p-Tolylimido rhenium(v) complexes with phenolate-based ligands: synthesis, X-ray studies and catalytic activity in oxidation with tert-butylhydroperoxide†
Abstract
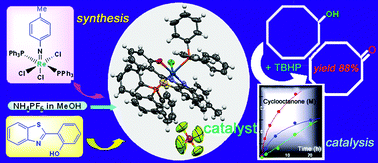
The reactions of mer-[Re(p-NTol)X3(PPh3)2] (X = Cl, Br) with chelating phenolate-based ligands (2-(2-hydroxy-5-methylphenyl)benzotriazole (HL1), 2-(2-hydroxyphenyl)benzothiazole (HL2) or 2-(2-hydroxyphenyl)benzoxazole (HL3)) afforded a series of p-tolylimido rhenium(V) complexes cis- or trans-(X,X)-[Re(p-NTol)X2(L)(PPh3)]·yMeCN (where X = Cl, Br; L = L1, L2, L3 and y = 0–2) and [Re(p-NTol)X(L)(PPh3)2]Z·pPPh3 (where X = Cl, Br; Z = ReO4, PF6; L = L1, L2, L3 and p = 0 or 1). The reported compounds were characterized by elemental analysis, FT-IR, NMR (1H, 13C and 31P) and X-ray crystallography. Interestingly, the halide ions of [Re(p-NTol)Cl2(L1)(PPh3)]·MeCN (1) and [Re(p-NTol)Cl2(L2)(PPh3)]·2MeCN (3) are in cis relative dispositions, whereas the complexes [Re(p-NTol)Br2(L)(PPh3)] (L1 for 2, L2 for 4 and L3 for 6) and [Re(p-NTol)Cl2(L3)(PPh3)] (5) were found to be trans-(X,X) isomers. The compounds [Re(p-NTol)X(L)(PPh3)2](PF6) (X = Cl, Br; L = L1 and L2) and [Re(p-NTol)X(L3)(PPh3)2](PF6)·PPh3 (X = Cl, Br) have been tested in oxidative catalysis. A few compounds exhibited very good catalytic properties in oxidation of alcohols with tert-BuOOH (TBHP) in acetonitrile solution at moderate temperatures. Complex [Re(p-NTol)Cl(L2)(PPh3)2]PF6 (13) is the catalyst of choice for oxidation of 1-phenylethanol to acetophenone (in 80% yield; turnover number attained 290 after 30 h) and cyclooctanol to cyclooctanone (in 88% yield). Notably lower activity has been found in the oxidation of alkanes with TBHP. Product distribution in the oxidation of methylcyclohexane indicates some steric hindrance around the reaction center.

 Please wait while we load your content...
Please wait while we load your content...