Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Environmental and health impact assessment of Liquid Organic Hydrogen Carrier (LOHC) systems – challenges and preliminary results†
M.
Markiewicz
*a,
Y. Q.
Zhang
a,
A.
Bösmann
b,
N.
Brückner
b,
J.
Thöming
a,
P.
Wasserscheid
b and
S.
Stolte
ac
aUFT – Centre for Environmental Research and Technology, University of Bremen, Leobener Straße, D-28359 Bremen, Germany. E-mail: martam@uni-bremen.de
bInstitute of Chemical Reaction Engineering, Friedrich Alexander University of Erlangen Nürnberg, Egerlandstraße 3, 91058 Erlangen, Germany
cDepartment of Environmental Analysis, Faculty of Chemistry, University of Gdańsk, ul. Sobieskiego 18, 80-952 Gdańsk, Poland
First published on 4th February 2015
Abstract
Liquid Organic Hydrogen Carrier (LOHC) systems offer a very attractive way to store and transport hydrogen, a technical feature that is highly desirable to link unsteady energy production from renewables with the vision of a sustainable, CO2-free, hydrogen-based energy system. LOHCs can be charged and discharged with considerable amounts of hydrogen in cyclic, catalytic hydrogenation and dehydrogenation processes. As their physico-chemical properties are very similar to diesel, today's infrastructure for liquid fuels can be used for their handling thus greatly facilitating the step-wise transition from today's fossil system to a CO2 emission free energy supply for both, stationary and mobile applications. However, for a broader application of these liquids it is mandatory to study in addition to their technical performance also their potential impact on the environment and human health. This paper presents the first account on the toxicological profile of some potential LOHC structures. Moreover, it documents the importance of an early integration of hazard assessment in technology development and reveals for the specific case of LOHC structures the need for additional research in order to overcome some challenges in the hazard assessment for these liquids.
Broader contextDue to increasing environmental awareness, many countries try to optimize their economies for a low-carbon growth turning towards renewable energy sources. Nevertheless, to fully exploit these sources fundamental change in our energy supplies is needed. Hydrogen is considered a main player in future energy systems, especially for mobile applications but its storage poses a technological challenge. Liquid Organic Hydrogen Carrier (LOHC) systems offer a very attractive way to store and transport hydrogen that links unsteady energy production from renewables with the vision of a sustainable, CO2-free, hydrogen-based energy system. LOHCs can be charged and discharged with considerable amounts of hydrogen in cyclic, catalytic hydrogenation and dehydrogenation processes. As their physico-chemical properties are very similar to those of diesel, today's infrastructure for liquid fuels can be used for their handling thus greatly facilitating the step-wise transition from today's fossil system to a CO2 emission free energy supply for both, stationary and mobile applications. However, for a broader application of these liquids it is mandatory to study in addition to their technical performance also their potential impact on the environment and human health. |
Introduction
Modern societies depend on steady and reliable supply of energy.1,2 Due to the increasing environmental awareness, many countries try to optimize their economies for a low-carbon growth, i.e. a growth that happens without a major increase in CO2 emissions, e.g. without burning additional fossil fuels. Currently, with its Roadmap 2050, the European Union has set new long-term goals in energy policy including an 80% reduction of domestic CO2 emissions.3 To comply with these goals a fundamental change in our energy system is needed.The amount of energy that can be harvested from renewable sources, such as sun, wind and hydropower is extremely high. It can satisfy the global energy demand over hundred times with the enormous benefit of being inexhaustible.4,5 Energy from these renewable sources has many socio-economic advantages over fossil fuel or nuclear based energy: (a) zero or very low variable costs of generation; (b) lower environmental impact since there are almost no emissions and no waste production associated with the power generation; (c) applicability for decentralized power generation.4
However, some major technical challenges remain to be addressed before the full transition to a renewable-based energy system can take place successfully:
• The production of most renewable energies is geographically limited, dependent on unforeseeable weather conditions and intermittent; even though in virtually every location on the globe some kind of sustainable energy can be produced it usually does not meet the spatiotemporal demand;5,6
• Energy systems with a high share of energy from wind or sun are characterised by periods in time when overproduction of energy from renewable resources causes very low or negative stock energy prices;7–9
• Renewable energies are currently mostly used to power stationary consumers to which they are transported via the electric grid; their use in mobile applications, though extensively researched and certainly very relevant,10–12 is still far less advanced.
Hydrogen is considered as a main player in future energy systems, especially for mobile applications as it is a clean fuel of very high gravimetric energy density (120 MJ kg−1). Its gravimetric energy density is three times higher than that of gasoline and any other liquid fuel.4,13 Furthermore, hydrogen powered cars using a fuel cell have efficiencies of energy conversion of 50–60%, much higher than today's cars using fossil fuels and an internal combustion engine where maximum efficiencies of about 25% are reported.8,14,15 In order to use hydrogen as a fuel for vehicles an on-board storage system is required that contains suitable amounts of hydrogen. Note that a medium size vehicle needs between 0.8 and 1 kg H2 per 100 km. The hydrogen storage system should be light, compact and safe. Moreover, the system should allow a dynamic hydrogen release on demand and a fast H2 filling of the storage system without the need for specific or new infrastructure.
Given these very tough requirements it is important to state that the use of hydrogen in a future energy systems is by far not restricted to its use in cars and trucks. Many other mobile (e.g. forklifts), transportable (e.g. portable electronics), and stationary applications (stand-alone energy systems, back-up systems) are discussed and attract high commercial interest. Apart from using hydrogen in fuel cells there remains the option to burn hydrogen in a combustion engine. Using lean-hydrogen mixtures at not too high temperatures (to disfavour formation of NOx) may also lead to more sustainable energy processes at somewhat lower investment cost and higher technical robustness.
While the gravimetric energy storage density of hydrogen is excellent its volumetric storage density suffers from the very low H2 density. Under ambient conditions one liter of gaseous hydrogen contains only 10.8 kJ of energy. Even under very high pressures (70 MPa H2, called “Compressed Gaseous Hydrogen” or CGH2) or in its liquid state which requires temperatures below 20 K (called “Liquid Hydrogen” or LH2) the volumetric energy density of hydrogen is low. Liquid hydrogen has a density of 71.2 kg m−3 resulting in an energy storage capacity under these very challenging conditions of 8.3 MJ L−1 which is by a factor of four lower than the volumetric storage density of typical fuels under ambient conditions. Note that the compression of hydrogen and especially the cooling of hydrogen are energy intensive and costly operations and that hydrogen losses by diffusion (high pressure storage) or by boil-off (cryogenic hydrogen storage) may lead to relevant hydrogen/energy losses. Under current technologically feasible conditions CGH2 storage uses around 15% of stored energy to achieve 70 MPa compression and LH2 as much as 30% for liquefaction (based on lower heating value of H2 of 120 MJ kg−1).16 Other disadvantages result from time consuming loading and unloading procedures (mainly for LH2) and the need for specific infrastructures.17
So far several technical options have been proposed to store and transport hydrogen in a more economic and efficient manner. These include for example physical sorption on high surface area materials (e.g. nanostructured materials like active carbon) or chemical adsorption to solids leading to solid hydride materials.12,14,18 When using hydrides there is always a trade-off between storage capacity and hydrogen desorption temperature, however, recent development especially regarding doped alanates brought these materials closer to fulfilling current hydrogen technology requirements.19,20 However, these solutions have a number of severe drawbacks with respect to their practicability: apart from limited hydrogen carrying capacity and time consuming loading/unloading procedures with the significant heat production/heat demand, the handling of solids is impractical for the storage and transport of larger amounts of energy.
The catalytic hydrogenation of hydrogen-lean molecules offers another option to store and transport energy in the form of hydrogen. Nitrogen can be hydrogenated to ammonia,21 CO2 can be hydrogenated to either formic acid,22 methane,23 methanol,24 or Fischer–Tropsch products.25 However, all these options have one thing in common, they use gaseous substances as energy-lean molecules and as a consequence they require isolation of CO2 or N2 from air or exhaust gas streams in appropriate quality and quantity for the hydrogen storage process and they release mixtures of hydrogen and the hydrogen-lean gas during dehydrogenation reaction instead of pure hydrogen.
This important drawback is circumvented if organic liquids of low vapour pressure are used as hydrogen-lean compounds, a concept for which the name “Liquid Organic Hydrogen Carrier (LOHC)” has been coined. Starting from pioneering work in the 1990s, later patents by Pez, Scott, Copper and Cheng from Air Products26,27 and continuous intensive research in the last couple of years,28 LOHC systems have developed to a very promising technology for hydrogen storage and transport. LOHC systems are formed by pairs of organic compounds, the hydrogen-lean one being typically an aromatic or heteroaromatic compound, the other hydrogen-rich one being typically an alicyclic or heterocyclic compound.26,29–32 LOHCs are loaded with hydrogen in analogy to large scale catalytic hydrogenation reactions of the chemical industry. Typical reaction conditions for the exothermic LOHC hydrogenation are hydrogen pressures of 1 to 5 MPa and temperatures of 373 to 523 K. Typical hydrogenation catalysts are Ni- or Ru on oxide supports applied in slurry phase tank reactors or trickle bed hydrogenation units.33,34 Typical reaction conditions for the endothermic LOHC dehydrogenation are hydrogen pressures of 1 to 0.5 MPa and temperatures of 423 to 673 K. Typical dehydrogenation catalysts are Pt or Pd on oxide supports applied in slurry phase tanks or tubular reactors.35,36
In the context of the storage and transport of renewable energy equivalents, excess renewable electric energy is converted into high pressure hydrogen by electrolysis (typical H2 pressures of electrolysers are 1 to 5 MPa) and the latter is used directly to hydrogenate the hydrogen-lean form of the LOHC. The hydrogen-charged LOHC can be regarded as a liquid transport form of hydrogen that can be handled in today's infrastructure for liquid fuels (pipelines, oil tanker and petrol stations).37 Thus, LOHCs enable long-time energy storage under ambient temperature and pressure conditions without significant losses. On energy or hydrogen demand, the hydrogen-rich LOHC molecule is heated to the dehydrogenation temperature and allowed to be in contact with the dehydrogenation catalyst. Further heat is added to the reactor to deal with the endothermic nature of the dehydrogenation reaction. The hydrogen-lean form of the LOHC-system is isolated by simple condensation from the dehydrogenation reactor together with very pure hydrogen. The hydrogen-lean LOHC compound is stored for its next charging cycle or transported to a place with cheap and available regenerative energy. Fig. 1 illustrates the storage and transport of renewable energy equivalents using LOHC systems.
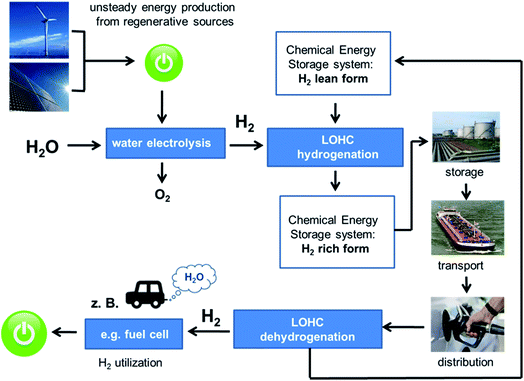 | ||
| Fig. 1 Schematic view on storage and transport of regenerative energy equivalents using Liquid Organic Hydrogen Carrier (LOHC) systems (reproduced with permission from ref. 38, Copyright American Chemical Society). | ||
Initially one or two six-membered ring compounds like benzene, toluene, naphthalene, biphenyl and their corresponding hydrogenated equivalents cyclohexane, methylcyclohexane, decaline (dodecahydronaphthalene), and bicyclohexyl were suggested as LOHC systems (Fig. 2).39–41 These compounds have storage capacities between 6 and 7 wt% H2 and can be hydrogenated under relatively mild conditions.31 However, some of these compounds are too toxic (e.g. benzene) or too volatile (benzene/cyclohexane, toluene/methylcyclohexane) to be of greater practical relevance, at least so far.26,40,42 More recently, thiophene, quinaldine and carbazole derivatives were also recommended as LOHC systems as the presence of a hetero atom reduces the heat of hydrogenation/dehydrogenation and thus allows for dehydrogenation at milder temperatures.43,44 In particular, N-ethylcarbazole (NEC)/perhydro-N-ethylcarbazole (H12-NEC) has found a lot of interest as a LOHC system due to its relatively high H2 storage capacity (5.8 wt% H2) and its good dehydrogenation characteristics at 453–533 K (ambient pressure, heterogeneous Pd- or Pt catalyst).26,30,37 The hydrogen-lean form, NEC is a solid with a melting point of 341 K. This is much lower than the melting point of carbazole (mp. = 518 K) but still not ideal. The solid nature of the fully dehydrogenated molecules complicates the technical use of this LOHC system as either the tank has to be heated to 343 K or the dehydrogenation degree has to be limited to ca. 90% for the mixture of fully and partially dehydrogenated substances to remain liquid. However, this reduces the effective hydrogen capacity to about 5.2 wt%. There is also a risk for NEC dealkylation at temperatures above 533 K. While these conditions are above the normal dehydrogenation conditions they still limit operating the hydrogen release reaction at very high temperature levels and thus much faster which would allow us to use smaller reactors.38
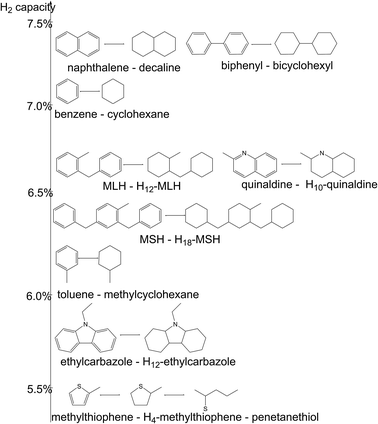 | ||
| Fig. 2 Examples of LOHCs systems (H2-lean and H2-rich forms) and their gravimetric hydrogen carrying capacity. | ||
Recently isomeric mixtures of perhydro-benzyltoluene and perhydro-dibenzyltoluene were also proposed as LOHCs.38 The hydrogen-lean form of these LOHC systems, benzyltoluene and dibenzyltoluene, are readily available and technically applied as heat transfer oils in the form of their isomeric mixtures. Typical trade names of these substances are Marlotherm LH (MLH, i.e. mixture of benzyltoluenes) or Marlotherm SH (MSH, i.e. mixture of dibenzyltoluenes). Dehydrogenation of the respective hydrogen-charged mixtures, H12-MLH and H18-MSH requires higher temperatures than for the dehydrogenation of H12-NEC (553–633 K for H18-MSH vs. 453–533 K for H12-NEC). However, the MSH/H18-MSH system offers low melting points of all relevant mixtures and species (<243 K), high hydrogen capacity (6.2 wt% H2), excellent technical availability, and a huge amount of available data concerning thermal stability and heat transfer properties.38
All the named compounds can undoubtedly store hydrogen, however, taking into account all possible structural variations of LOHC molecules, the enormous dimension of potential LOHC applications in the energy system and the cost related to implementation of the LOHC technology, industry can only afford to develop a very limited number of the most promising LOHC candidates to a full commercial scale. Therefore intense investigations to limit the set of potential structures to the most promising candidates are in progress.
Apart from technological and economic aspects safety and environmental criteria must also be taken into account for the selection of the most promising LOHC system. The hazard assessment should be performed at the early research and development stages – moving from “end of pipe” solutions (addressing environmental problems after they have manifested themselves) to proactive environmental protection – in order to anticipate and assess the hazards that each involved chemical might pose. This approach might open the chance to focus research and development efforts on such LOHC systems with reduced hazard potential and higher intrinsic safety. Regardless of which of the hundreds of possible LOHC structures will finally make it to the market, it will be handled, processed, stored and transported in vast quantities. An average stationary storage application will need to process 1 kg LOHC material for ca. 7.2 MJ of thermal energy stored in the form of its releasable hydrogen. For potential future mobile applications of the technology a car would need to dehydrogenate between 12 and 20 litres of LOHC material per 100 km driving range. Shall these technologies penetrate markets in an extended manner, LOHC chemicals will become high production volume chemicals (HPVC) that are globally used by the public with potential release into the biosphere, for example, during fuelling, via leakages or in accidental spills. In recent decades the extensive production, use and release of man-made chemicals have resulted in serious environmental problems and have raised public awareness of the hazards arising from chemical substances and technologies in general. Therefore especially HPVC are subjected to strict health/environmental regulations such as the European Union Regulation REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals). Generally, the public opinion and acceptance is of highest importance when implementing new technologies – as the controversy concerning risk of hydraulic fracturing currently shows.45
Therefore this paper aims to demonstrate an approach for the early integration of hazard assessment into the development of the LOHC technology. First data give indications of possible hazards for some potential LOHC structures. More important we want to address the need for research and the challenges that have to be faced when assessing the environmental impact of LOHC systems. Hereby, we hope to encourage further research in this important field which will help to facilitate the LOHC selection on technological, economic and environmental grounds to provide the base for a broad public acceptance of this highly promising hydrogen storage and transport technology with the potential to contribute to a CO2-free energy system.
Short introduction to risk assessment
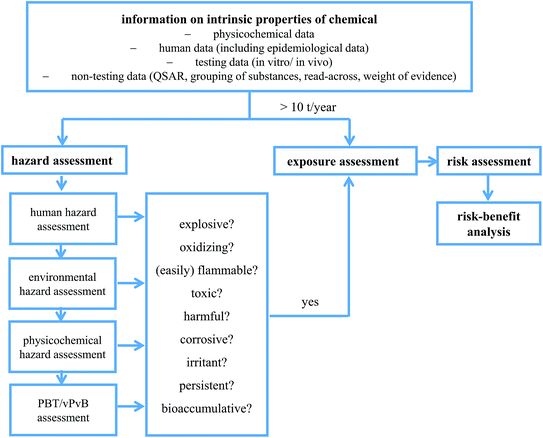
In order to protect human health and the environment, chemicals produced or imported into European Union in quantities higher than 1 ton per year have to be subjected to REACH. REACH, depending on the production volume, requires varying extents of information on identity, physicochemical properties, mammalian toxicity, ecotoxicity, environmental fate (including biotic and abiotic degradation), manufacturing and applications which are used for the assessment of risk associated with chemicals. In risk assessment there are two important building blocks: hazard and release/exposure. In simple words risk assessment is based on identifying harmful effects that can be exerted by the chemical substance (hazard) and assessing the likelihood of these effects to occur based on predicted release (exposure). Fig. 3 shows a simplified scheme of assessment of chemicals.For a chemical to be recognized as environmentally safe it should be biodegradable, non-toxic and non-accumulative so that it can be assured that whenever it is released it will breakdown quickly to non-harmful products and will not persist in any of the environmental compartments including living organisms. In order to make that declaration possible it has to be made sure, at the very least, that the substance does not fulfil Persistent, Bioaccumaulative, Toxic/very Persistent, very Bioaccumaulative, Toxic (PBT/vPvBT) or Carcinogenic Mutagenic Toxic for Reproduction (CMR) criteria and does not act as an endocrine disrupter as those are the substances of particular concern.
When assessing the risk associated with chemicals we are most often interested in effects on humans and on the environment. Since it is not possible to test the effect of the chemicals in question directly on humans as well as under every relevant environmental condition some kind of model or approximation has to be made. The more distant this model is from the subject of interest the more uncertainty it carries. Therefore defining the risks associated with chemicals is always affected by uncertainties resulting mainly from the difficulty in extrapolating from model test results to real subjects. Note that the assessment of known pollutants, like the “dirty dozen” (twelve chemicals recognised in 2001 by United Nations Stockholm Convention as persistent organic pollutants of particular concern) is easier due to the fact that historical or epidemiological data for those compounds exist together with a multitude of model tests conducted under different conditions. The risk assessment of “new chemicals”, that were or are not regularly detected in the environment is challenging. No historical data and often limited model test data are available and the assessment has to be started from scratch by e.g. numerical models (Quantitative Structure-Activity Relationship QSAR) which – though very useful – result in a greater degree of uncertainty. The results obtained by QSARs can act as indicators or guidelines but have to be verified.
Specific challenges when assessing risks associated with LOHC systems
The aim of performing e.g. the ecotoxicological test with model organisms is to reduce the uncertainty factor in risk assessment by indicating hazards, possible modes of actions and organisms which might be particularly prone to the action of the chemical under investigation.Even though some potential LOHC chemicals suggested in the literature are relatively common organic compounds, the amount of data that are needed for their risk/fate assessment is scarce. There is a strong need not only for basic ecotoxicological parameters but also for information as simple as the solubility in water or the octanol/water partition coefficient. As most LOHC structures suggested so far are organic, uncharged chemicals, they are somehow volatile (with a technical tendency for using representatives of relatively low vapour pressure to allow easy hydrogen/LOHC separation), will have an affinity to organic phases (i.e. organic matter, biological membranes etc.) and their aqueous solubility will be somehow limited.
In all future scenarios of LOHC application, the LOHC molecules are expected to be produced in technical quantities and technical qualities. This means that the LOHC systems will also include some amounts of contaminants. Apart from impurities in the starting material, the working LOHC systems will always contain a mixture of hydrogenated, partially hydrogenated and hydrogen-lean compounds depending on the degree of hydrogen-loading.
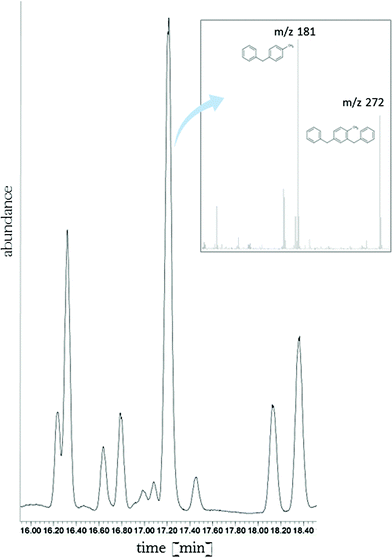
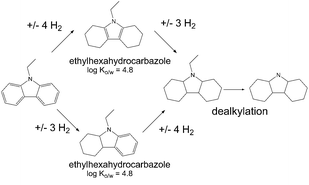
For example, our initial examination of the LOHC system NEC/H12-NEC revealed that octahydro compounds are dominant in partially dehydrogenated mixtures but some small amounts of the hexahydro form are also present (Fig. 5). It is worth noting that the physicochemical properties of those two forms are quite different, as an example the Ko/w of octahydro-N-ethylcarbazole is approximately one order of magnitude lower than Ko/w of hexahydro-N-ethylcarbazole. Even though most reported LOHC systems show very high stability under operation conditions, the fact that they are meant to be recycled many times in hydrogen charging and uncharging cycles suggests that some degree of breakdown or aging should be expected. This effect can be simulated by catalytic dehydrogenation under very harsh temperature conditions (>543 K) for the NEC/H12-NEC system and leads to NEC dealkylation to carbazole. The properties of all those diverse structures in the LOHC system can be very different therefore they should all actually be assessed as separate entities or mixtures prior to a large scale use of these systems.
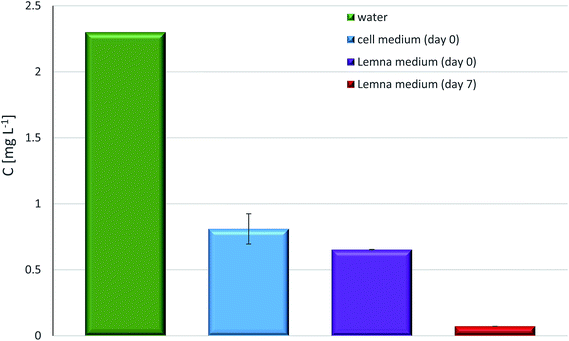
Due to these complications it is necessary to confirm in tests with poorly water soluble compounds the real concentration that was available during the toxicology test. This very often, and most certainly in the case of some of the proposed LOHC structures, requires sophisticated analytics as the nominal concentrations in the test can be in the μg L−1 to ng L−1 range (predicted aqueous solubility of H18-MSH is in ng L−1 range48) and become even lower as the result of sorption. An order of magnitude differences depending on composition of medium can be expected as shown in our results with the NEC/H12-NEC system using different contact times and different biological assays (Fig. 6).
The environmental distribution should be taken into account at the very beginning of hazard assessment as it defines the possible routes of release and exposure. Substances that are well water soluble and non-volatile e.g. inorganic salts will be predominantly released with water streams and will remain dissolved to a large extent. Their main route of exposure will be via water and to some extent diet. Substances that are poorly water soluble e.g. neutral, organic compounds like LOHCs compounds, might be released with water streams but after that they will most probably find some kind of a sink (e.g. sediments) and become adsorbed which can decrease their bioavailability. Their main route of exposure will be via diet and the exposure via water will be of limited importance. Considering distribution will therefore be of importance in selecting the most meaningful tests and most realistic routes of exposure.
The main route of exposure in acute aquatic toxicity tests is passive diffusion through integumentum. Therefore only the fraction of test compounds that is truly dissolved in water can exert the toxic effect in this way.49 On the other hand higher Ko/w results in higher affinity to hydrophobic phases, including biological membranes, and implies higher toxicity. Many potential LOHCs can be classified as poorly water soluble based on predictions as for most of them basic physicochemical properties like aqueous solubility or Ko/w are missing.
In extreme cases the aqueous solubility might be so low that the highest concentration which can be obtained in water/medium is too low for any acute toxic effects to be observed, so that it is not possible to obtain a full dose–response curve and derive an half maximal effective concentration (EC50) value. In fact for some LOHCs solubility can hardly be measured as in the case of H18-MSH whose predicted aqueous solubility lies in low ng L−1 range.48 From this extremely low water solubility it does not necessarily follow, however, that the compound is not toxic. For very hydrophobic compounds (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ko/w > 5) chronic toxicity cannot be excluded even if there are no observable effects in acute tests as the compound might not have been sufficiently taken up by the test organism during the test duration. As they might be accumulating in living organisms these types of compounds have to be investigated for chronic effects. It is conceivable that poorly soluble compounds can form biphasic systems in the environment like e.g. oil spills forming droplets or layers on the surface of water. It is also possible that during prolonged exposure the compound will be concentrated in hydrophobic phases of living organisms (like fatty tissue, biological membranes) as a result of partitioning or will be ingested as droplets or in the particle bound form (sorbed on humic matter or biomass on which they feed) and build up in the body. In such a case the amount of compound that acts upon the test subjects might significantly exceed the water solubility limit. Building the concentration up in a longer food chain – biomagnification – might have even more pronounced ecological effects and can influence humans directly. Therefore, it is advisable to include the chronic test or the multi-generations test in addition to acute tests in the toxicity evaluation especially for poorly water soluble substances as the same processes take place in the environment.
Ko/w > 5) chronic toxicity cannot be excluded even if there are no observable effects in acute tests as the compound might not have been sufficiently taken up by the test organism during the test duration. As they might be accumulating in living organisms these types of compounds have to be investigated for chronic effects. It is conceivable that poorly soluble compounds can form biphasic systems in the environment like e.g. oil spills forming droplets or layers on the surface of water. It is also possible that during prolonged exposure the compound will be concentrated in hydrophobic phases of living organisms (like fatty tissue, biological membranes) as a result of partitioning or will be ingested as droplets or in the particle bound form (sorbed on humic matter or biomass on which they feed) and build up in the body. In such a case the amount of compound that acts upon the test subjects might significantly exceed the water solubility limit. Building the concentration up in a longer food chain – biomagnification – might have even more pronounced ecological effects and can influence humans directly. Therefore, it is advisable to include the chronic test or the multi-generations test in addition to acute tests in the toxicity evaluation especially for poorly water soluble substances as the same processes take place in the environment.
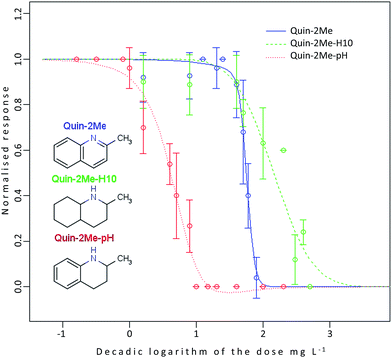
Table 1 summarizes EC50 values for the same compounds obtained experimentally and predicted using QSAR. What is evident is that EC50 values differ by around two orders of magnitude for the different hydrogenated forms indicating differences in their toxicities. Nevertheless in this example the EC50s are rather high (meaning low acute toxicity towards this organism) and in general one to three orders of magnitude higher than a cut-off value of 0.1 mg L−1 for classification as ‘T’.50 This is the first indication that those compounds would not be classified as environmentally toxic in PBT assessment based on the Daphnia test. However, tests with algae and/or fish are still necessary to make definite conclusion. Moreover, the QSAR model used for prediction works quite well for aromatic and partially hydrogenated quinaldines but seems to overestimate the toxicity of the fully hydrogenated compound by an order of magnitude. To further illustrate this point, literature EC50 values experimentally obtained in the same test system using loadings (water accommodated fraction) of diesel fuel and gasoline are also given.51 Based on this comparison aromatic (Quin-2Me) and fully hydrogenated (Quin-2Me-H10) forms of quinaldine show toxicity comparable to diesel fuel. The EC50 value of the partially hydrogenated form (Quin-2Me-pH) is nearly an order of magnitude lower, which makes it more toxic than the fully H2 loaded and unloaded forms and approximately as toxic as gasoline. However, none of the compounds listed in Table 1 has to be classified as “T” based on the results from the Daphnia magna test.
| Compound | Measured EC50 [mg L−1] | Predicted EC50 [mg L−1] |
|---|---|---|
| a Source of data: European Chemical Agency.51 b Diesel fuel no. 2 (CAS 68476-34-6). c Natural gasoline (CAS 8006-61-9), n/a not available. | ||
| Quin-2Me | 56 (53–59) | 17 |
| Quin-2Me-pH | 2.7 (2.3–3.2) | 5.1 |
| Quin-2Me-H10 | 204 (155–204) | 10 |
| Diesel fuel no. 2b | 138a | n/a |
| Natural gasolinec | 4.5a | n/a |
As mentioned before another important element of environmental assessment is PBT evaluation which includes biodegradability. This is to make sure that target compounds can be biologically degraded in a reasonable time frame and will not be accumulating in e.g. surface waters. Additional information that can be derived from the biodegradation test is an indication that target compounds can be degraded during standard wastewater treatment. An inoculum used for e.g. ready biodegradability testing is often a diluted microbial community obtained from an activated sludge aeration tank – a core part of wastewater treatment. Therefore positive results of biodegradation testing indicate that removal in the wastewater treatment plant will most probably be possible although no assumptions regarding degradation rates or time frames can directly be made.52
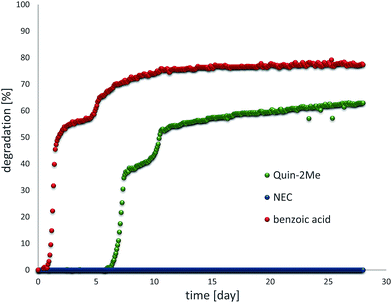
There is a set of rules of thumb allowing to “guesstimate” if compounds will be degradable or not. Aromatic forms of LOHC structures presented here generally do not possess structural features that are considered to hinder biodegradability (high degree of halogenation, more than 3 aromatic rings, excessive branching etc.), except the presence of the heteroatom.53 The heteroatom itself is usually not very problematic unless it is substituted like in the case of N-ethylcarbazole.54 Indeed, our preliminary biodegradation study revealed that Quin-2Me containing unsubstituted nitrogen in the ring is degradable to a high extent but N-ethylcarbazole bearing an ethyl group on N atom does not show any biodegradation even though its unsubstituted parent compound carbazole is known to be degradable (Fig. 8).55
| NEC | H8-NEC | H12-NEC | |
|---|---|---|---|
| a Models used for generating screening data: aBiowin2,48bBiowin3,48cBiowin6,48dKOWWIN,48eECOSAR,48 (data for baseline toxicity). Decision making criteria: (1) for classifying as P the outcome of Biowin2 has to be “does not biodegrade fast” and Biowin3 predicted the biodegradation time frame ≥ months or outcome of Biowin2 has to be “does not biodegrade fast” and Biowin6 predicted the ultimate biodegradation time frame ≥ months; (2) for classifying as not B Ko/w < 4.5; (3) for classifying as potentially T the EC50 or LC50 < 0.1 mg L−1 in the algae, daphnia or fish test.50 | |||
| Persistence assessment | |||
| Biodegradation probability a | Biodegrades fast | Biodegrades fast | Does not biodegrade fast |
| Ultimate biodegradation time frame b | Weeks | Weeks–months | Weeks–months |
| Ready biodegradation probability c | Not readily degradable | Not readily degradable | Not readily degradable |
| P indicator | Not P1 | Not P1 | Not P1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Bioaccumulation assessment | |||
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ko/wd Ko/wd |
4.33 | 5.85 | 3.44 |
| B indicator | Not B2 | Potentially B/vB2 | Not B2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Toxicity assessment | |||
| EC50 [mg L−1] algaee | 1.8 | 0.15 | 7.3 |
| EC50 [mg L−1] daphniae | 1.0 | 0.05 | 5.6 |
| EC50 [mg L−1] fishe | 1.5 | 0.06 | 8.7 |
| T indicator | Presumably not T3 | Potentially T3 | Presumably not T3 |
The higher the quantity of a given chemical circulating on the markets the more data has to be gathered for its risk assessment. In the case of LOHC technology the amount of carrier needed and the potential for release will be relatively high if the technology becomes a technical success. Therefore, it is particularly important to choose a system which does not raise significant concerns in terms of PBT/CMR assessment. In general any QSAR derived indicators are ‘class specific’ and will perform relatively good if the chemical in question is structurally similar to the training set used to establish that QSAR. In this context ‘similar’ very often means having similar Ko/w at least for ecotoxicity assessment. Since the universe is not made of both water and octanol one can easily imagine that the reality can be much more complex. In terms of compliance with REACH QSAR derived data are so far only admissible as the supporting information†, therefore generation of test data cannot be avoided.
LOHCs suggested to date are a group of structurally diverse chemicals therefore it is difficult to make general statements with regard to their overall environmental and health impact. Further testing is needed for most structures of interest.
Conclusion
A proper communication of risk associated with using LOHC compounds to the general public is a key in gaining social acceptance for a future LOHC-based energy and hydrogen transport. This new technology promises a link between unsteady renewable electricity production and a CO2-free energy supply for stationary and mobile applications and thus offers multiple benefits for the society.Many different organic molecules can serve as potential LOHC structures. Most of them are uncharged organics, thus volatile, flammable and lipophilic – but so are the gasoline and diesel fuels that we use with great success every day.
In order to facilitate broad introduction of LOHC-based hydrogen distribution systems, all precautions have to be taken to select not only the carrier that performs technically the best but also the carrier that is least toxic and most environmentally friendly.
Since LOHCs are supposed to be a cleaner alternative to fossil fuels, the latter are a good reference point for assessing the ‘greenliness’ of LOHC – in this comparison it should be still taken into account that fuels are burned while the LOHC systems act like a “deposit bottle” for hydrogen. Conventional fossil fuels, like diesel fuel or gasoline, usually contain hundreds of species, their composition is mostly unknown and defined on the basis of boiling point range only. Even though individual components of crude oil can be quite toxic (e.g. naphthalene) and reading the Material Safety Data Sheet of diesel no. 2 gives every layman the creeps, those products are circulating on the market since years in billions of tonnes. History knows numerous accidents involving fossil fuels that had catastrophic and far reaching consequences. Despite obvious and multiple risks associated with fossil fuels and high uncertainty linked with their unknown composition our civilisation relies heavily on them simply because of the lack of better options and the overruling socio-economic benefit. One important and unquestioned benefit of LOHC systems in comparison with crude oil based fuels is that the amount of components in LOHCs is limited and known which makes the assessment and risk management much less complex.
If, in addition to being more sustainable than fossil fuels, selected LOHCs can be shown in the future to have much better environmental profiles this would be already a giant improvement. A clear first point in favour of the LOHC systems vs. fossil fuels is the fact that the amount of components in LOHCs is limited and known which makes the assessment and risk management much less complex than in the case of crude oil with varying compositions depending on the oil origin.
Several challenges have to be faced when assessing the risk of LOHCs which result mostly from high Kow of some LOHCs as reported throughout this manuscript. However, at the current stage of development (eco)toxicological screening and environmental fate assessment of potential candidates should not only aim to exclude chemicals with enhanced hazards, but should also derive design criteria for better LOHC structures with regard to the ecotox profile. Testing should be focused on especially CMR (carcinogenic, mutagenic or toxic for reproduction) assessment as this is of high importance and very difficult to model or predict via QSAR. The avoidance of CMR chemicals would already give LOHCs an enormous competitive advantage in comparison to fossil fuels that contain carcinogens. The data from CMR and PBT assessment as well as economic data should be used for sound risk-benefit and socio-economic analyses. The results of the latter should decide on the type of LOHC system that should be industrially used and, finally, on the degree the LOHC technology should be applied in a future hydrogen-based economy.
Acknowledgements
We would like to thank the whole UFT Team, especially Dr Jürgen Arning for helpful discussion and Ulrike Bottin-Weber for their experimental support. The authors from Bremen would like to acknowledge financial support of Universität Bremen and European Union 7FP COFOUND BREMEN-TRAC Fellowship Program under the grant number 600411 and the German Federal Foundation for the Environment (Deutsche Bundesstiftung Umwelt (DBU). The authors from Erlangen would like to acknowledge support from the Bavarian Hydrogen Center and the Cluster of Excellence “Engineering of Advanced Materials”.Notes and references
- B. D. Solomon and A. Banerjee, Energy Policy, 2006, 34, 781–792 CrossRef PubMed.
- M. S. Dresselhaus and I. L. Thomas, Nature, 2001, 414, 332–337 CrossRef CAS PubMed.
- European Comission, A Roadmap for Moving to a Competitive Low Carbon Economy in 2050, 2011 Search PubMed.
- I. Dincer, Renewable Sustainable Energy Rev., 2000, 4, 157–175 CrossRef.
- M. Grätzel, Philos. Trans. R. Soc., A, 2007, 365, 993–1005 CrossRef PubMed.
- A. Evans, V. Strezov and T. J. Evans, Renewable Sustainable Energy Rev., 2009, 13, 1082–1088 CrossRef PubMed.
- N. Mayer, N. Kreifels and B. Burger, Kohleverstromung zu Zeiten nidriger Böresnstrompreise – Kurzstudie, 2013 Search PubMed.
- U. Eberle, B. Müller and R. Von Helmolt, Energy Environ. Sci., 2012, 5, 8780 Search PubMed.
- D. U. Eberle and D. R. von Helmolt, Energy Environ. Sci., 2010, 3, 689 Search PubMed.
- J. Van Mierlo and G. Maggetto, Energy Convers. Manage., 2006, 47, 2748–2760 CrossRef CAS PubMed.
- European Hydrogen and Fuel Cell Technology Platform - Strategic Research Agenda, 2005.
- C. Liu, F. Li, L.-P. Ma and H.-M. Cheng, Adv. Energy Mater., 2010, 22, 28–62 CrossRef PubMed.
- J. Yang, A. Sudik, C. Wolverton and D. J. Siegel, Chem. Soc. Rev., 2010, 39, 656–675 RSC.
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353–358 CrossRef CAS PubMed.
- E. Robert, H. Heinz, L. Jean-Francois, L. Laura, M. Heiko and R. David, Well - to - Wheels Analysis of Future Automotive Fuels and Powertrains in the European Context, 2014 Search PubMed.
- M. Felderhoff, C. Weidenthaler, R. Von Helmolt and U. Eberle, Phys. Chem. Chem. Phys., 2007, 9, 2643–2653 RSC.
- D. Teichmann, W. Arlt and P. Wasserscheid, Int. J. Hydrogen Energy, 2012, 37, 18118–18132 CrossRef CAS PubMed.
- S. Satyapal, J. Petrovic, C. Read, G. Thomas and G. Ordaz, Catal. Today, 2007, 120, 246–256 CrossRef CAS PubMed.
- B. Bogdanović and M. Schwickardi, J. Alloys Compd., 1997, 253–254, 1–9 CrossRef.
- F. Schüth, B. Bogdanović and M. Felderhoff, Chem. Commun., 2004, 2249–2258 RSC.
- T. Q. Hua, R. K. Ahluwalia, J.-K. Peng, M. Kromer, S. Lasher, K. McKenney, K. Law and J. Sinha, Int. J. Hydrogen Energy, 2011, 36, 3037–3049 CrossRef CAS PubMed.
- B. Loges, A. Boddien, F. Gärtner, H. Junge and M. Beller, Top. Catal., 2010, 53, 902–914 CrossRef CAS.
- S. Saxena, S. Kumar and V. Drozd, Int. J. Hydrogen Energy, 2011, 36, 4366–4369 CrossRef CAS PubMed.
- T. Kobayashi and H. Takahashi, Energy Fuels, 2004, 18, 285–286 CrossRef CAS.
- P. Kaiser, R. B. Unde, C. Kern and A. Jess, Chem. Ing. Tech., 2013, 85, 489–499 CrossRef CAS.
- G. P. Pez, A. Scott, A. C. Cooper, and H. Cheng, US 7101530, 2006.
- G. P. Pez, A. C. Cooper and R. Scott, Pat. Appl. Publ., 2008, 1–23 Search PubMed.
- US Department of Energy, Hydrogen and Fuel Cell Program.
- A. C. Cooper, K. M. Campbell and G. P. Pez, 16th World Hydrog. Energy Conf., 2006 Search PubMed.
- C. M. Araujo, D. L. Simone, S. J. Konezny, A. Shim, R. H. Crabtree, G. L. Soloveichik and V. S. Batista, Energy Environ. Sci., 2012, 5, 9534–9542 CAS.
- K. Müller, J. Völkl and W. Arlt, Energy Technol., 2013, 1, 20–24 CrossRef.
- F. Sotoodeh, B. J. M. Huber and K. J. Smith, Appl. Catal., A, 2012, 419–420, 67–72 CrossRef CAS PubMed.
- C. Wan, Y. An, G. Xu and W. Kong, Int. J. Hydrogen Energy, 2012, 37, 13092–13096 CrossRef CAS PubMed.
- F. Sotoodeh and K. J. Smith, Can. J. Chem. Eng., 2013, 91, 1477–1490 CrossRef CAS.
- M. Amende, C. Gleichweit, K. Werner, S. Schernich, W. Zhao, M. P. A. Lorenz, O. Höfert, C. Papp, M. Koch, P. Wasserscheid, M. Laurin, H.-P. Steinrück and J. Libuda, ACS Catal., 2014, 4, 657–665 CrossRef CAS PubMed.
- M. Amende, C. Gleichweit, S. Schernich, O. Höfert, M. P. A. Lorenz, W. Zhao, M. Koch, K. Obesser, C. Papp, P. Wasserscheid, H.-P. Steinrück and J. Libuda, J. Phys. Chem. Lett., 2014, 5, 1498–1504 CrossRef CAS.
- D. Teichmann, W. Arlt, P. Wasserscheid and R. Freymann, Energy Environ. Sci., 2011, 4, 2767–2773 CAS.
- N. Brückner, K. Obesser, A. Bösmann, D. Teichmann, W. Arlt, J. Dungs and P. Wasserscheid, ChemSusChem, 2013, 7, 229–235 CrossRef PubMed.
- J. N. Dumont and T. W. Schultz, Bull. Environ. Contam. Toxicol., 1979, 22, 159–166 CrossRef CAS.
- S. Hodoshima, S. Takaiwa, A. Shono, K. Satoh and Y. Saito, Appl. Catal., A, 2005, 283, 235–242 CrossRef CAS PubMed.
- N. Kariya, A. Fukuoka and M. Ichikawa, Appl. Catal., A, 2002, 233, 91–102 CrossRef CAS.
- M. Taube, D. W. T. Rippin, D. L. Cresswell and W. Knecht, Int. J. Hydrogen Energy, 1983, 8, 213–225 CrossRef CAS.
- H. Y. Zhao, S. T. Oyama and E. D. Naeemi, Catal. Today, 2010, 149, 172–184 CrossRef CAS PubMed.
- R. Yamaguchi, C. Ikeda, Y. Takahashi and K.-i. Fujita, J. Am. Chem. Soc., 2009, 131, 8410–8412 CrossRef CAS PubMed.
- H. Boudet, C. Clarke, D. Bugden, E. Maibach, C. Roser-Renouf and A. Leiserowitz, Energy Policy, 2013, 65, 57–67 CrossRef PubMed.
- OECD, Guideline for Testing of Chemicals 105-Water Solubility, 1995 Search PubMed.
- OECD, Guidance Document on Aquatic Toxicity Testing of Difficult Substanes and Mixtures, 2000, vol. 23 Search PubMed.
- US EPA Estimation Programs Interface SuiteTM for Microsoft® Windows EPI SuiteTM V. 4.1, 2014 Search PubMed.
- H. Rufi, P. R. Fisk, A. E. Girling, J. M. H. King, R. Länge, E. X. Lejeune, N. Stelter, C. Stevens, P. Suteau, J. Tapp, J. Thus, D. J. Versteeg and H. J. Niessen, Ecotoxicol. Environ. Saf., 1998, 39, 72–77 CrossRef.
- ECHA, Chapter R.11 PBT Assess., 2012.
- Source: European Chemicals Agency, http://echa.europa.eu/.
- OECD, Guideline Fortesting of Chemicals 301-Ready Biodegradability, 1992 Search PubMed.
- R. S. Boethling, E. Sommer and D. DiFiore, Chem. Rev., 2007, 107, 2167–2820 CrossRef PubMed.
- B. Philip, M. Hoff, F. Germa, B. Schink, D. Beimborn and V. Mersch-Sudermann, Environ. Sci. Technol., 2007, 41, 1390–1398 CrossRef.
- C. Zhao, Y. Zhang, X. Li, D. Wen and X. Tang, J. Hazard. Mater., 2011, 190, 253–259 CrossRef CAS PubMed.
- P. A. Vieira, R. B. Vieira, S. Faria, E. J. Ribeiro and V. L. Cardoso, J. Hazard. Mater., 2009, 168, 1366–1372 CrossRef CAS PubMed.
- R. Benigni and C. Bossa, Chem. Rev., 2011, 111, 2507–2536 CrossRef CAS PubMed.
- M. Kapuci, Z. Ulker, S. Gurkan and L. Alpsoy, Toxicol. Ind. Health, 2014, 30, 82–89 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ee03528c |
| This journal is © The Royal Society of Chemistry 2015 |