Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High pressure synthesis of polar and non-polar cation-ordered polymorphs of Mn2ScSbO6†
E.
Solana-Madruga
*a,
A. J.
Dos santos-García
b,
A. M.
Arévalo-López
c,
D.
Ávila-Brande
a,
C.
Ritter
d,
J. P.
Attfield
c and
R.
Sáez-Puche
a
aDpto. Química Inorgánica, Universidad Complutense de Madrid, Av. Complutense sn, 28040-Madrid, Spain. E-mail: esolana@ucm.es
bDpto. Ingeniería Mecánica, Química y Diseño Industrial, Universidad Politécnica de Madrid, C/ Ronda de Valencia 3, 28012-Madrid, Spain
cCentre for Science at Extreme Conditions and School of Chemistry, University of Edinburgh, Mayfield Road, EH9 3JZ, UK
dInstitut Laue-Langevin, 38042 Grenoble Cedex, France
First published on 13th October 2015
Abstract
Two new cation-ordered polymorphs of Mn2ScSbO6 have been synthesised at high-pressure. At 5.5 GPa and 1523 K Mn2ScSbO6 crystallizes in the Ni3TeO6-type structure with the polar R3 space group and cell parameters a = 5.3419 (5) Å and c = 14.0603 (2) Å. Below TC = 42.0 K it exhibits ferrimagnetic order with a net magnetization of 0.6μB arising from unusual site-selective Mn/Sc disorder and is thus a potential multiferroic material. A double perovskite phase obtained at 12 GPa and 1473 K crystallizes in the non-polar P21/n monoclinic space group with cell parameters a = 5.2909 (3) Å, b = 5.4698 (3) Å, c = 7.7349 (5) Å and β = 90.165 (6) °. Magnetization and neutron diffraction experiments reveal antiferromagnetic order below TN = 22.3 K with the spins lying in the ac plane.
I. Introduction
Material properties are dependent upon atomic arrangement and the degree of order can be tailored by controlling the pressure–temperature conditions and bulk composition. At a given pressure P and temperature T, there are different possible atomic arrangements that correspond to local minima of free energy, the lowest-energy conformation being the thermodynamically-stable phase. However, high pressure and high temperature (HPHT) synthesis conditions may favour higher-energy minima and metastable phases, making them kinetically stable under ambient conditions and therefore recoverable.Amongst transition metal oxides, HPHT helps to stabilize unusual oxidation states and environments that result in useful properties, e.g. the room temperature ferromagnetic metal CrO2.1 Moreover, HPHT promotes interesting structural mechanisms. The ilmenite (IL) FeTiO3, for example, crystallizes in the space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) with Fe and Ti stacked into alternate layers along the c-axis.2 It transforms into an unquenchable distorted perovskite (Pv) structure (space group Pbnm) at 16 GPa and converts back into a polar LiNbO3-type (LN, space group R3c) with Fe and Ti cations being ordered in the same layers.3,4
with Fe and Ti stacked into alternate layers along the c-axis.2 It transforms into an unquenchable distorted perovskite (Pv) structure (space group Pbnm) at 16 GPa and converts back into a polar LiNbO3-type (LN, space group R3c) with Fe and Ti cations being ordered in the same layers.3,4
Moreover, ABO3 oxides with Mn2+ on the A site are of fundamental interest due to the electronic and magnetic phenomena that emerge from the coupling of spin, charge and orbital degrees of freedom. HPHT conditions are often needed to stabilize MnBO3 materials, e.g. perovskite-type MnVO3 with an incommensurate magnetic structure and metallic conductivity,5 and LiNbO3-type MnTiO3-II with a weak ferromagnetism through anisotropic exchange interactions.6 The use of HPHT on ordered quaternary systems (AA′B2O6 or A2BB′O6) is a relatively less explored area with only a few reports.7,8 Amongst these, Mn2FeB′O6 (B′ = Ta and Nb) with a LN-type structure9 or (B′ = Mo, W) with the Ni3TeO6-type (NTO)10,11 order shows polar and magnetic properties, while Mn2BSbO6(B = Fe and Cr) shows polymorphism between the IL- and double perovskite-types (DPv) depending on whether they are synthesized at moderate (3–5 GPa) or higher pressures (5.5–8 GPa).12,13
In this work, we present the order–disorder effects on the high pressure polymorphs of Mn2ScSbO6 oxide. Mn and Sc cations are randomly distributed in a corundum-related type structure when this material is synthesized under ambient conditions and it shows no long-range magnetic ordering.14 The double perovskite (DPv) can be achieved with pressures higher than 10 GPa, but below 5.5 GPa the polar NTO-structure is obtained. NTO_Mn2ScSbO6 shows an unusual ferrimagnetism due to partial substitution of non-magnetic Sc3+ at just one of the two Mn2+ sites, whereas DPv_Mn2ScSbO6 is antiferromagnetic. Combined X-ray and powder neutron diffraction refinements and electron micro-diffraction experiments confirm the non-centrosymmetry of the NTO_Mn2ScSbO6 polymorph allowing a structural polarization that is predicted to be 28.3 μC cm−2 at room temperature. These results demonstrate that cation ordering can be induced by high pressure synthesis providing access to new metastable phases with unusual properties including multiferroicity. We also show that site selective disorder provides an unusual way to induce ferrimagnetism.
II. Experimental section
Mn2ScSbO6 was previously synthesized at ambient pressure by Kosse et al.15,16 and subsequently studied by Ivanov et al.14 NPD studies showed a random distribution of Mn and Sc crystallizing in a corundum-related structure (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) with no long-range magnetic ordering. We prepared a sample at 1373 K and Supplementary Fig. 1 (SF1†) shows the Rietveld fit to the XRD pattern that agrees with a random cation distribution.
) with no long-range magnetic ordering. We prepared a sample at 1373 K and Supplementary Fig. 1 (SF1†) shows the Rietveld fit to the XRD pattern that agrees with a random cation distribution.
Both double perovskite and Ni3TeO6-type polymorphs were synthesized under high pressure and high temperature conditions. The precursor, prepared by grinding stoichiometric amounts of Mn2O3, Sc2O3 and Sb2O3 oxides, was treated at 12 GPa and 1473 K for 20 minutes in a Walker-type multianvil apparatus for the preparation of the perovskite phase. The application of 5.5 GPa and 1523 K for 35 minutes in a belt-type press led to the formation of the Ni3TeO6 polymorph. In both cases, the sample was quenched by rapid cooling and the pressure was progressively released down to ambient conditions.
The crystal structures were first characterized by Rietveld refinement of powder X-ray diffraction collected on a Phillips X'Pert Pro Alfa 1 diffractometer using Cu Kα1 radiation, equipped with a Ge (111) monochromator working in Bragg–Brentano geometry. The diffractograms were collected between 10 and 120 degrees with a step size of 0.017°.
For transmission electron microscopy (TEM) studies, samples were ground in n-butyl alcohol and ultrasonically dispersed. A few drops of the resulting suspension were deposited on a carbon-coated grid. The study of reciprocal space by selected area electron diffraction (SAED) and microdiffraction was carried out using a JEOL JEM2100 microscope operating at 200 kV with a double tilt (±42°) goniometer. High resolution transmission electron microscopy (HRTEM) and electron energy loss spectroscopy (EELS) experiments were performed with a JEOL JEM 3000F microscope operating at 300 kV (double tilt (±25°) point resolution 0.17 nm), fitted with an energy-dispersive X-ray spectroscopy (XEDS) microanalysis system (OXFORD INCA) and an ENFINA spectrometer with an energy resolution of 1.3 eV.
Simulations of the HRTEM images were performed with the software MacTempas X,17 using the refined structures from neutron diffraction data. The oxidation state of Mn in both materials was determined by EELS with the relationship between the white-line intensity ratio (L3/L2) and the oxidation state.18
Neutron powder diffraction (NPD) patterns were collected at 300 K on a high resolution D2B diffractometer (Institut Laue-Langevin, Grenoble) between 0 and 160° with a step-width of 0.05°, using a neutron wavelength λ = 1.594 Å and a standard He cryostat. The NTO polymorph was also cooled down to 4 K, where a long scan was measured under the same conditions. The evolution of the magnetic structures was studied using the sequential patterns collected at each degree from 3 K up to 55 K on the high intensity D1B instrument. Each pattern was measured in the angular range 0° ≤ 2θ ≤ 130° with a step-width of 0.1° using λ = 2.520 Å. Long scans were also taken at 3 K.
The nuclear structures were refined from room temperature D2B data, using Rietveld analysis through the FullProf software package19 and considering a Thomson–Cox–Hastings function to optimize the shape of the peaks. The magnetic symmetry analysis of the low temperature D1B data was performed by means of the program BasIreps.20
Magnetic susceptibility measurements were performed on a Quantum Design XL-MPMS SQUID magnetometer under DC Zero-Field-Cooling (ZFC) and Field-Cooling (FC) conditions in the temperature range 2 K < T < 300 K under a magnetic field of 3 kOe. Magnetization dependence on the magnetic field strength was studied at 5, 20, 40 and 90 K for the perovskite polymorph up to 5 T and at 2, 20 and 40 K for the NTO-type oxide up to 7 T.
III. Results and discussion
(A) NTO_Mn2ScSbO6
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) and LN-type (R3c) structures, suggesting an ilmenite-type ordering (R
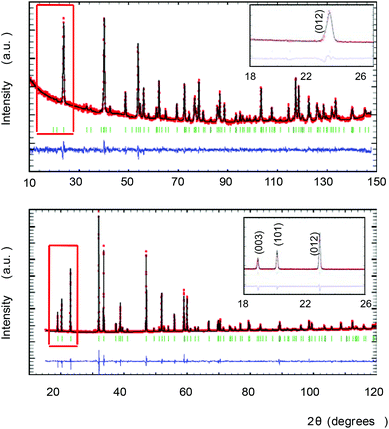
c) and LN-type (R3c) structures, suggesting an ilmenite-type ordering (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ). Rietveld refinement was performed with the IL-Mn2FeSbO6 structure as a starting model. The refinement converged to Rwp = 11.3% and Rp = 6.24% agreement factors.
). Rietveld refinement was performed with the IL-Mn2FeSbO6 structure as a starting model. The refinement converged to Rwp = 11.3% and Rp = 6.24% agreement factors.
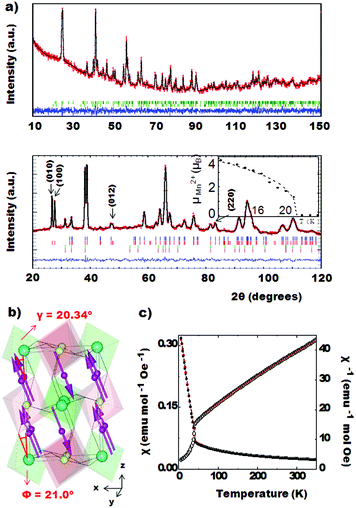
However, NPD experiments on the same compound revealed no intensity for the (003) and (101) reflections (Fig. 1-top) and the IL-type model obtained from XRD refinement showed a poor fit to the neutron data. The data can be fitted assuming a LN-type ordering (R3c) with Rwp = 4.40% and Rp = 3.41% agreement factors.
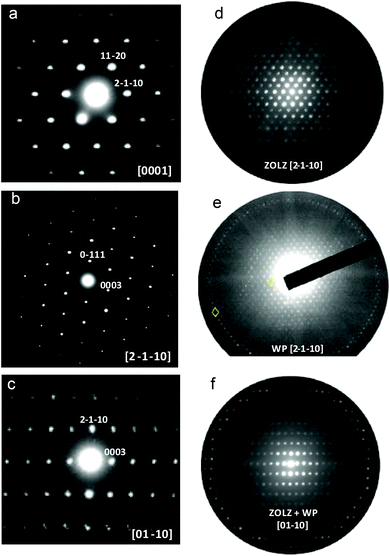
In order to clarify this situation, electron microscopy experiments were performed. The SAED patterns exhibit the reflection conditions: hkil: −h + k + l = 3n, hki0: −h + k = 3n, 000l: l = 3n, h − h00: h = 3n, h − h0l: h + l = 3n and the only feasible space groups are R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) and R3 (Fig. 2a–c).
and R3 (Fig. 2a–c).
Fig. 2d and e show the microdiffraction of the [2 −1 −1 0] zone axis. The no periodicity difference between the zero and first order Laue zones (ZOLZ and FOLZ) indicates the absence of a c-glide plane, in agreement with XRD. Moreover, the analysis of the whole pattern (WP) of the [2 −1 −1 0] and [0 1 −1 0] zone axes (Fig. 2f) unequivocally distinguishes between R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (two-fold axis) and R3 (no symmetry).21
(two-fold axis) and R3 (no symmetry).21
A model with R3 symmetry was then tested. In order to break the inversion centre of the IL-type model, we assumed a complete ordering within the (00z)-layers between Mn1/Sc and Mn2/Sb; this cation arrangement is adopted in the Ni3TeO6-type structure with the polar R3 space group. The NTO-type structure has also been observed in the related compounds Mn2FeMoO6 and Ni2ScSbO6.10,22 The refinement converged smoothly; Fig. 1 shows the Rietveld refinements of the NPD (top) and XRD (bottom) data for the NTO_Mn2ScSbO6 model and Table 1 summarizes the structural details and agreement factors resulting from this combined refinement.
| a = 5.3419 (5) (Å), c = 14.0603 (2) (Å) | |||||
|---|---|---|---|---|---|
| Atom | Site | x | y | z | Occ |
| Mn1 | 3a | 1/3 | 2/3 | 0.1619(8) | 1 |
| Mn2/Sc | 3a | 2/3 | 1/3 | 0.3423(8) | 0.877(2)/0.123(2) |
| Sc/Mn2 | 3a | 0 | 0 | 0.2224(8) | 0.877(2)/0.123(2) |
| Sb | 3a | 1/3 | 2/3 | 0.3823(8) | 1 |
| O1 | 9b | 0.641(1) | 0.695(2) | 0.2926(7) | 1 |
| O2 | 9b | 0.998(2) | 0.622(2) | 0.4555(5) | 1 |
| (Mn1–O1) × 3 | 2.42(1) | (Mn2–O1) × 3 | 2.12(1) | Δ[Mn1O6] | 5.5 × 10−3 |
| (Mn 1–O2) × 3 | 2.09 (1) | (Mn2–O2) × 3 | 2.31(1) | Δ[SbO6] | 1.1 × 10−4 |
| (Sb–O1) × 3 | 2.02(1) | (Sc–O1) × 3 | 2.04(1) | Δ[Mn2O6] | 1.7 × 10−3 |
| (Sb–O2) × 3 | 1.98(1) | (Sc–O2) × 3 | 2.18 (1) | Δ[ScO6] | 1.1 × 10−4 |
| <Mn1–O1–Mn2> | 121.0 (4) | <Mn1–O2–Mn2> | 118.4 (3) |
|---|---|---|---|
| a Fitting residuals: Rp = 3.04%, Rwp = 3.86%, RB = 6.18% and RF = 5.04%,. b V i = ∑jSiji = exp(r0 − rij/0.37). Values calculated using rij = 1.79 Å for Mn2+, 1.849 Å for Sc3+ and 1.942 Å for Sb5+. Polyhedral distortions calculated from Δ = 1/n × ∑[(di − dav)/dav]2. | |||
| BVS(Mn1)b = 1.89 | BVS (Sb) = 5.18 | BVS (Mn2) = 2.01 | BVS (Sc) = 2.93 |
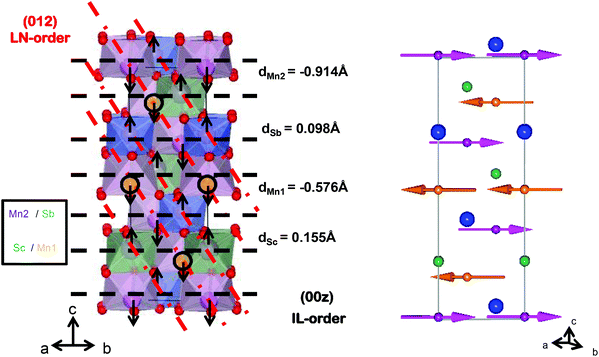
The final model clarifies the apparent contradiction between the XRD and NPD results. The cation arrangement of the R3 NTO-type structure is illustrated in Fig. 3 (left). The (00z) layers alternate between Mn1/Sc and Mn2/Sb composition. For XRD, Sc (Z = 21) and Mn (Z = 25) look similar when compared with the consecutive layer of Mn and Sb (Z = 50). Therefore, IL-type ordering is apparent and the (003) and (101) reflections are observable. On the other hand, the difference in neutron scattering lengths for Mn, Sc and Sb (−3.73, 12.29 and 5.57 fm respectively) breaks the apparent inversion centre, as the (012) planes are alternatively constituted by Mn or by Sc/Sb, which makes the scattering distribution resemble that of an LN-type. For clarity, both IL- and LN-type orders are highlighted in Fig. 3 (left) with black and red dashed lines respectively. In conclusion, the combination of SAED, NPD and XRD techniques unequivocally leads to the true NTO-type structure of this compound.
EELS analysis performed on several crystallites yields a +2 oxidation state for Mn (see ESI Fig. SF2a†). A structural HRTEM image along the [2 −1 −1 0] zone axis (SF2b†) shows a well ordered material and the absence of extended defects.
The structure of NTO_Mn2ScSbO6 can be described as a corundum derivative with (00z) layers alternatively occupied by [Mn1O6]/[ScO6] and [Mn2O6]/[SbO6] edge sharing octahedra. The order is such that the polyhedra sharing faces along the c axis are always Mn1 against Sb and Sc against Mn2 (see Fig. 3 left). All site occupancies were tested by allowing cation exchange among the different sites, but the only significant result was found between Sc and Mn2 positions and the final refinement converged to a value of 12.3 (2)% of anti-site mixing. The insertion of this small amount of Sc3+ in the Mn2 site decreases its octahedral distortion from 5.5 × 10−3 observed for Mn1 down to 1.7 × 10−3, while the observed Δ[ScO6] value remains 1.1 × 10−4 as that observed for [SbO6].
The displacement of the different cations from their ideal sites with reference to the oxygen octahedra are labeled in Fig. 3(left). Mn cations show larger displacements in comparison with the d0 Sc or d10 Sb, and in accordance with the greater distortion found for their polyhedra (see Table 1).
The theoretical value of polarization along the z axis has been calculated from 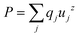 , where qj stands for the charge over a generic j ion and uzj for its displacement along the z axis, resulting in Pexpected = 28.3 μC cm−2. This estimate neglects the effects of Mn/Sc disorder. Attempts were made to measure the experimental polarization, using a modified Sawyer–Tower circuit.23 Due to the low resistivity, sample polarization is masked by conducting effects and typical inconclusive P vs. E ellipse-shaped cycles were obtained.
, where qj stands for the charge over a generic j ion and uzj for its displacement along the z axis, resulting in Pexpected = 28.3 μC cm−2. This estimate neglects the effects of Mn/Sc disorder. Attempts were made to measure the experimental polarization, using a modified Sawyer–Tower circuit.23 Due to the low resistivity, sample polarization is masked by conducting effects and typical inconclusive P vs. E ellipse-shaped cycles were obtained.
From the structural point of view, Mn2+ cations are located in different octahedral sites within (00z) layers and are connected to each other through sharing corners. This allows cooperative AFM Mn–O–Mn superexchange interactions that are governed by <Mn1–O1–Mn2> = 121.0 (4)° and <Mn1–O2–Mn2> = 118.4 (3)° angles.
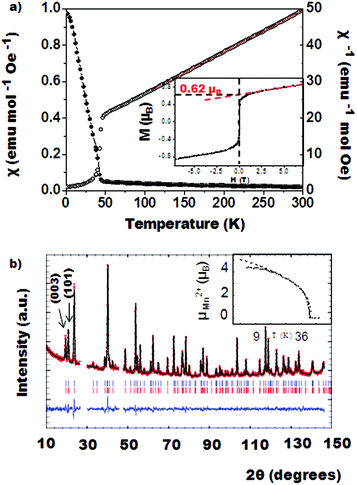
The 4 K NPD data collected for NTO_Mn2ScSbO6 at D2B instrument are shown in Fig. 4b. The magnetic structure of this polymorph (Fig. 3 right) was determined from the fit to these data, where 27°–30° and 46°–48° angular ranges are excluded due to the presence of peaks arising from the sample holder. Additional magnetic neutron diffraction peaks are observed below TC = 42 K, the most intense being (003) and (101), which can be indexed with the propagation vector k = [0 0 0]. Fits to the magnetic peaks confirm a FM coupling of Mn1 spins within 00z layers, where spins are confined to the ab plane, but antiferromagnetically coupled with the Mn2 cations of the adjacent layers. The refined moment per Mn2+ cation is 4.62 (3) μB. The spin order and Mn site occupancies observed by NPD reveal the origin of the ferromagnetic behavior of NTO_Mn2ScSbO6. Antiparallel Mn1 and Mn2 spins are symmetry-inequivalent and so a small uncompensated ferromagnetic moment is expected, but this is greatly enhanced by site-selective disorder as Mn1 sites are fully occupied by Mn2+ spins, but 12.3% of Mn2 sites are substituted by non-magnetic Sc3+. The predicted uncompensated moment (12.3% × 4.62 μB = 0.57 μB) is in excellent agreement with the observed magnetization of 0.6 μB. Hence, the ferromagnetic component arises from an unusual site-selective substitution of non-magnetic Sc3+ at just one of the two Mn2+ sites. A similar ferrimagnetism due to selective disorder has been recently observed in DPv-type La3Ni2SbO9.24 Magnetic diffraction from the ferromagnetic component in NTO_Mn2ScSbO6 is expected to be very weak and is not observed in the difference pattern between NPD data sets collected at 50 K and 3 K on the D1B instrument (SF4† lower panel).
The evolution of the magnetic moment below the Curie temperature (inset on Fig. 4b), was fitted to a critical law μ(T) = μ(0) × [1 − (T/TC)]β in the (TC/2) < T < TC temperature range between 21 K and 41 K. It led to the parameters TC = 42.0 K and β = 0.37, consistent with 3D Heisenberg behaviour for which β = 0.36 is predicted.
Overall, NTO_Mn2ScSbO6 is notable as a potential multiferroic material, with ferroelectricity arising from the acentric cation order in the NTO-type arrangement, and ferrimagnetism from the uncompensated antiparallel order of Mn2+ spins enhanced by site-selective Mn/Sc disorder. This material seems to be a type I multiferroic as the two orders are decoupled. The magnetoelectric coupling between the electrical and magnetic polarizations may be small as they are orthogonal, being respectively parallel and perpendicular to the c-axis, although this could lead to an unusual switching mechanism as predicted for LiNbO3-type MnTiO3-II.6
(B) DPv_Mn2ScSbO6
Fig. 5a (top) shows the Rietveld refinement of NPD data of DPv_Mn2ScSbO6 collected at room temperature. It was fitted using the Mn2BSbO6 (B = Fe, Cr)12,13 structure as the starting model and crystallizes in the P21/n monoclinic space group with lattice parameters a = 5.2909 (3) Å, b = 5.4698 (3) Å, c = 7.7349 (5) Å and β = 90.165 (6)°. The resulting crystallographic parameters, interatomic distances and bond angles are summarized on Table 2. Anti-site disorder was found between Sc and Mn, resulting in 9.1% of the A-site occupied by Sc3+. The secondary phase included in the refinement of DPv_Mn2ScSbO6 is the NTO polymorph formed at lower pressures during the synthesis reaction. A 20.1% of this phase has been found to crystallize within this high pressure compound. The presence of such a high proportion of NTO_Mn2ScSbO6 is due to the high stability of the NTO-type structure at high temperatures. The effect of this secondary phase on the magnetic behaviour of DPv_Mn2ScSbO6 is discussed below.
| a = 5.2909 (3) (Å), b = 5.4698 (3) (Å), c = 7.7349 (5) (Å), β = 90.165 (6) (°) | |||||
|---|---|---|---|---|---|
| Atom | Site | x | y | Z | Occ |
| a Fitting residuals: Rp = 2.00%, Rwp = 2.51%, RB = 6.04% and RF = 4.36%. b V i = ∑jSiji = exp(r0 − rij/0.37). Values calculated using rij = 1.79 Å for Mn2+, 1.849 Å for Sc3+ and 1.942 Å for Sb5+. Polyhedral distortions calculated from Δ = 1/n × ∑[(di − dav)/dav]2. | |||||
| Mn/Sc | 4e | −0.009(3) | 0.038(2) | 0.749(4) | 0.909(4)/0.091(4) |
| Sc/Mn | 2d | ½ | 0 | 0 | 0.909(4)/0.091(4) |
| Sb | 2c | 0 | ½ | 0 | 1 |
| O1 | 4e | 0.313(2) | 0.321(2) | 0.940(1) | 1 |
| O2 | 4e | 0.327(2) | 0.307(2) | 0.570(1) | 1 |
| O3 | 4e | 0.878(1) | 0.427(1) | 0.760(2) | 1 |
| (B–O1) × 2 | 2.07(1) | (Sb–O1) × 2 | 1.982(9) | ||
| (B–O2) × 2 | 2.10(1) | (Sb–O2) × 2 | 1.99(1) | Δ[BO6] | 2.9 × 10−4 |
| (B–O3) × 2 | 2.15(1) | (Sb–O3) × 2 | 2.00(1) | Δ[SbO6] | 1.6 × 10−5 |
| <A–O1–A> | 113 (1) | <A–O2–A> | 104 (1) | <A–O3–A> | 123 (1) |
| <B–O1–B′> | 140.9 (3) | <B–O2–B′> | 137.1 (3) | <B–O3–B′> | 136.8 (3) |
| BVS(Mn)b | 1.7 | BVS (Sc) | 2.9 | BVS (Sb) | 5.3 |
Fig. 5b depicts the nuclear (and magnetic) structure of DPv_Mn2ScSbO6; Sb and Sc are six-fold coordinated and ordered in a rock-salt configuration. Mn cations occupy the highly distorted cuboctahedral voids. The tilt angle, calculated as Φ = (180° − θ)/2 from <B–O–B′> bond angles (see Table 2) is Φ = 21.0 (3)°. This value is indicative of the high degree of distortion attained in this polymorph. The polyhedral distortions (Δ in Table 2) are also abnormally large (Δ[ScO6] = 2.9 × 10−4 and Δ[SbO6] = 1.6 × 10−5), close to those observed in other perovskites containing small-sized cations as Mn2+ and Sc3+ at the A site, as MnVO3 or ScVO3 with Φ = 18.02° and 26° and Δ = 4.4 × 10−4 and 4.0 × 10−4 respectively.5,26 The calculated BVS values are close to the expected values for the nominal composition Mn2+2Sc3+Sb5+O6.
The microstructure of this double perovskite is clearly observed in the HRTEM image recorded along the [010] zone axis (see SF2c†). The image and its corresponding FFT indicate the absence of extended defects and a good agreement with the calculated image is found. EELS experiments performed on the Mn L-edge in several crystals of DPv_Mn2ScSbO6 revealed an average oxidation state of +2 for Mn, in correspondence with BVS calculations.
The magnetic structure of DPv_Mn2ScSbO6 was determined by NPD using data from the D1B instrument. Additional magnetic neutron diffraction peaks are observed below 22 K.
Fig. 5a (bottom) shows the fit to the 3 K data. The magnetic reflections can be indexed with the k = [0 0 0] propagation vector in the P21/n space group. Irreducible representations and their basis vectors were obtained by using the program BASIREPS. Fig. 5b shows the resulting magnetic structure. It can be described as an antiparallel orientation of Mn2+ spins located in the ac-plane, with a total moment of 4.54 (1) μB at 3 K. The deviation of the spins with respect to the c axis has been calculated to be γ = 20.34 (1)°, resulting in 1.58 (1) μB and 4.26 (1) μB components along the a- and c-axes respectively. The inset in Fig. 5a shows the thermal evolution of the ordered magnetic moment, fitted to the critical law. The fitting results in TN = 22.3 K and β = 0.37, where β agrees again with 3D Heisenberg antiferromagnetic behaviour.
IV. Conclusions
Our results demonstrate that two new phases of Mn2ScSbO6 are accessible by high pressure–high temperature synthesis and their structural and microstructural details and magnetic properties have been investigated.Although cations are completely randomly located within the room pressure polymorph, frustrating any long range magnetic order, the synthesis of this oxide under high pressure conditions induces different cationic arrangements leading to interesting properties. The NTO-type moderate pressure modification crystallizes in the rhombohedral R3 polar space group, which allows a predicted room temperature polarization of 28.3 μC cm−2. It has ferrimagnetic order below 42 K, with the spins lying in the ab plane. A ferromagnetic component of 0.6 μB has been determined to arise from unusual site-selective Mn/Sc disorder. NTO_Mn2ScSbO6 is thus a potential multiferroic material. The high pressure phase has a double perovskite structure crystallizing in the P21/n space group, which exhibits an antiferromagnetic order below 22.3 K. Its magnetic structure has antiparallel Mn2+ spins in the ac plane.
Acknowledgements
The authors thank MINECO and Comunidad de Madrid for foundation through projects MAT2013-44964 and S-2013/MIT-12753, respectively. EPSRC and the Royal Society are also acknowledged for support, and Dr Gallardo-Amores and Dr Romero-de Paz for HPHT synthesis and magnetic measurements.Notes and references
- V. Srivastava, M. Rajagopalan and S. P. Sanyal, Eur. Phys. J. B, 2008, 61, 131 CrossRef CAS.
- R. J. Harrison, U. Becker and S. A. T. Redfern, Am. Mineral., 2000, 85, 1694 CrossRef CAS.
- K. Leinenweber, Phys. Chem. Minerals, 1991, 18, 244 CrossRef CAS.
- L. C. Ming, Y. H. Kim, T. Uchida, Y. Wang and M. Rivers, Am. Mineral., 2006, 91, 120 CrossRef CAS.
- M. Markkula, A. M. Arevalo-Lopez, A. Kusmartseva, J. A. Rodgers, C. Ritter, H. Wu and J. P. Attfield, Phys. Rev. B: Condens. Matter, 2011, 84, 094450 CrossRef.
- A. M. Arévalo-López and J. P. Attfield, Phys. Rev. B: Condens. Matter, 2013, 88, 104416 CrossRef.
- S. Vasala and M. Karppinen, Prog. Solid State Chem., 2015, 43, 1 CrossRef CAS.
- R. H. Mitchell, Perovskites: modern and ancient, Almaz Press Inc., 2002 Search PubMed.
- M. R. Li, D. Walker, M. Retuerto, T. Sarkar, J. Hadermann, P. W. Stephens, M. Croft, A. Ignatov, C. P. Grams, J. Hemberger, I. Nowik, P. S. Halasyamani, T. T. Tran, S. Mukherjee, T. S. Dasgupta and M. Greenblatt, Angew. Chem., Int. Ed., 2013, 52, 8406 CrossRef CAS PubMed.
- M. R. Li, M. Retuerto, D. Walker, T. Sarkar, P. W. Stephens, S. Mukherjee, T. S. Dasgupta, J. P. Hodges, M. Croft, C. P. Grams, J. Hemberger, J. Sánchez-Benítez, A. Huq, F. O. Saouma, J. I. Jang and M. Greenblatt, Angew. Chem., Int. Ed., 2014, 53, 10774 CrossRef CAS PubMed.
- M. R. Li, M. Croft, P. W. Stephens, M. Ye, D. Vanderbilt, M. Retuerto, Z. Deng, C. P. Grams, J. Hemberger, J. Hadermann, W. M. Li, C. Q. Jin, F. O. Saouma, J. I. Jang, H. Akamatsu, V. Gopalan, D. Walker and M. Greenblatt, Adv. Mater., 2015, 27, 2177 CrossRef CAS PubMed.
- A. J. Dos santos-García, C. Ritter, E. Solana-Madruga and R. Sáez-Puche, J. Phys.: Condens. Matter., 2013, 25, 206004 CrossRef PubMed.
- A. J. Dos santos-García, E. Solana-Madruga, C. Ritter, D. Ávila-Brande, O. Fabelo and R. Sáez-Puche, Dalton Trans., 2015, 44, 10665 RSC.
- S. Ivanov, P. Nordblad, R. Mathieu, R. Tellgren, E. Politova and G. André, Eur. J. Inorg. Chem., 2011, 4691 CrossRef CAS.
- L. I. Kosse, E. D. Politova and V. V. Chechkin, Izv. AN SSSR, Neorg. Mater., 1982, 18, 1879 CAS.
- L. I. Kosse, E. D. Politova and Yu. N. Venevtsev, Zh. Neorg. Khim., 1983, 28, 1689 CAS.
- Mac Tempas X. Version 2.3.7.A program for simulating HRTEM images and diffraction patterns Search PubMed.
- H. K. Schmid and W. Mader, Micron, 2006, 37, 426 CrossRef CAS PubMed.
- J. Rodriguez-Carvajal, Physica B, 1993, 192, 55 CrossRef CAS.
- J. Rodriguez-Carvajal, BASIREPS: a program for calculating irreducible representations of space groups and basis functions for axial and polar vector properties. Part of the FullProf Suite of programs, http://www.ill.eu/sites/fullprof/ Search PubMed.
- J. P. Morniroli and J. W. Steeds, Ultramicroscopy, 1992, 45, 219 CrossRef CAS.
- S. A. Ivanov, R. Mathieu, P. Nordblad, R. Tellgren, C. Ritter, E. Politova, G. Kaleva, A. Mosunov, S. Stefanovich and M. Weil, Chem. Mater., 2013, 25, 935 CrossRef CAS.
- N. A. Spaldin, J. Solid State Chem., 2012, 195, 2 CrossRef CAS.
- P. D. Battle, M. Avdeev and J. Hadermann, J. Solid State Chem., 2014, 220, 163 CrossRef CAS.
- G. V. Bazuev, B. G. Golovkin, N. V. Lukin, N. I. Kadyrova and Yu. G. Zainulin, J. Solid State Chem., 1996, 124, 333 CrossRef CAS.
- E. Castillo-Martínez, M. Bieringer, S. P. Shafi, L. M. D. Cranswick and M. A. Alario-Franco, J. Am. Chem. Soc., 2011, 133, 8552 CrossRef PubMed.
- J. B. Goodenough, Magnetism and the chemical bond. Interscience monographs on chemistry, 1963 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1428909–1428911. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt03445k |
| This journal is © The Royal Society of Chemistry 2015 |