appl. Energy, 2013, 102, 950–961 CrossRef CAS PubMed.
- J. Eilers and S. T. Sie, Catal. Lett., 1990, 7, 253–270 CrossRef CAS.
- M. S. Peters, K. D. Timmerhaus and R. E. West, Plant Design and Economics for Chemical Engineers, McGraw-Hill, New York, 5th edn, 2003 Search PubMed.
- OANDA, http://www.oanda.com/currency/historical-rates/, 2014.
- UK petroleum industry association (UKPIA), http://www.ukpia.com/fuel-prices-historic-data.aspx, 2014.
| This journal is © The Royal Society of Chemistry 2015 |