 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
General cooperative effects of single atom ligands on a metal: a 195Pt NMR chemical shift as a function of coordinated halido ligands’ ionic radii overall sum
M.
Benedetti
*,
F.
de Castro
,
D.
Antonucci
,
P.
Papadia
and
F. P.
Fanizzi
*
Dipartimento di Scienze e Tecnologie Biologiche ed Ambientali, Università del Salento, Via Monteroni, I-73100 Lecce, Italy. E-mail: michele.benedetti@unisalento.it; fp.fanizzi@unisalento.it; Fax: +39 0832 298626; Tel: +39 0832 298867
First published on 24th July 2015
Abstract
An inverse linear relationship between the experimentally observed 195Pt NMR signals and the overall sum of coordinated halido ligands’ ionic radii was discovered in Pt(II) and Pt(IV) complexes. The reduction of 195Pt NMR frequencies parallels the increase of coordinated halido ligands’ ionic radii sum. This suggests that each halido ligand may act as a conducting ring whose induced electric current shields the 195Pt NMR signals proportionally to the ionic radius of the coordinated halido ligand.
In previous studies we analyzed the NMR properties of pentacoordinate complexes, formed by the interaction of Zeise's anion, [PtCl3(η2-CH2
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)]−, with N,N-chelate ligands.1–14 These specific complexes are generally considered to be interesting since platinum bonded unsaturated ligands are useful models in the study of the interaction of alkenes and alkynes with metals.14–22 In particular, in the study of the single crystal X-ray structures and NMR signals of the symmetric pentacoordinate complexes [PtX2(η2-CH2
CH2)]−, with N,N-chelate ligands.1–14 These specific complexes are generally considered to be interesting since platinum bonded unsaturated ligands are useful models in the study of the interaction of alkenes and alkynes with metals.14–22 In particular, in the study of the single crystal X-ray structures and NMR signals of the symmetric pentacoordinate complexes [PtX2(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)] (X = Cl, Br, I; Me2phen = 2,9-dimethyl-1,10-phenanthroline), we described the evidence of pseudo-ring currents circulating around the Pt–X axes, Fig. 1 and 2. These currents seemed to be modulated by the ionic radii of the halido ligands coordinated at trans positions, above and below the trigonal equatorial plane. Indeed, in these complexes, characterized by negligible Pt–η2-ethene and Pt–N bond length variations in the trigonal plane, the two trans-axial halido ligands clearly influence the observed 1H, 13C, 15N and 195Pt NMR chemical shifts of neighbour atoms.20 Platinum chemical shift correlations with other parameters such as coordinated halido ligands electronegativity were previously reported.23,24 Nevertheless recently we could evidence the existence of a linear relationship between the ionic radii of coordinated halido ligands and the 195Pt NMR chemical shifts.20
CH2)(Me2phen)] (X = Cl, Br, I; Me2phen = 2,9-dimethyl-1,10-phenanthroline), we described the evidence of pseudo-ring currents circulating around the Pt–X axes, Fig. 1 and 2. These currents seemed to be modulated by the ionic radii of the halido ligands coordinated at trans positions, above and below the trigonal equatorial plane. Indeed, in these complexes, characterized by negligible Pt–η2-ethene and Pt–N bond length variations in the trigonal plane, the two trans-axial halido ligands clearly influence the observed 1H, 13C, 15N and 195Pt NMR chemical shifts of neighbour atoms.20 Platinum chemical shift correlations with other parameters such as coordinated halido ligands electronegativity were previously reported.23,24 Nevertheless recently we could evidence the existence of a linear relationship between the ionic radii of coordinated halido ligands and the 195Pt NMR chemical shifts.20
 | ||
Fig. 1 Schematic representation of the structure of pentacoordinate complexes of the type [PtXY(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)], (X,Y = Cl, Br, I; Me2phen = 2,9-dimethyl-1,10-phenanthroline). CH2)(Me2phen)], (X,Y = Cl, Br, I; Me2phen = 2,9-dimethyl-1,10-phenanthroline). | ||
The problem of understanding the phenomena influencing the NMR signal frequencies, which can also be related to the physical properties of a molecule, has deserved particular attention in the recent literature.23–28 In this work, we evaluated the NMR shielding properties of coordinated halido ligands in trigonal–bipyramidal Pt(II) and octahedral Pt(IV) complexes (Fig. 1 and 3). 195Pt NMR data for pentacoordinate Pt(II) complexes of the type [PtXY(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)] (X≠Y = Cl, Br, I), where two non-equivalent axial halido ligands are bonded to the metal (Fig. 1), have been collected together with those of the previously reported symmetrical analogues (X = Y = Cl, Br, I), Table 1.20 In a further step, octahedral Pt(IV) complexes of the type [PtXnY6−n]2− (1 ≤ n ≤ 6; X,Y = F, Cl, Br, I) were also studied by 195Pt NMR spectroscopy, in order to analyse the effect of changing the oxidation state and the coordination number and geometry of the metal which also includes cis, trans, fac and mer isomers, Table 2.
CH2)(Me2phen)] (X≠Y = Cl, Br, I), where two non-equivalent axial halido ligands are bonded to the metal (Fig. 1), have been collected together with those of the previously reported symmetrical analogues (X = Y = Cl, Br, I), Table 1.20 In a further step, octahedral Pt(IV) complexes of the type [PtXnY6−n]2− (1 ≤ n ≤ 6; X,Y = F, Cl, Br, I) were also studied by 195Pt NMR spectroscopy, in order to analyse the effect of changing the oxidation state and the coordination number and geometry of the metal which also includes cis, trans, fac and mer isomers, Table 2.
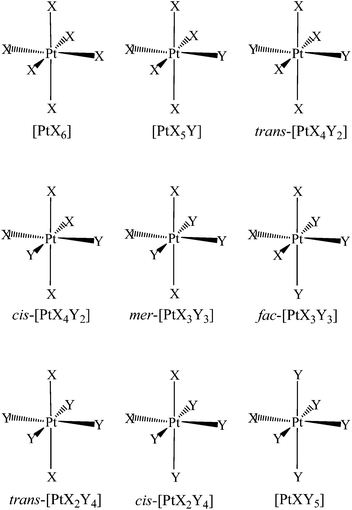 | ||
| Fig. 3 Representation of the general structures of octahedral complexes [PtXnY6−n]2− (1 ≤ n ≤ 6; X,Y = F, Cl, Br, I). | ||
| Complex | 195Pt δ (ppm) | Ref. |
|---|---|---|
| [PtF6]2− | 7090 | 25 |
| [PtClF5]2− | 5856 | 25 |
| [PtBrF5]2− | 5669 | 25 |
| trans-[PtCl2F4]2− | 4887 | 25 |
| trans-[PtBr2F4]2− | 4551 | 25 |
| cis-[PtCl2F4]2− | 4547 | 25 |
| cis-[PtBr2F4]2− | 4136 | 25 |
| mer-[PtCl3F3]2− | 3551 | 25 |
| fac-[PtCl3F3]2− | 3191 | 25 |
| mer-[PtBr3F3]2− | 2907 | 25 |
| trans-[PtCl4F2]2− | 2534 | 25 |
| fac-[PtBr3F3]2− | 2487 | 25 |
| cis-[PtCl4F2]2− | 2171 | 25 |
| trans-[PtBr4F2]2− | 1157 | 25 |
| cis-[PtBr4F2]2− | 1142 | 25 |
| [PtCl5F]2− | 1111 | 25 |
| [PtCl6]2− | 0 | 23 |
| [PtCl5Br]2− | −286 | 23 |
| [PtBr5F]2− | −311 | 25 |
| cis-[PtCl4Br2]2− | −583 | 23 |
| trans-[PtCl4Br2]2− | −585 | 23 |
| [PtCl5I]2− | −854 | 23 |
| fac-[PtCl3Br3]2− | −892 | 23 |
| mer-[PtCl3Br3]2− | −894 | 23 |
| cis-[PtCl2Br4]2− | −1213 | 23 |
| trans-[PtCl2Br4]2− | −1216 | 23 |
| [PtClBr5]2− | −1547 | 23 |
| cis-[PtCl4I2]2− | −1806 | 23 |
| trans-[PtCl4I2]2− | −1884 | 23 |
| [PtBr6]2− | −1891 | 23 |
| [PtBr5I]2− | −2545 | 23 |
| fac-[PtCl3I3]2− | −2849 | 23 |
| mer-[PtCl3I3]2− | −2849 | 23 |
| trans-[PtBr4I2]2− | −3132 | 23 |
| cis-[PtBr4I2]2− | −3252 | 23 |
| mer-[PtBr3I3]2− | −3898 | 23 |
| cis-[PtCl2I4]2− | −3932 | 23 |
| trans-[PtCl2I4]2− | −3932 | 23 |
| fac-[PtBr3I3]2− | −4014 | 23 |
| trans-[PtBr2I4]2− | −4603 | 23 |
| cis-[PtBr2I4]2− | −4719 | 23 |
| [PtClI5]2− | −5081 | 23 |
| [PtBrI5]2− | −5483 | 23 |
| [PtI6]2− | −6293 | 23 |
The general structure of the considered pentacoordinate complexes is reported in Fig. 1. The 195Pt NMR chemical shifts of these pentacoordinate species are reported in Table 1. A clear inverse linear relationship between 195Pt NMR frequencies and the overall sum of coordinated halido ligands’ ionic radii (R2 = 0.998) can be observed in the plot of 195Pt NMR data (Table 1) as a function of coordinated halido ligands’ ionic radii sum (Fig. 4). This means that in [PtXY(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)] complexes the shielding produced by each halido ligand on the observed 195Pt NMR signal frequency, depends in first approximation only on its ionic radius, as previously stated for the symmetric species [PtX2(η2-CH2
CH2)(Me2phen)] complexes the shielding produced by each halido ligand on the observed 195Pt NMR signal frequency, depends in first approximation only on its ionic radius, as previously stated for the symmetric species [PtX2(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)].20 On the other hand, it appears that the 195Pt NMR shielding is not directly related to the mutual trans influence of the axial X− ligands, potentially able to affect the Pt–X(Y) bond lengths in [PtXY(η2-CH2
CH2)(Me2phen)].20 On the other hand, it appears that the 195Pt NMR shielding is not directly related to the mutual trans influence of the axial X− ligands, potentially able to affect the Pt–X(Y) bond lengths in [PtXY(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)] complexes.
CH2)(Me2phen)] complexes.
The correlation of 195Pt NMR frequencies, with the overall sum of coordinated halido ligands’ ionic radii, also in platinum complexes with a different oxidation state, coordination number and geometry was therefore investigated. We studied with a similar approach the 195Pt NMR frequency variation observed in octahedral Pt(IV) complexes of the type [PtXnY6−n]2− (1 ≤ n ≤ 6; X,Y = F, Cl, Br, I), Fig. 3. The 195Pt NMR signals of the investigated Pt(IV) model complexes are reported in Table 2. Also for octahedral Pt(IV) complexes [PtXnY6−n]2− a clear inverse linear relationship between 195Pt NMR frequencies and the overall sum of coordinated halido ligands’ ionic radii was found. This is evident in the linear plot (R2 = 0.996) of 195Pt NMR data, as a function of coordinated halido ligands’ ionic radii sum, reported in Fig. 5. The pure additional effect of halido ligands’ ionic radii on 195Pt NMR frequencies, in the studied Pt(IV) species, is further evidenced in Fig. 6, where the 195Pt chemical shifts are reported as a function of the X− halido ligand ionic radius, for six different groups of complexes of the type [PtClnX6−n]2−. In each group, the platinum complexes bear a specific number (n; 0 ≤ n ≤ 5) of chlorido ligands and a variable X− halido ligand for the remaining 6 − n coordination sites. Six different lines interpolating the 195Pt NMR chemical shifts, as a function of the remaining specific X− ionic radius, in [PtClnX6−n]2− complexes, are reported in Fig. 6A. Interestingly, each unit increase of the chlorido ligand number (n), which results in a nearly constant increase of the slope of the specific interpolating line. The linear relationship between the slopes of the six lines in Fig. 6A and the chlorido ligand number (n) (R2 = 0.999) is reported in Fig. 6B. It is also noteworthy that all the six interpolating lines of Fig. 6A pass exactly through the data point representing in the graph the 195Pt NMR chemical shift of the [PtCl6]2− complex, the only one that belongs to all the six groups of model complexes. These results further confirm the regular trend of the observed correlation. Consistently, similar trends are observed in the analogue [PtFnX6−n]2−, [PtBrnX6−n]2− and [PtInX6−n]2− series of complexes.
Our hypothesis on the 195Pt NMR chemical shift linear dependence from the overall halido ligands’ ionic radius sum therefore holds not only for Pt(II) trigonal-bipyramidal [PtXY(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)] but also for octahedral Pt(IV) [PtXnY6−n]2− (X,Y = halido ligand) complexes. As a further confirmation of the simple additional effect of the overall halido ligands’ ionic radius sum, only slight variations of the 195Pt NMR frequency are observed here on passing from cis to trans and from fac to mer geometric isomers. Therefore, at least for the studied model complexes, it is also possible, based on the 195Pt NMR data for some complexes within a series, to easily predict the 195Pt NMR shifts for analogous complexes bearing a different combination of coordinated halido ligands.
CH2)(Me2phen)] but also for octahedral Pt(IV) [PtXnY6−n]2− (X,Y = halido ligand) complexes. As a further confirmation of the simple additional effect of the overall halido ligands’ ionic radius sum, only slight variations of the 195Pt NMR frequency are observed here on passing from cis to trans and from fac to mer geometric isomers. Therefore, at least for the studied model complexes, it is also possible, based on the 195Pt NMR data for some complexes within a series, to easily predict the 195Pt NMR shifts for analogous complexes bearing a different combination of coordinated halido ligands.
Experimental
All solvents and reagents, except otherwise stated, were purchased from Aldrich Chemical Company and used as received. The symmetric pentacoordinate complexes [PtX2(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)] (X = Cl, Br, I) were prepared with the previously reported methods.29 These complexes [PtXY(η2-CH2
CH2)(Me2phen)] (X = Cl, Br, I) were prepared with the previously reported methods.29 These complexes [PtXY(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)] (X≠Y = Cl, Br, I) were obtained in equilibrium mixtures with symmetric species [PtX2(η2-CH2
CH2)(Me2phen)] (X≠Y = Cl, Br, I) were obtained in equilibrium mixtures with symmetric species [PtX2(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)] (X = Cl, Br, I), following the previously reported procedures.29 Alternatively, similar mixtures of complexes could be also obtained by direct reaction of symmetric [PtX2(η2-CH2
CH2)(Me2phen)] (X = Cl, Br, I), following the previously reported procedures.29 Alternatively, similar mixtures of complexes could be also obtained by direct reaction of symmetric [PtX2(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)] (X = Cl, Br) derivatives with KBr or KI. The asymmetric [PtXY(η2-CH2
CH2)(Me2phen)] (X = Cl, Br) derivatives with KBr or KI. The asymmetric [PtXY(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)] species, with mixed halido ligands, could be identified by 1H and 195Pt NMR spectroscopy. NMR (CDCl3, 600 MHz (1H), 130 MHz (195Pt), 300 K): [PtClBr(η2-CH2
CH2)(Me2phen)] species, with mixed halido ligands, could be identified by 1H and 195Pt NMR spectroscopy. NMR (CDCl3, 600 MHz (1H), 130 MHz (195Pt), 300 K): [PtClBr(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)]. δ(1H) 3.48 (s, 6H, CH3); 3.68 (m, 2H, CH, η2-ethene, 2JPt–H = 70 Hz); 3.78 (m, 2H, CH, η2-ethene, 2JPt–H = 70 Hz); 7.79 (d, 2H, CH, 3JH–H = 8 Hz); 7.85 ppm (s, 2H, CH); 8.31 (d, 2H, CH, 3JH–H = 8 Hz). δ (195Pt) −2456 ppm. [PtClI(η2-CH2
CH2)(Me2phen)]. δ(1H) 3.48 (s, 6H, CH3); 3.68 (m, 2H, CH, η2-ethene, 2JPt–H = 70 Hz); 3.78 (m, 2H, CH, η2-ethene, 2JPt–H = 70 Hz); 7.79 (d, 2H, CH, 3JH–H = 8 Hz); 7.85 ppm (s, 2H, CH); 8.31 (d, 2H, CH, 3JH–H = 8 Hz). δ (195Pt) −2456 ppm. [PtClI(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)]. δ (1H) 3.47 (s, 6H, CH3); 3.68 (m, 2H, CH, η2-ethene, 2JPt–H = 70 Hz); 3.90 (m, 2H, CH, η2-ethene, 2JPt–H = 70 Hz); 7.79 (d, 2H, CH, 3JH–H = 8 Hz); 7.85 ppm (s, 2H, CH); 8.29 (d, 2H, CH, 3JH–H = 8 Hz). δ (195Pt) −3087 ppm. [PtBrI(η2-CH2
CH2)(Me2phen)]. δ (1H) 3.47 (s, 6H, CH3); 3.68 (m, 2H, CH, η2-ethene, 2JPt–H = 70 Hz); 3.90 (m, 2H, CH, η2-ethene, 2JPt–H = 70 Hz); 7.79 (d, 2H, CH, 3JH–H = 8 Hz); 7.85 ppm (s, 2H, CH); 8.29 (d, 2H, CH, 3JH–H = 8 Hz). δ (195Pt) −3087 ppm. [PtBrI(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(Me2phen)]. δ (1H) 3.46 (s, 6H, CH3); 3.68 (m, 2H, CH, η2-ethene, 2JPt–H = 70 Hz); 3.90 (m, 2H, CH, η2-ethene, 2JPt–H = 70 Hz); 7.79 (d, 2H, CH, 3JH–H = 8 Hz); 7.85 ppm (s, 2H, CH); 8.27 (d, 2H, CH, 3JH–H = 8 Hz). δ (195Pt) −3426 ppm.
CH2)(Me2phen)]. δ (1H) 3.46 (s, 6H, CH3); 3.68 (m, 2H, CH, η2-ethene, 2JPt–H = 70 Hz); 3.90 (m, 2H, CH, η2-ethene, 2JPt–H = 70 Hz); 7.79 (d, 2H, CH, 3JH–H = 8 Hz); 7.85 ppm (s, 2H, CH); 8.27 (d, 2H, CH, 3JH–H = 8 Hz). δ (195Pt) −3426 ppm.
The University of Salento (Italy), the PON 254/Ric. Potenziamento del “CENTRO RICERCHE PER LA SALUTE DELL'UOMO E DELL'AMBIENTE” Cod. PONa3_00334, and the Consorzio Interuniversitario di Ricerca in Chimica dei Metalli nei Sistemi Biologici (CIRCMSB), Bari (Italy) are acknowledged for financial support.
Notes and references
- M. Benedetti, F. P. Fanizzi, L. Maresca and G. Natile, Chem. Commun., 2006, 1118 RSC.
- M. Benedetti, C. R. Barone, C. R. Girelli, F. P. Fanizzi, G. Natile and L. Maresca, Dalton Trans., 2014, 43, 3669 RSC.
- M. Benedetti, C. R. Girelli, D. Antonucci and F. P. Fanizzi, J. Organomet. Chem., 2014, 771, 40 CrossRef CAS PubMed.
- V. M. Vecchio, M. Benedetti, D. Migoni, S. A. de Pascali, A. Ciccarese, S. Marsigliante, F. Capitelli and F. P. Fanizzi, Dalton Trans., 2007, 5720 RSC.
- C. R. Barone, M. Benedetti, V. M. Vecchio, F. P. Fanizzi, L. Maresca and G. Natile, Dalton Trans., 2008, 5313 RSC.
- M. Benedetti, D. Antonucci, S. A. de Pascali, C. R. Girelli and F. P. Fanizzi, J. Organomet. Chem., 2012, 714, 60 CrossRef CAS PubMed.
- M. Benedetti, D. Antonucci, S. A. de Pascali, G. Ciccarella and F. P. Fanizzi, J. Organomet. Chem., 2012, 714, 104 CrossRef CAS PubMed.
- J. S. Saad, M. Benedetti, G. Natile and L. G. Marzilli, Inorg. Chem., 2010, 49, 5573 CrossRef CAS PubMed.
- J. S. Saad, M. Benedetti, G. Natile and L. G. Marzilli, Inorg. Chem., 2011, 50, 4559 CrossRef CAS PubMed.
- M. Benedetti, C. R. Barone, D. Antonucci, V. M. Vecchio, A. Ienco, L. Maresca, G. Natile and F. P. Fanizzi, Dalton Trans., 2012, 41, 3014 RSC.
- M. Benedetti, D. Antonucci, C. R. Girelli, F. Capitelli and F. P. Fanizzi, Inorg. Chim. Acta, 2014, 409, 427 CrossRef CAS PubMed.
- M. Benedetti, C. R. Girelli, D. Antonucci, S. A. de Pascali and F. P. Fanizzi, Inorg. Chim. Acta, 2014, 413, 109 CrossRef CAS PubMed.
- M. Benedetti, D. Antonucci, D. Migoni, V. M. Vecchio, C. Ducani and F. P. Fanizzi, ChemMedChem, 2010, 5, 46 CrossRef CAS PubMed.
- F. P. Fanizzi, N. Margiotta, M. Lanfranchi, A. Tiripicchio, G. Pacchioni and G. Natile, Eur. J. Inorg. Chem., 2004, 1705 CrossRef CAS PubMed.
- M. Benedetti, V. Lamacchia, D. Antonucci, P. Papadia, C. Pacifico, G. Natile and F. P. Fanizzi, Dalton Trans., 2014, 43, 8826 RSC.
- S. Mecking, L. K. Johnson, L. Wang and M. Brookhart, J. Am. Chem. Soc., 1998, 120, 888 CrossRef CAS.
- C. Hahn, M. E. Cucciolito and A. Vitagliano, J. Am. Chem. Soc., 2002, 124, 9038 CrossRef CAS PubMed.
- F. Ragaini, M. Gasperini, S. Cenini, L. Arnera, A. Caselli, P. Macchi and N. Casati, Chem. – Eur. J., 2009, 15, 8064 CrossRef CAS PubMed.
- M. Benedetti, D. Antonucci, C. R. Girelli and F. P. Fanizzi, Eur. J. Inorg. Chem., 2015, 2308 CrossRef CAS PubMed.
- M. Benedetti, P. Papadia, C. R. Girelli, F. De Castro, F. Capitelli and F. P. Fanizzi, Inorg. Chim. Acta, 2015, 428, 8 CrossRef CAS PubMed.
- V. G. Albano, G. Natile and A. Panunzi, Coord. Chem. Rev., 1994, 133, 67 CrossRef CAS.
- H. van der Poel and G. van Koten, Inorg. Chem., 1981, 20, 2950 CrossRef CAS.
- M. R. Burger, J. Kramer, H. Chermette and K. R. Koch, Magn. Reson. Chem., 2010, 48, S38 CrossRef CAS PubMed.
- A. C. Tsipis and I. N. Karapetsas, Dalton Trans., 2014, 43, 5409 RSC.
- (a) H. Drews and W. Preetz, Z. Naturforsch., 1997, 52, 435 CAS; (b) W. Preetz, G. Peters and D. Bubitz, Chem. Rev., 1996, 96, 977 CrossRef CAS PubMed.
- K. R. Koch, M. R. Burger, J. Kramer and A. N. Westra, Dalton Trans., 2006, 3277 RSC.
- B. M. Still, P. G. Anil Kumar, J. R. Aldrich-Wright and W. S. Price, Chem. Soc. Rev., 2007, 36, 665 RSC.
- E. Gabano, E. Marengo, M. Bobba, E. Robotti, C. Cassino, M. Botta and D. Osella, Coord. Chem. Rev., 2006, 250, 2158 CrossRef CAS PubMed.
- F. P. Fanizzi, F. P. Intini, L. Maresca, G. Natile, M. Lanfranchi and A. Tiripicchio, J. Chem. Soc., Dalton Trans., 1991, 1007 RSC.
| This journal is © The Royal Society of Chemistry 2015 |




