Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dinuclear lanthanide(III)/zinc(II) complexes with methyl 2-pyridyl ketone oxime†
Nikolaos C.
Anastasiadis
ab,
Christina D.
Polyzou
ab,
George E.
Kostakis
 ac,
Vlasoula
Bekiari
d,
Yanhua
Lan
e,
Spyros P.
Perlepes
b,
Konstantis F.
Konidaris
ac,
Vlasoula
Bekiari
d,
Yanhua
Lan
e,
Spyros P.
Perlepes
b,
Konstantis F.
Konidaris
 *a and
Annie K.
Powell
*ae
*a and
Annie K.
Powell
*ae
aInstitute of Nanotechnology, Karlsruhe Institute of Technology, Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany. E-mail: konstantis.konidaris@gmail.com
bDepartment of Chemistry, University of Patras, GR-265 04 Patras, Greece
cDepartment of Chemistry, School of Life Sciences, University of Sussex, Brighton BN1 9QJ, UK
dDepartment of Aquaculture, Technological Educational Institute of Western Greece, GR-30200 Messolonghi, Greece
eInstitute of Inorganic Chemistry, Karlsruhe Institute of Technology, Engesserstrasse 15, 76131 Karlsruhe, Germany. E-mail: annie.powell@kit.edu
First published on 14th October 2015
Abstract
The first use of methyl 2-pyridyl ketone oxime (mpkoH) in zinc(II)/lanthanide(III) chemistry leads to the [ZnLn(mpko)3(mpkoH)3](ClO4)2 and [ZnLn(NO3)2(mpko)3(mpkoH)] families of dinuclear ZnIILnIII complexes displaying blue-green, ligand-based photoluminescence; the ZnIIDyIII compound shows field-induced relaxation of magnetization.
Dinuclear and polynuclear MII or III/LnIII coordination cluster complexes, where MII or III are paramagnetic 3d-metal ions and LnIII is a trivalent lanthanide ion,1 occupy a unique place among mixed-metal molecular materials as a result of the interaction between 3d and 4f electron systems giving rise, for example, to alternatives2 to homometallic 3d-metal Single-Molecule Magnets (SMMs)3 and magnetic refrigerants.4 An important feature here is the fact that 4f metal ions can contribute a large spin and, for most 4f ions, also the magnetic anisotropy needed for SMM behaviour. Furthermore, the coupling between 3d and 4f metal ions can be relatively strong in terms of superexchange interaction.
However, in contrast to the plethora of studies concerning discrete 3d/4f-metal complexes, where both metal ions are paramagnetic, there is rather limited information on complexes containing ZnII (a diamagnetic 3d10 metal ion) and paramagnetic LnIII centres.5 Such complexes are extremely useful because (i) they can help scientists to elucidate the LnIII⋯LnIII magnetic exchange interactions in a series of isostructural MIIxLnIIIy coordination clusters (M = Mn, Fe, Co, Ni, Cu; y ≥ 2)1c,6 and (ii) they often exhibit interesting photoluminescence properties and phenomena7 distinctly different from those of analogous complexes containing only LnIII ions.
From a synthetic inorganic chemistry viewpoint, methods must be devised to combine 3d- and 4f-metal ions within a dinuclear or polynuclear molecule. One of our preferred routes is a “one-pot” procedure involving a mixture of 3d- and 4f-metal starting materials and an organic ligand possessing distinct functionalities, or “pockets”, for preferential bonding of the 3d and 4f ions. For example, the various anionic 2-pyridylmonoximes (Scheme 1) have been widely used to date in the synthesis of structurally and magnetically interesting 3d-,8 3d/3d′-![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and 3d/4f-metal10 complexes [MII = paramagnetic 3d-metal ion]. However, there are no reports of their use in ZnII/LnIII chemistry. These ligands are, in fact, attractive for ZnII/LnIII chemistry because the hard, deprotonated O atom will favour binding to strongly oxophilic LnIII ions, whereas the softer 2-pyridyl and oximate N atoms will favour coordination to the ZnII centre.
9 and 3d/4f-metal10 complexes [MII = paramagnetic 3d-metal ion]. However, there are no reports of their use in ZnII/LnIII chemistry. These ligands are, in fact, attractive for ZnII/LnIII chemistry because the hard, deprotonated O atom will favour binding to strongly oxophilic LnIII ions, whereas the softer 2-pyridyl and oximate N atoms will favour coordination to the ZnII centre.
We have been recently involved in a new research programme aiming to prepare, characterize and study discrete (i.e. non-polymeric), mixed ZnII/LnIII coordination cluster complexes with 2-pyridylmonoximate (Scheme 1) and 2,6-pyridylbisoximate bridging ligands. Our short-term goal is to establish routes for such complexes and to isolate the maximum number of products from a given ligand. Our longer-term goal is to force the carefully designed ZnII-ligand moiety of the heterometallic complex to act as an efficient sensitizer, and one that is more efficient than the organic-only chromophore in exciting LnIII ions (mainly EuIII, TbIII and DyIII) for emission in the visible region of the spectrum.11 It is also well known12 that highly luminescent LnIII complexes are of interest for a wide variety of photonic applications such as planar waveguide amplifiers, light-emitting diodes and bio-inspired luminescent probes. Most of the electronic transitions of the LnIII ions involve a redistribution of electrons within the 4f sub-shell. Electric dipole selection rules forbid such transitions but these rules are relaxed by several mechanisms, such as coupling with vibrational states, J-mixing and mixing with opposite-parity wave functions (5d orbitals, ligand orbitals or charge transfer states). The coupling between these vibrational and electronic states and the 4f wavefunctions depends on the strength of the interaction between the 4f orbitals and the surrounding ligands; in view of the shielding of the 4f orbitals, the degree of mixing remains small, and so are the oscillator strengths of the f–f transitions.12b As a consequence, even if many LnIII compounds display a good quantum yield, direct excitation of the LnIII ions rarely yields highly luminescent materials. This disadvantage may be overcome by employing suitable organic12,13 or d-block14 (making use of fully-allowed, low-energy charge transfer transitions from p-character systems to d-character systems, e.g. luminescent complexes of d6 and d8 metal ions) chromophores as antenna groups to generate sensitised emission from LnIII ions. In this communication we describe our preliminary efforts towards the realisation of the short-term goal mentioned above, i.e. to establish the chemistry of the ZnII/LnIII/2-pyridylmonoxime system.
Reactions of Zn(ClO4)2·6H2O, mpkoH, Et3N and Ln(NO3)3·6H2O in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio in MeOH at room temperature gave pale yellow (Ln = Eu, Gd, Tb) or pale orange (Ln = Dy, Ho) solutions, which upon storage at 15 °C gave pale yellow [ZnLn(mpko)3(mpkoH)3](ClO4)2·2H2O (Ln = Eu, 1·2H2O; Ln = Gd, 2·2H2O; Ln = Tb, 3·2H2O) and almost colourless [ZnLn(NO3)2(mpko)3(mpkoH)] (Ln = Dy, 4; Ln = Ho, 5) complexes; typical yields were in the 50–60% range. The structures of 1·2H2O and 4 were solved by single-crystal X-ray crystallography,‡ while the identity of 2·2H2O, 3·2H2O and 5 was confirmed by unit cell determination, IR spectroscopy and elemental (C, H, N) analyses.
2 molar ratio in MeOH at room temperature gave pale yellow (Ln = Eu, Gd, Tb) or pale orange (Ln = Dy, Ho) solutions, which upon storage at 15 °C gave pale yellow [ZnLn(mpko)3(mpkoH)3](ClO4)2·2H2O (Ln = Eu, 1·2H2O; Ln = Gd, 2·2H2O; Ln = Tb, 3·2H2O) and almost colourless [ZnLn(NO3)2(mpko)3(mpkoH)] (Ln = Dy, 4; Ln = Ho, 5) complexes; typical yields were in the 50–60% range. The structures of 1·2H2O and 4 were solved by single-crystal X-ray crystallography,‡ while the identity of 2·2H2O, 3·2H2O and 5 was confirmed by unit cell determination, IR spectroscopy and elemental (C, H, N) analyses.
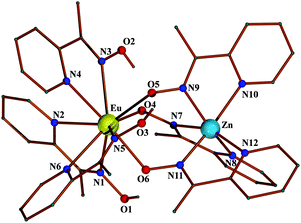
Complex 1·2H2O crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Its structure consists of dinuclear cations [ZnEu(mpko)3(mpkoH)3]2+ (Fig. 1), ClO4− anions and solvate H2O molecules. The metal ions are bridged by the three oximate groups of the η1:η1:η1:μ mpko− ligands. The ZnII ion is coordinated by six nitrogen atoms belonging to the “chelate” part of these ligands. This metal ion has a facial distorted octahedral geometry, the trans coordination angles being in the range 155.2(3)–158.4(3)°. The EuIII centre is bound to an O3N6 set of donor atoms. The oxygen atoms (O4, O5, O6) belong to the deprotonated oximate groups of the {Zn(mpko)3}− unit, while the six nitrogen atoms belong to the three bidentate chelating (η1:η1) mpkoH ligands. Using the Continuous Shape Measure (CShM) approach,15 the coordination geometry of EuIII can be best described as spherical capped square antiprismatic (Fig. S1†), the capping atom being N5; since the CShM value for this geometry (1.80735) is very close to that for the spherical tricapped trigonal prismatic geometry (1.80967), the EuIII ion can also be considered as having the latter. Most 9-coordinate metal ions possessing three 5-membered chelating rings and three monodentate ligands (the three monodentate “ligands” for Eu are the terminally coordinated oximate oxygen atoms O4, O5 and O6) form polyhedra that appear very close to the interconversion path between the capped square antiprism and tricapped trigonal prism.15
. Its structure consists of dinuclear cations [ZnEu(mpko)3(mpkoH)3]2+ (Fig. 1), ClO4− anions and solvate H2O molecules. The metal ions are bridged by the three oximate groups of the η1:η1:η1:μ mpko− ligands. The ZnII ion is coordinated by six nitrogen atoms belonging to the “chelate” part of these ligands. This metal ion has a facial distorted octahedral geometry, the trans coordination angles being in the range 155.2(3)–158.4(3)°. The EuIII centre is bound to an O3N6 set of donor atoms. The oxygen atoms (O4, O5, O6) belong to the deprotonated oximate groups of the {Zn(mpko)3}− unit, while the six nitrogen atoms belong to the three bidentate chelating (η1:η1) mpkoH ligands. Using the Continuous Shape Measure (CShM) approach,15 the coordination geometry of EuIII can be best described as spherical capped square antiprismatic (Fig. S1†), the capping atom being N5; since the CShM value for this geometry (1.80735) is very close to that for the spherical tricapped trigonal prismatic geometry (1.80967), the EuIII ion can also be considered as having the latter. Most 9-coordinate metal ions possessing three 5-membered chelating rings and three monodentate ligands (the three monodentate “ligands” for Eu are the terminally coordinated oximate oxygen atoms O4, O5 and O6) form polyhedra that appear very close to the interconversion path between the capped square antiprism and tricapped trigonal prism.15
There are three strong intramolecular (intracationic) hydrogen bonds with uncoordinated oxime oxygens (O1, O2, O3) as donors and the coordinated oximate oxygens (O6, O4 and O5, respectively) as acceptors. There are also H-bonded parallelograms of the type HOH⋯O(ClO2)O⋯HOH⋯O(ClO2)O (Fig. S2†) and single HOH⋯O(ClO3) H bonds in the crystal structure of the complex, which is further stabilized by weak intermolecular π–π stacking interactions and C–H⋯π interactions to form 2D honeycomb layers parallel to the plane formed by c and the bisection line of the a0b.
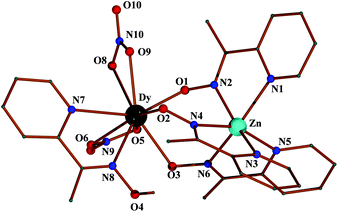
Complex 4 crystallizes in the monoclinic space group Cc. Its structure consists of dinuclear molecules [ZnDy(NO3)2(mpko)3(mpkoH)]. The molecular structure of 4 is similar to that of the cation [ZnEu(mpko)3(mpkoH)3]2+, the main difference being the replacement of two N,N′-bidentate chelating mpkoH ligands of the latter with two bidentate chelating nitrato groups in the former (Fig. 2). This replacement gives neutral molecules (and not cations) in 4 and the DyIII centre is thus bound to an O7N2 donor set. The coordination polyhedron of DyIII can be described as a spherical capped square antiprism (Fig. S3†) with the oxime nitrogen N8 as the capping atom, although descriptions as a spherical tricapped trigonal prism and muffin are equally acceptable. Again there is evidence for a strong intramolecular O4–H(O4)⋯O3 H bond; two weak C–H⋯π interactions (Fig. S4†) extend the structure into two dimensions forming layers parallel to the ab plane and giving a sql topology.
The lanthanide(III)–N(mpkoH) and –O(oximato) bond lengths around EuIII and DyIII in 1·2H2O and 4 show the expected tendency towards shorter values in the latter in line with the lanthanide(III) contraction. Compounds 1·2H2O and 4 are the first structurally characterized heterometallic ZnII/LnIII complexes with 2-pyridyloxime/oximate ligation.
Upon maximum excitation at 400 nm, solid 4 displays photoluminescence at 448, 483 and 540 nm at room temperature (Fig. 3). The most probable origin of emission is ligand-based as the excitation and emission spectra of both mpkoH and 4 are observed in the same region. Thus, somewhat to our disappointment, no significant DyIII emission was detected. Almost identical excitation and emission spectra are observed for solid samples of 1·2H2O and 3·2H2O (Fig. S5 and S6†), supporting our view that no LnIII emission appears. Although not desirable, this is not an unusual situation for lanthanide(III) complexes with certain types of organic ligands.16 In our case, this means that the energy transfer from the organic ligands to LnIII is not efficient, probably because the energy levels of the excited states of these ions lie higher than that of the excited state of the {Zn-mpko−}moiety; this requires a change of the ligand in the ZnII moiety of the complex.
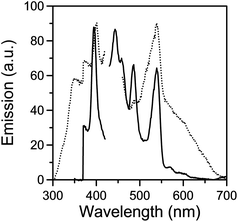 | ||
| Fig. 3 Solid-state room-temperature excitation (emission at 540 nm, left) and emission (excitation at 400 nm, right) of mpkoH (dotted curves) and complex 4 (solid curves). | ||
The room temperature χMT product for 4 (14.30 cm3 K mol−1) under an applied dc field of 1000 Oe is consistent with the expected value of 14.17 cm3 K mol−1 for one isolated DyIII ion (6H15/2 free ion, S = 5/2, L = 5, gJ = 4/3). The product decreases slowly to a value of 12.58 cm3 K mol−1 at 2.2 K, before a small upturn at 2.0 K (Fig. 4, left). The decrease in χMT is due to the progressive depopulation of the DyIII excited-state Stark sublevels.16 The small upturn below 2 K could be due to a weak ferromagnetic interaction between the complexes.17 The field dependence of magnetization shows that the magnetization reaches 5.4 μB at 70 kOe after a rapid increase at low fields (Fig. 4, right). The observed non-saturated magnetization at the highest field is much lower than the expected 10 μB for the DyIII ion indicating some anisotropy in the system.17,18
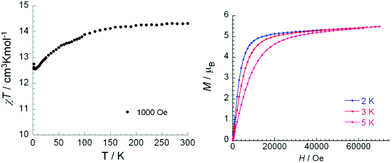 | ||
| Fig. 4 Temperature dependence of χMT for 4 (left) and molar magnetization vs. field at indicated temperatures (right, the solid lines are guides for the eyes). | ||
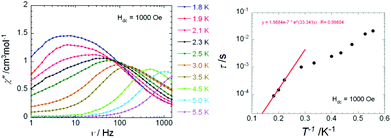
The dynamic magnetic properties of 4 were probed using ac susceptometry. Practically no signals for the out-of-phase component of the ac susceptibility were observed in the absence of a dc field at 1.8 K (Fig. S7†). However, an intense signal is observed with the application of an external dc field of 1000 Oe. Thus, on application of this field the positions of the maxima of the out-of-phase signals become strongly frequency-dependent (Fig. 5) as expected for a Single-Ion Magnet (SIM). The application of this field suppresses fast zero-field tunnelling of the magnetization, which is a well-documented behaviour for LnIII-based SIMs and SMMs.17 To calculate the characteristic time and the barrier to relaxation of 4, the relaxation times were fitted with the frequencies occurring at the χ′′max of the frequency-dependent ac susceptibility data (Fig. 5, left) by using the Orbach thermally activated relaxation law τ = τ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(Ueff/kBT). Linear data following this law were only obtained between 4 K and 5.5 K (solid line, Fig. 5, right), with an effective energy barrier of Ueff = 33.3 K and τ0 = 2.0 × 10−7 s. This suggests that the relaxation might follow a quantum regime below 4 K or that there is more than one thermally activated relaxation process in the complex. Thus, complex 4 can be termed as an “emissive field-induced SMM/SIM”.1c,19 The observation of more than one well-resolved pathway for magnetic relaxation remains a rarity among mononuclear SMMs.20
exp(Ueff/kBT). Linear data following this law were only obtained between 4 K and 5.5 K (solid line, Fig. 5, right), with an effective energy barrier of Ueff = 33.3 K and τ0 = 2.0 × 10−7 s. This suggests that the relaxation might follow a quantum regime below 4 K or that there is more than one thermally activated relaxation process in the complex. Thus, complex 4 can be termed as an “emissive field-induced SMM/SIM”.1c,19 The observation of more than one well-resolved pathway for magnetic relaxation remains a rarity among mononuclear SMMs.20
Conclusions
In conclusion, the members of the first two families of ZnII/LnIII/2-pyridylmonoximate complexes have been prepared. The complexes exhibit ligand-based blue-green photoluminescence, while ZnII/DyIII is considered to be a field-induced single-ion magnet. We are currently trying to investigate the possible presence of members of a third family of products in the Ln(NO3)3·xH2O/Zn(ClO4)2·xH2O/mpkoH general reaction system (we have strong evidence for this). Work is also in progress to enhance the aromatic content of the dinuclear ZnIILnIII complexes by employing anionic phenyl 2-pyridine ketone oxime (R = Ph in Scheme 1) as a bridging ligand and PhCO2− (instead of NO3− or mpkoH) as terminal groups, with the hope of achieving efficient energy transfer from the ZnII-ligand moiety to the lanthanide and “switch on” LnIII-based emission. Achieving DyIII-based emission in a ZnIIDyIII SIM would enable us to correlate luminescence and magnetism20a,21 since, in theory, the highest energy f–f transitions in the emission spectra of mononuclear DyIII SIMs can be modelled to provide a direct picture of the splitting of the ground J multiplet.Acknowledgements
This work was supported by the Alexander von Humboldt Foundation (postdoctoral research fellowship to KFK) and the DFG through SFB TR88 “3MET” (AKP). CDP gratefully acknowledges the Alexander Onassis Public Benefit Foundation for a PhD fellowship (G ZG 034-2/2012-2013).Notes and references
- For review-type articles, see: (a) C. D. Polyzou, C. G. Efthymiou, A. Escuer, L. Cunha-Silva, C. Papatriantafyllopoulou and S. P. Perlepes, Pure Appl. Chem., 2013, 85, 315 CrossRef CAS; (b) J. W. Sharples and D. Collison, Coord. Chem. Rev., 2014, 260, 1 CrossRef CAS PubMed; (c) H. L. C. Feltham and S. Brooker, Coord. Chem. Rev., 2014, 276, 1 CrossRef CAS.
- For example, see: (a) C. G. Efthymiou, Th. C. Stamatatos, C. Papatriantafyllopoulou, A. J. Tasiopoulos, W. Wernsdorfer, S. P. Perlepes and G. Christou, Inorg. Chem., 2010, 49, 9737 CrossRef CAS PubMed; (b) S. Schmidt, D. Prodius, V. Mereacre, G. E. Kostakis and A. K. Powell, Chem. Commun., 2013, 49, 1696 RSC; (c) K. Chandra Mondal, A. Sundt, Y. Lan, G. E. Kostakis, O. Waldmann, L. Ungur, L. F. Chibotaru, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2012, 51, 7550 CrossRef PubMed; (d) G. J. Sopasis, A. B. Canaj, A. Philippidis, M. Siczek, T. Lis, J. R. O'Brien, M. M. Antonakis, S. Pergantis and C. J. Milios, Inorg. Chem., 2012, 51, 5911 CrossRef CAS PubMed; (e) D. Dermitzaki, G. Lorusso, C. P. Raptopoulou, V. Psycharis, A. Escuer, M. Evangelisti, S. P. Perlepes and Th. C. Stamatatos, Inorg. Chem., 2013, 52, 10235 CrossRef CAS PubMed.
- C. J. Milios and R. E. P. Winpenny, Struct. Bonding, 2015, 164, 1 CrossRef.
- M. Evangelisti and E. K. Brechin, Dalton Trans., 2010, 39, 4672 RSC.
- For example, see: (a) W.-K. Wong, X. Yang, R. A. Jones, J. H. Rivers, V. Lynch, W.-K. Lo, D. Xiao, M. M. Oye and A. L. Holmes, Inorg. Chem., 2006, 45, 4340 CrossRef CAS PubMed; (b) S. Akine, F. Utsuno, T. Taniguchi and T. Nabeshima, Eur. J. Inorg. Chem., 2010, 3143 CrossRef CAS.
- W.-R. Yu, G.-H. Lee and E.-C. Yang, Dalton Trans., 2013, 42, 3941 RSC.
- (a) C. E. Burrow, T. J. Burchell, P.-H. Lin, F. Habib, W. Wernsdorfer, R. Clerac and M. Murugesu, Inorg. Chem., 2009, 48, 8051 CrossRef CAS PubMed; (b) T. D. Pasatoiu, A. M. Madalan, M. Zamfirescu, C. Tiseanu and M. Andruh, Phys. Chem. Chem. Phys., 2012, 14, 11448 RSC.
- K. F. Konidaris, V. Bekiari, E. Katsoulakou, C. P. Raptopoulou, V. Psycharis, E. Manessi-Zoupa, G. E. Kostakis and S. P. Perlepes, Dalton Trans., 2012, 41, 3797 RSC.
- S. Ross, T. Weyhermuller, E. Bill, K. Wieghardt and P. Chaudhuri, Inorg. Chem., 2001, 40, 6656 CrossRef CAS PubMed.
- C. D. Polyzou, H. Nikolaou, C. Papatriantafyllopoulou, V. Psycharis, A. Terzis, C. P. Raptopoulou, A. Escuer and S. P. Perlepes, Dalton Trans., 2012, 41, 13755 RSC.
- (a) T. D. Pasatoiu, A. M. Madalan, M. U. Kumke, C. Tiseanu and M. Andruh, Inorg. Chem., 2010, 49, 2310 CrossRef CAS PubMed; (b) T. D. Pasatoiu, C. Tiseanu, A. M. Madalan, B. Jurca, C. Duhayon, J. P. Sutter and M. Andruh, Inorg. Chem., 2011, 50, 5879 CrossRef CAS PubMed; (c) M. A. Palacios, S. Titos-Padilla, J. Ruiz, J. M. Herrera, S. T. A. Pope, E. K. Brechin and E. Colacio, Inorg. Chem., 2014, 53, 1465 CrossRef CAS PubMed.
- (a) J.-C. Bunzli, Acc. Chem. Res., 2006, 39, 53 CrossRef PubMed; (b) J.-C. G. Bűnzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048 RSC.
- S. Swavey and R. Swavey, Coord. Chem. Rev., 2009, 253, 2627 CrossRef CAS.
- D. Sykes and M. D. Ward, Chem. Commun., 2011, 47, 2279 RSC.
- A. Ruiz-Martinez, D. Casanova and S. Alvarez, Chem. – Eur. J., 2008, 14, 1291 CrossRef CAS PubMed.
- N. C. Anastasiadis, C. M. Granadeiro, N. Klouras, L. Cunha-Silva, C. P. Raptopoulou, V. Psycharis, V. Bekiari, S. S. Balula, A. Escuer and S. P. Perlepes, Inorg. Chem., 2013, 52, 4145 CrossRef CAS PubMed.
- H. L. C. Feltham, Y. Lan, F. Klower, L. Ungur, L. F. Chibotaru, A. K. Powell and S. Brooker, Chem. – Eur. J., 2011, 17, 4362 CrossRef CAS PubMed.
- M. Ren, D. Pinkowicz, M. Yoon, K. Kim, L.-M. Zheng, B. K. Breedlove and M. Yamashita, Inorg. Chem., 2013, 52, 8342 CrossRef CAS PubMed.
- As has been suggested,1c the term “field-induced SMM” appears to be misleading as such compounds are not magnets as they show no slow relaxation (and no hysteresis) in the absence of a field.
- (a) J. Long, J. Rouquette, J.-M. Thibaud, R. A. S. Ferreira, L. D. Carlos, B. Donnadieu, V. Vieru, L. F. Chibotaru, L. Konczewicz, J. Haines, Y. Guari and J. Larionova, Angew. Chem., Int. Ed., 2015, 54, 2236 CrossRef CAS PubMed; (b) A. Watanabe, A. Yamashita, M. Nakano, T. Yammura and T. Kajiwara, Chem. – Eur. J., 2011, 17, 7428 CrossRef CAS PubMed; (c) M. Jeletic, P.-H. Lin, J. J. Le Roy, I. Korobkov, S. I. Gorelsky and M. Murugesu, J. Am. Chem. Soc., 2011, 133, 19286 CrossRef CAS PubMed; (d) J. Ruiz, A. J. Mota, A. Rodriguez-Diéguez, S. Titos, J. M. Herrera, E. Ruiz, E. Cremades, J. P. Costes and E. Colacio, Chem. Commun., 2012, 48, 7916 RSC.
- (a) J. Long, R. Vallat, R. A. S. Ferreira, L. D. Carlos, F. A. Almeida Paz, Y. Guari and J. Larionova, Chem. Commun., 2012, 48, 9974 RSC; (b) G. Cucinotta, M. Perfetti, J. Luzon, M. Etiene, P.-E. Car, A. Caneschi, G. Calvez, K. Bernot and R. Sessoli, Angew. Chem., Int. Ed., 2012, 51, 1606 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental details of the synthesis of complexes 1·2H2O and 4, analytical data for all complexes, short experimental for single-crystal X-ray crystallography, metric parameters for the H bonds in 1·2H2O, and structural (Fig. S1–S4), optical (Fig. S5 and S6) and magnetic (Fig. S7 and S8) plots. CCDC 1021513 (1·2H2O) and 1021514 (4). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt03663a |
‡ Crystallographic data for complex 1·2H2O: C42H49EuZnN12O16Cl2, Mr = 1266.16, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 13.8641(15) Å, b = 14.5627(19) Å, c = 15.190(2) Å, α = 96.519(11)°, β = 113.081(10)°, γ = 109.850(9)°, V = 2543.6(6) Å3, Z = 2, Dc = 1.653 g cm−3, μ (Mo Kα, λ = 0.71073 Å) = 1.878 mm−1, T = 180(2) K, 17 , a = 13.8641(15) Å, b = 14.5627(19) Å, c = 15.190(2) Å, α = 96.519(11)°, β = 113.081(10)°, γ = 109.850(9)°, V = 2543.6(6) Å3, Z = 2, Dc = 1.653 g cm−3, μ (Mo Kα, λ = 0.71073 Å) = 1.878 mm−1, T = 180(2) K, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 067 reflections collected, 10 067 reflections collected, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 653 unique (Rint = 0.0919), R1 on F (wR2 on F2) = 0.0606 (0.1164) for 4678 observed (I > 2σ(I)) reflections. Crystallographic data for 4: C28H29DyZnN10O10, Mr = 893.48, monoclinic, Cc, a = 15.9387(11) Å, b = 9.3158(4) Å, c = 22.5168(17) Å, β = 94.590(6)°, V = 3332.6(4) Å3, Z = 4, Dc = 1.781 g cm−3, μ (Mo Kα, λ = 0.71073 Å) = 3.015 mm−1, T = 180(2) K, 11 653 unique (Rint = 0.0919), R1 on F (wR2 on F2) = 0.0606 (0.1164) for 4678 observed (I > 2σ(I)) reflections. Crystallographic data for 4: C28H29DyZnN10O10, Mr = 893.48, monoclinic, Cc, a = 15.9387(11) Å, b = 9.3158(4) Å, c = 22.5168(17) Å, β = 94.590(6)°, V = 3332.6(4) Å3, Z = 4, Dc = 1.781 g cm−3, μ (Mo Kα, λ = 0.71073 Å) = 3.015 mm−1, T = 180(2) K, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 098 reflections collected, 6259 unique (Rint = 0.0265), R1 on F (wR2 on F2) = 0.0191 (0.0480) for 6209 observed (I > 2σ(I)) reflections. Unit cell information: complex 2·2H2O: triclinic, P 098 reflections collected, 6259 unique (Rint = 0.0265), R1 on F (wR2 on F2) = 0.0191 (0.0480) for 6209 observed (I > 2σ(I)) reflections. Unit cell information: complex 2·2H2O: triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 13.8894 Å, b = 14.6063 Å, c = 15.2384 Å, α = 96.985°, β = 112.719°, γ = 109.824°, V = 2566.45 Å3. Complex 3·2H2O: triclinic, P , a = 13.8894 Å, b = 14.6063 Å, c = 15.2384 Å, α = 96.985°, β = 112.719°, γ = 109.824°, V = 2566.45 Å3. Complex 3·2H2O: triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 13.8574 Å, b = 14.5554 Å, c = 15.1713 Å, α = 96.684°, β = 112.834°, γ = 109.960°, V = 2539.08 Å3. Complex 5: monoclinic, Cc, a = 15.9450 Å, b = 9.3192 Å, c = 22.499 Å, β = 95.061°, V = 3330.2 Å3. CCDC numbers 1021513 (1·2H2O) and 1021514 (4). , a = 13.8574 Å, b = 14.5554 Å, c = 15.1713 Å, α = 96.684°, β = 112.834°, γ = 109.960°, V = 2539.08 Å3. Complex 5: monoclinic, Cc, a = 15.9450 Å, b = 9.3192 Å, c = 22.499 Å, β = 95.061°, V = 3330.2 Å3. CCDC numbers 1021513 (1·2H2O) and 1021514 (4). |
| This journal is © The Royal Society of Chemistry 2015 |