Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Water stable triazolyl phosphonate MOFs: steep water uptake and facile regeneration†
S.
Begum
ab,
S.
Horike
b,
S.
Kitagawa
*bc and
H.
Krautscheid
*a
aFakultät für Chemie und Mineralogie, Universität Leipzig, Johannisallee 29, Leipzig 04103, Germany. E-mail: krautscheid@rz.uni-leipzig.de; Fax: +0049-3419736199; Tel: +0049-3419736172
bDepartment of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan. E-mail: kitagawa@icems.kyoto-u.ac.jp; Fax: +81-75-383-2732; Tel: +81-75-383-2733
cInstitute for Integrated Cell-Material Sciences (WPI-iCeMS), Kyoto University, Yoshida, Sakyo-ku, Kyoto 606-8501, Japan
First published on 5th October 2015
Abstract
The new microporous cobalt triazolyl phosphonate MOF 3α[Co4L3(μ3-OH)(H2O)3](SO4)0.5·xH2O (L2− = 4-(4H-1,2,4-triazol-4-yl)-phenyl phosphonate) exhibits exciting features including high water stability, reversible hydration–dehydration, steep water uptake in repeated cycles at low water vapor pressures and reactivation at room temperature under mild evacuation.
The design and development of water stable porous materials are crucial for industrial applications that require efficient capture and release of water such as solid desiccants, heat pumps and electric dehumidifiers.1–5 The well known solid sorption materials, i.e. silica gels and zeolites, are used on a large scale; however, with their limitations such as silica gels are not very energy efficient since most of the water sorption occurs outside their operating pressure windows,5 and zeolites require high temperature for regeneration.6 In contrast to pure inorganic porous materials, recently metal–organic frameworks have been recognized as alternative sorption materials for adsorptive heat transformation processes used in thermally driven adsorption chillers or adsorption heat pumps.2,5,7
In the last two decades MOFs have been extensively studied for their application in gas sorption and separation properties.8 However, water sorption in MOFs remained underexplored as the majority of the reported MOFs are not stable in water.9,10 One of the earliest water sorption studies in MOFs was reported in 2002 for HKUST-1 with the primary focus on evaluating stability rather than its sorption behavior.11 Until now most of the water sorption studies have focused on well known carboxylate based MOFs, i.e. HKUST-1,12,13 MIL-100/101,2,14 MIL-53, CPO-27, UiO-66,12,15 MOF-801 and MOF-841.1 Although MOFs have attained special attention for water sorption properties in the last couple of years, it remains a challenge to find or design materials with higher water uptake in a narrow relative humidity range that resists hydrolysis and maintains high structural integrity.
In this regard a number of strategies have been reported to improve the water stability of MOFs. The MIL series and zirconium based UiO-series with tri- or tetravalent metal ions, respectively, are amongst the most stable poly-carboxylate MOFs. Other approaches include functionalization of organic linkers, i.e. incorporation of fluorinated linkers and use of longer alkyl side chains in order to enhance the hydrophobic character, and consideration of catenation in MOFs.10,16 These attempts increased the water stability but decreased the water uptake capacity due to enhanced hydrophobicity and reduction in surface areas.
In comparison with polycarboxylate MOFs, in spite of the known fact that phosphonate MOFs intrinsically impart remarkable hydrostability,17–19 CAU-14 is the only example of phosphonate MOFs recently reported for steep water uptake of 39.1 wt% at p/po = 0.2.18 Therefore, the synthesis of new phosphonate MOFs with regular micropores for improved water sorption properties is of great interest. Furthermore, phosphonate MOFs exhibit a greater tendency for forming polar pores; this would allow a different selectivity than observed for non-polar pores that are typical for the majority of carboxylate and imidazolate MOFs.20
Herein, we report on a new porous water stable cobalt triazolyl phosphonate MOF 3α[Co4L3(μ3-OH)(H2O)3](SO4)0.5·xH2O (1, L2− = 4-(4H-1,2,4-triazol-4-yl)phenyl phosphonate) with hydrophilic nanochannels. 1 exhibits excellent water stability and maintains its high structural integrity with water uptake already at low humidity and in a narrow relative humidity range. In contrast to zeolites, 1 demonstrates facile regeneration.
1 crystallizes in the hexagonal space group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c (no. 190) with a sixth of a formula unit [Co4L3(μ3-OH)(H2O)3](SO4)0.5 in the asymmetric unit.‡ The charge balancing sulfate ion is disordered in the crystal structure of 1, which was identified by ion chromatography.
2c (no. 190) with a sixth of a formula unit [Co4L3(μ3-OH)(H2O)3](SO4)0.5 in the asymmetric unit.‡ The charge balancing sulfate ion is disordered in the crystal structure of 1, which was identified by ion chromatography.
One of the two crystallographically independent Co2+ ions (Co1) and the C, N and P atoms of the linker anion L2− are coplanar (Fig. 1a). Two nitrogen atoms each of three triazole rings connect three cobalt ions to a typical μ3-OH and triazole bridged Co3 triangle (Fig. 1a). Such triangles are connected via three O–P–O units of phosphonate groups to linear stacks along [001] as shown in Fig. 1b.
In addition, π⋯π stacking interactions with 327 pm (= c/2) between the aromatic rings of the linker L2− stabilize the crystal structure. With Co–O bond lengths from 206 to 210(1) pm, and Co–N bond lengths 212 and 215(1) pm, Co1 exhibits octahedral coordination. The centres of six trigonal prismatically arranged21 but disordered phosphonate oxygen atoms are occupied by the cobalt ions Co2 (![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) position; Co2–O 219(1) pm, please see the ESI†). Coordinated water molecules complete the octahedral coordination of Co1, and non-coordinating phosphonate oxygen atoms protrude from each stack towards the interior of the 1D pores. Six such stacks connected via organic linkers result in hydrophilic hexagonal channels that retain physisorbed water molecules inside the pores. These channels are arranged like a hexagonal packing of rods and show a visible open diameter of 1.3 nm.
position; Co2–O 219(1) pm, please see the ESI†). Coordinated water molecules complete the octahedral coordination of Co1, and non-coordinating phosphonate oxygen atoms protrude from each stack towards the interior of the 1D pores. Six such stacks connected via organic linkers result in hydrophilic hexagonal channels that retain physisorbed water molecules inside the pores. These channels are arranged like a hexagonal packing of rods and show a visible open diameter of 1.3 nm.
In order to evaluate the behavior of the coordinated and non-coordinated water molecules in the pores with temperature, TGA studies were performed (Fig. 2). The TGA curve of air dried 1 shows a mass loss of 20 wt% in two steps in the temperature range of 40 to 225 °C. These steps are attributed to the loss of non-coordinated and coordinated water molecules at about 90 °C and at 200 °C, respectively. A mass loss of 19.4 wt% corresponds to 13 water molecules per formula unit. Under mild evacuation conditions (25 °C, 1 × 10−2 mbar) only non-coordinated water molecules leave the framework ensured by the presence of the long plateau in the TGA curve of 1′ (1 after activation at 25 °C, 1 × 10−2 mbar) until the first mass loss of about 5.5% around 200 °C. The experimental mass loss is consistent with the loss of three water molecules per formula unit (calc. 5.3%). To test the framework hydro-stability, 1 was refluxed in water for 24 hours; the PXRD patterns confirm the stability of 1 in boiling water (Fig. S2†).
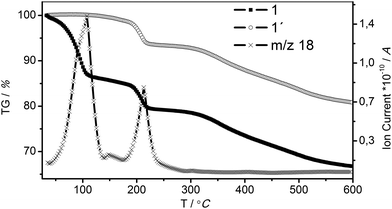 | ||
| Fig. 2 TG-DTA curves of 1 (pristine), water molecules leave the structure in two steps confirmed by m/z = 18 signals. 1′ is 1 after activation (25 °C, 1 × 10−2 mbar). | ||
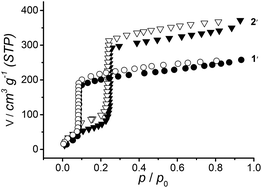
Recently we reported on a lanthanum MOF, [La3L4(H2O)6]Cl·xH2O (2), with the same linker as 1 with a pore diameter of 1.9 nm.17 Both compounds, 1 and 2, exhibit a similar robust structural behavior and a steep water uptake (Fig. 3) at a relative water vapor pressure p/po = 0.08 and 0.21 for 1 (pore size 1.3 nm) and 2 (pore size 1.9 nm), respectively.
The volumetric water sorption isotherms for evacuated (at r.t.) samples of 1 and 2, denoted 1′ and 2′, were measured at 293 K and are shown in Fig. 3. Both, 1′ and 2′, exhibit sorption isotherms close to type I behavior, characterized by a small increase followed by a steep water uptake of 230 and 330 cm3 (STP) g−1 at low relative pressures and a long saturation plateau.4,22 This behavior is similar to that observed in CPO-27 (Ni, Mg, Co)10,23 as well as in zeolites. However, in CPO-27 water adsorption proceeds via chemisorption at coordinatively unsaturated metal sites followed by physisorption. CPO-27 MOFs retain their structural stability when thermally treated in the flow of N2, however, thermal treatment at temperatures higher than 100 °C in the presence of air leads to structural deformation.24 CPO-27 and 1 share the common feature of their honeycomb structure with a channel diameter of 1.1 and 1.3 nm, respectively. In contrast to zeolites, in phosphonate MOFs 1 and 2, the most exciting and unique feature is their easy regeneration in 2 hours under mild evacuation conditions (10−2 mbar) at room temperature. 1′ and 2′ both show robust cycling performance as indicated by the reproducible water uptake in repeated cycles of adsorption and desorption shown in Fig. S7 and S8,† respectively.
These promising results of steep water uptake capacity at low pressures in repeated cycles make 1 and 2 suitable hydrophilic adsorbents that require mild regeneration conditions with retention of their structural integrity. Adsorption profiles of abrupt water uptake in a single adsorption step as observed in 1 and 2 are rare in the literature, exhibited by very few other MOFs such as CAU-14,18 CAU-10-H,25 MIL-125-NH2,7 UiO-67,26 and In-MIL-68,5b at p/po values of 0.2, 0.2, 0.2, 0.58 and 0.58, respectively, in comparison with 0.08 for 1 and 0.21 for 2. The absence of hysteresis loops in the sorption isotherms of 1 and 2 is an indicator of structural integrity in these phosphonate MOFs as well as continuous and reversible physisorption in a microporous material. The water stability of 1 and 2 arises from the bond strengths of phosphonate oxygen metal bonds and further from their extension in one dimensional metal phosphonate stacks connected via organic linkers. Furthermore, the presence of coordinated water protruding towards the 1D pores determines the hydrophilic nature of the pores and offers great affinity for the incoming guest water molecules during adsorption. Water uptake by 1 and 2 is found in the ideal pressure range for applications (0.05 < p/po < 0.4) and desorption temperatures at or below 80 °C.2,3
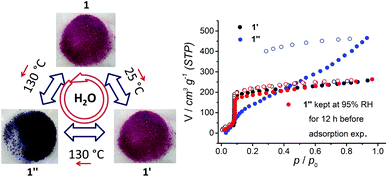
Taking into account the second weight loss in the TGA curve of 1, we heated compound 1 at 130 °C under vacuum (10−2 mbar) in order to lose coordinated water molecules and generate unsaturated metal centers. We observed that 1 experiences a reversible color change from pink (pristine 1 and after activation at 25 °C under 10−2 mbar, denoted 1′) to dark purple (heated at 130 °C at 10−2 mbar or at 150 °C at ambient pressure, denoted 1′′, Fig. 4). PXRD patterns of 1′′ (Fig. S4†) retain lower angle peaks whereas at higher angles the peaks almost vanished. Crystals of 1 retained their shape but the crystallinity was no longer adequate for single crystal diffraction studies. Nevertheless, we studied 1′′ for its water sorption behaviour. The water sorption isotherm of 1′′ shown in Fig. 4 exhibits a significantly higher and gradual water uptake capacity compared to 1′, probably due to structural rearrangement. Water is retained in 1′′ as hysteresis is observed in adsorption and desorption cycles. In comparison with the steep water uptake in 1′, 1′′ shows gradual water uptake with almost doubled capacity. Samples of 1′′ kept at 95% RH for 12 h at 90 °C turned back from dark purple to pink. The PXRD pattern confirms that 1′′ changed back to 1. Furthermore, water sorption studies performed on the same sample confirmed reversible conversion of 1′′ to 1 as the sorption isotherms of 1′ and 1′′, after keeping under 95% RH at 90 °C, exactly matched as shown in Fig. 4. We infer from these observations that 1 is highly stable under humid conditions, and as a function of temperature 1 can be transformed to 1′ or 1′′ and under humid conditions 1′ and 1′′ reversibly convert back to 1.
Conclusions
In conclusion, we introduced highly water stable triazolyl phosphonate MOFs, 1 and 2, as potentially efficient water sorption materials that exhibit steep water uptake in the ideal range for applications (0.05 < p/po < 0.4). Our studies confirm that 1 and 2 with hydrophilic nano-channels sustain their structure in boiling water as well as in saturated water vapors. After water sorption 1 and 2 can be activated under mild evacuation at room temperature and show reproducible water uptake in repeated cycles of adsorption and desorption. These results render porous triazolyl phosphonate MOFs interesting candidates for water sorption based applications such as adsorptive heat transformation processes.Acknowledgements
We thank Dr J. Möllmer and U. Mikow (INC Leipzig) for support in the quantification of cobalt, sulphate and phosphate ions (AAS and ion chromatography). S. Begum acknowledges the graduate school BuildMoNa and Research Academy Leipzig (RAL) for supporting her research visit at Kyoto University. She extends her acknowledgement for financial support by the Higher Education Commission, Pakistan and a fellowship from DAAD. Financial support from Deutsche Forschungsgemeinschaft (SPP 1362 – Poröse Metall-organische Gerüstverbindungen) and Universität Leipzig (PbF-1) is gratefully acknowledged.Notes and references
- H. Furukawa, F. Gándara, Y. B. Zhang, J. Jiang, W. L. Queen, M. R. Hudson and O. M. Yaghi, J. Am. Chem. Soc., 2014, 136, 4369 CrossRef CAS PubMed.
- F. Jeremias, A. Khutia, S. K. Henninger and C. Janiak, J. Mater. Chem., 2012, 22, 10148 RSC.
- A. Khutia, H. U. Rammelberg, T. Schmidt, S. Henninger and C. Janiak, Chem. Mater., 2013, 25, 790 CrossRef CAS.
- N. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114, 10575 CrossRef CAS PubMed.
- (a) J. Canivet, A. Fateeva, Y. Guo, B. Coasne and D. Farrusseng, Chem. Soc. Rev., 2014, 43, 5594 RSC; (b) J. Canivet, J. Bonnefoy, C. Daniel, A. Legrand, B. Coasne and D. Farrusseng, New J. Chem., 2014, 38, 3102 RSC.
- (a) P. L. Llewellyn, F. Schüth, Y. Grillet, F. Rouquerol, J. Rouquerol and K. K. Unger, Langmuir, 1995, 11, 574 CrossRef CAS; (b) R. Gopal, B. R. Hollebone, C. H. Langford and R. A. Shigeishi, Sol. Energy, 1982, 28, 421 CrossRef CAS; (c) O. M. Dzhigit, A. V. Kiselev, K. N. Mikos, G. G. Muttik and T. A. Rahmanova, Trans. Faraday Soc., 1971, 67, 458 RSC; (d) M. D. Olga, V. K. Andrie, A. R. Tamara and S. P. Zhdanov, J. Chem. Soc., Faraday Trans., 1979, 75, 2662 RSC; (e) S. T. Wilson, B. M. Lok, C. A. Messina, T. R. Cannan and E. M. Flanigen, J. Am. Chem. Soc., 1982, 104, 1146 CrossRef CAS; (f) X. Benjing, Z. Chenxi and Y. Zifeng, J. Nat. Gas Chem., 2005, 14, 65 Search PubMed; (g) S. Komarneni, R. Pidugu and V. C. Menon, J. Porous Mater., 1996, 3, 99 CrossRef CAS.
- F. Jeremias, D. Fröhlich, C. Janiak and S. K. Henninger, New J. Chem., 2014, 38, 1846 RSC.
- (a) D. Lässig, J. Lincke, J. Moellmer, C. Reichenbach, A. Moeller, R. Gläser, G. Kalies, K. A. Cychosz, M. Thommes, R. Staudt and H. Krautscheid, Angew. Chem., Int. Ed., 2011, 50, 10344 CrossRef PubMed; (b) S. Ma, Pure Appl. Chem., 2009, 81, 2235 CrossRef CAS; (c) J. Liu, P. K. Thallapally, B. P. McGrail, D. R. Brown and J. Liu, Chem. Soc. Rev., 2012, 41, 2308 RSC; (d) J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC; (e) N. Nijem, H. Wu, P. Canepa, A. Marti, K. J. Balkus, T. Thonhauser, J. Li and Y. J. Chabal, J. Am. Chem. Soc., 2012, 134, 15201 CrossRef CAS PubMed; (f) J. Moellmer, A. Moeller, C. Patzschke, K. Stein, D. Lässig, J. Lincke, R. Gläser, H. Krautscheid and R. Staudt, J. Mater. Chem., 2012, 22, 10274 RSC.
- (a) T. L. Kinnibrugh, A. A. Ayi, V. I. Bakhmutov, J. Zoń and A. Clearfield, Cryst. Growth Des., 2013, 13, 2973 CrossRef CAS; (b) J. E. Mondloch, M. J. Katz, N. Planas, D. Semrouni, L. Gagliardi, J. T. Hupp and O. K. Farha, Chem. Commun., 2014, 50, 8944 RSC.
- K. Tan, N. Nijem, Y. Gao, S. Zuluaga, J. Li, T. Thonhauser and Y. J. Chabal, CrystEngComm, 2014, 17, 247 RSC.
- Q. Min Wang, D. Shen, M. Bülow, M. Ling Lau, S. Deng, F. R. Fitch, N. O. Lemcoff and J. Semanscin, Microporous Mesoporous Mater., 2002, 55, 217 CrossRef.
- J. B. DeCoste, G. W. Peterson, B. J. Schindler, K. L. Killops, M. A. Browe and J. J. Mahle, J. Mater. Chem. A, 2013, 1, 11922 CAS.
- L. Grajciar, O. Bludský and P. Nachtigall, J. Phys. Chem. Lett., 2010, 1, 3354–3359 CrossRef CAS.
- G. Akiyama, R. Matsuda, H. Sato, A. Hori, M. Takata and S. Kitagawa, Microporous Mesoporous Mater., 2012, 157, 89 CrossRef CAS PubMed.
- P. Ghosh, Y. J. Colón and R. Q. Snurr, Chem. Commun., 2014, 50, 11329 RSC.
- H. Jasuja and K. S. Walton, Dalton Trans., 2013, 42, 15421 RSC.
- S. Begum, Z. Wang, A. Donnadio, F. Costantino, M. Casciola, R. Valiullin, C. Chmelik, M. Bertmer, J. Kärger, J. Haase and H. Krautscheid, Chem. – Eur. J., 2014, 20, 8862 CrossRef CAS PubMed.
- N. Hermer and N. Stock, Dalton Trans., 2015, 44, 3720 RSC.
- (a) M. Taddei, F. Costantino, F. Marmottini, A. Comotti, P. Sozzani and R. Vivani, Chem. Commun., 2014, 50, 14831 RSC; (b) S. F. Tang, J. J. Cai, L. J. Li, X. X. Lv, C. Wang and X. B. Zhao, Dalton Trans., 2014, 43, 5970 RSC; (c) J. M. Taylor, K. W. Dawson and G. K. H. Shimizu, J. Am. Chem. Soc., 2013, 135, 1193 CrossRef CAS PubMed.
- S. Paranthaman, F. X. Coudert and A. H. Fuchs, Phys. Chem. Chem. Phys., 2010, 12, 8123 RSC.
- (a) O. Gourdon, V. Petricek, M. Dusek, P. Bezdicka, S. Durovic, D. Gyepesova and M. Evain, Acta Crystallogr., Sect. B: Struct. Sci., 1999, 55, 841 Search PubMed; (b) S. A. Kubow, K. J. Takeuchi, J. J. Grzybowski, A. J. Jircitano and V. L. Geodken, Inorg. Chim. Acta, 1996, 241, 21 CrossRef CAS; (c) C. Wendelstorf and R. Kraemer, Angew. Chem., Int. Ed. Engl., 1997, 36, 2791 CrossRef CAS PubMed; (d) H. A. Sagher, I. Fallis, L. J. Farrugia and R. D. Peacock, J. Chem. Soc., Chem. Commun., 1993, 1499 RSC.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- A. Das, P. D. Southon, M. Zhao, C. J. Kepert, A. T. Harris and D. M. D. Alessandro, Dalton Trans., 2012, 41, 11739 RSC.
- P. D. C. Dietzel, B. Panella, M. Hirscher, R. Blom and H. Fjellvåg, Chem. Commun., 2006, 959 RSC.
- D. Fröhlich, S. K. Henninger and C. Janiak, Dalton Trans., 2014, 43, 15300 RSC.
- N. Ko, J. Hong, S. Sung, K. E. Cordova, H. J. Park, J. K. Yang and J. Kim, Dalton Trans., 2015, 44, 2047 RSC.
- PLATON; A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1411211. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt02651b |
‡ Crystal data: 1 (C24H49N9Co4O27P3S0.5): Stoe IPDS-2 T Diffractometer, Mm = 1240.38 g mol−1, crystal size 0.30 × 0.05 × 0.05 mm3, hexagonal, space group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c (no. 190), a = 2059.40(16), c = 653.04(4), Z = 2, T = 180(2) K, V = 2398.6(4) × 106 pm3, dcalc = 1.717 g cm–3, μ = 1.576 mm−1, λ = 71.073 pm (Mo-Kα), 5525 measured, 1465 independent reflections, Rint = 0.0713, 1176 with I > 2σ(I), 100 parameters, phosphonate oxygen atoms, coordinated water and the μ3-OH group are disordered (please see the ESI for details), H atoms of phosphonate ligands in idealized positions. Since the sulfate anion and water molecules are disordered in the pores the SQUEEZE27 routine was applied. R1 (observed reflections) = 0.0540, wR2 (all data) = 0.1416, absolute structure parameter = 0.47(7); max. and min. residual electron density peaks 0.6 and −1.3 e−/106 pm3. CCDC 1411211 contains the supplementary crystallographic data for this paper. 2c (no. 190), a = 2059.40(16), c = 653.04(4), Z = 2, T = 180(2) K, V = 2398.6(4) × 106 pm3, dcalc = 1.717 g cm–3, μ = 1.576 mm−1, λ = 71.073 pm (Mo-Kα), 5525 measured, 1465 independent reflections, Rint = 0.0713, 1176 with I > 2σ(I), 100 parameters, phosphonate oxygen atoms, coordinated water and the μ3-OH group are disordered (please see the ESI for details), H atoms of phosphonate ligands in idealized positions. Since the sulfate anion and water molecules are disordered in the pores the SQUEEZE27 routine was applied. R1 (observed reflections) = 0.0540, wR2 (all data) = 0.1416, absolute structure parameter = 0.47(7); max. and min. residual electron density peaks 0.6 and −1.3 e−/106 pm3. CCDC 1411211 contains the supplementary crystallographic data for this paper. |
| This journal is © The Royal Society of Chemistry 2015 |

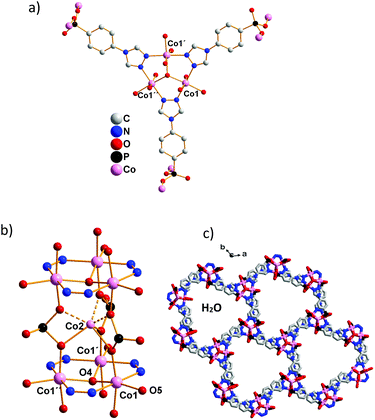
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ] showing the hexagonal arrangement of metal linker chains and the 1D pores; symmetry operations: ′ 1 −
] showing the hexagonal arrangement of metal linker chains and the 1D pores; symmetry operations: ′ 1 −